Abstract
The operation and performance of a biotrickling filter for methanol (MeOH) and hydrogen sulfide (H2S) removal at acid pH was studied. Excess biomass in the filter bed, causing performance loss and high pressure drop, was controlled by intermittent addition, of ozone (O3) and hydrogen peroxide (H2O2). The results showed that after adaptation to acid pH, the maximum elimination capacity (EC) reached for MeOH was 565 g m−3 h −1 (97 % RE). High MeOH loads resulted in increased biomass concentration within the support, triggering reductions in the removal efficiency (RE) for both compounds close to 50 %, and high pressure drop. At this stage, an inlet load of 150.2 ± 16.7 g m−3 h−1 of O3 was fed by 38 days favoring biomass detachment, and EC recovery and lower pressure dropped with a maximum elimination capacity of 587 g m−3 h−1 (81 % RE) and 15.8 g m−3 h−1 (97 % RE) for MeOH and H2S, respectively. After O3 addition, a rapid increase in biomass content and higher fluctuations in pressure drop were observed reducing the system performance. A second treatment with oxidants was implemented feeding a O3 load of 4.8 ± 0.1 g m−3 h−1 for 7 days, followed by H2O2 addition for 23 days, registering 607.5 gbiomass L−1 packing before and 367.5 gbiomass L−1 packing after the oxidant addition. PCR-DGGE analysis of different operating stages showed a clear change in the bacterial populations when O3 was present while the fungal population was less affected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gaseous pollutants from various industrial activities are frequently released to the atmosphere, causing health and environmental problems. Increased concern has driven the quest to improve and find new alternatives to treat these industrial emissions. Gaseous pollutants can be removed by different technologies including physical and chemical methods, such as adsorption, absorption, condensation, and combustion, and by biological-based technologies (Revah and Morgan-Sagastume 2005; Mudliar et al. 2010). Biological methods are considered cost-effective and reliable technologies for the control of waste gases contaminated with different chemical compounds. They are environmentally friendly as they operate at normal conditions and do not require or generate toxic products. These systems include, among others, conventional biofilters (BF); biotrickling filters (BTFs), bioscrubbers, and hollow fiber and membrane bioreactors (Revah and Morgan-Sagastume 2005, Yang et al. 2010). These systems allow treating airstreams at various temperatures (Hu et al. 2015; Kong et al. 2013) and mixtures of pollutants with different chemical properties where diverse microbial populations (i.e., autotrophic and heterotrophic bacteria, yeast, and molds) can develop (Chung 2007; Ding et al. 2006; González-Sanchez et al. 2008; Mudliar et al. 2010; Prenafeta-Boldú et al. 2012).
Although biofiltration has many advantages, it also presents some problems affecting its long-term performance. Excessive biomass accumulation, causing fluid clogging and channeling, is considered among the major problems in BTF as it induces excessive head loss and low performance and increased the operational costs (Sempere et al. 2008; Yang et al. 2010). There are different alternatives for removing excess biomass to improve the performance in biofiltration systems including physical, chemical, and biological methods. Although they are generally effective, some of them could affect the system performance or present implementation problems in real systems (Xi et al. 2014; Yang et al. 2010; Zehraoui et al. 2013). In this sense, chemical compounds such as NaOH and NaClO have been effective to remove or control the excessive biomass; however, the use of chemical solutions often leads to considerable drops in biofilter performance, requiring a period of up to several days to reacclimatize.
Methods based on the use of oxidants, such as ozone, have shown to be effective in biofiltration systems (Moussavi and Mohseni 2007; Wang et al. 2009). Hydrogen peroxide has also been used to remove or prevent biofilm accumulation by removing exopolysaccharides (EPS) by the disruption of the polymeric backbone. Examples include the detachment and removal of biofilms in reactors with low (<0.1 mM H2O2) concentrations (Christensen et al. 1990) and the reduction of head loss in granular activated carbon/sand filters for drinking water (Lauderdale et al. 2012). Furthermore, the combination of both oxidants was studied for removal of biofilms formed by Pseudomonas fluorescens. The results suggest that the hydroxyl radicals (•OH) generated in the biofilm by the sequential treatment (O3 followed by H2O2) might react with biofilm constituents, such as EPS rather than with intracellular components (Tachikawa and Yamanaka 2014). The use of oxidants for biomass control has mainly been demonstrated in BF system treating of individual volatile organic compounds, such as toluene, xylene, and formaldehyde, at neutral pH. Nonetheless, clogging or excessive biomass accumulation can also be present with mixed pollutants in BTF operating at different pH conditions. The treatment of methanol and hydrogen sulfide released by the pulp and paper industry induces both a decrease in the pH of the system due to the oxidation of H2S to sulfate and excessive growth of fungal biomass on MeOH causing clogging and operational problems (Jin et al. 2007; Rene et al. 2010; Sologar et al. 2003).
Increased knowledge on BF performance and associated microbial populations can be obtained by using molecular techniques to evidence the dynamics of microbial communities in response to changes in operating conditions such as the addition of a new substrate or changes in temperature or pH (Ding et al. 2006). Biochemical methods such as respirometry have also been used to evaluate the microbial activity in the reactors under different conditions (Arellano-Garcia et al. 2010).
As further research is necessary to evaluate the use of oxidants in the biofiltration system, the main objective of this work was to investigate the use of O3 and H2O2 to remove the excess of biomass in a BTF treating MeOH and H2S under different loads and the impact of these oxidants on the performance of the system and structure and activity of the microbial community.
Materials and methods
Inoculum preparation and mineral medium composition
A mixture of aerobic and anaerobic sludges obtained from two different wastewater treatment plants located in the municipality of Iztapalapa in Mexico City and an acid mine drainage sludge from the state of San Luis Potosi, Mexico, were used as the inoculum source. The mixed culture was acclimatized for 3 months at acid pH (2.5–3) in the mineral medium (MM) described below supplemented with MeOH at 0.5 % (v/v) and 8 g L−1 of Na2S2O3.5H2O as the source of sulfur. The MM used in the reactor was modified from Jin et al. (2007) and contained (g L−1) KH2PO4 (4.0), K2HPO4 (4.0), NH4Cl (0.8), MgCl2·6H2O (0.4), FeSO4·7H2O (0.02), adjusting the pH to 2.5 with a solution of HCl.
Experimental setup
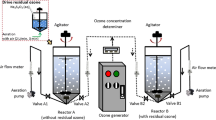
The BTF system, shown in Fig. 1, comprises an acrylic rectangular reactor (12 × 12 × 52 cm) packed with polyurethane foam (PUF) cubes (4 × 4 × 4 cm, Fig. S1) similar to the one previously described by González-Sánchez et al. (2008). The reactor was divided into two sections with a total packing volume of 2.3 L and several ports for biomass sampling. An air stream was bubbled through a vessel containing MeOH and then mixed with a stream containing H2S. The resulting waste gas was introduced through the bottom of the column for counter-current flow. The pH was maintained by feeding a solution of 0.5 M of NaOH with a metering pump (Barnant-HD-MA-Pump). The nutrient solution was renewed twice a week. Pressure drop measurements were made using a U-tube manometer and are expressed as millimeters of H2O per meter of support. Experiments were conducted at room temperature. The operational conditions in the BTF are described in Table 1.
For the O3 experiments, a corona discharge ozone generator (A2Z Ozone, Inc., USA: A2ZS-1GLAB) with a maximum production of 1000 mg h−1 was used. Ambient air was passed through the generator (800 mL min−1 for the first period and 200 mL min−1 for the second one) and then mixed with the regular air stream before feeding the biofilter. Table 1 shows the O3 inlet loads. H2O2 was added in the mineral medium during recirculation once a day and determined as reported by Tachikawa and Yamanaka (2014). H2O2 was fed at concentrations of 1 mg L−1 in two time intervals: 437–438 and 442–451 days and then at concentration of 5 mg L−1 from day 453 to day 459.
Biomass content and activity
The total biomass within the reactor was measured at different stages to evaluate the effect of oxidants. The liquid flow was stopped for 12 h to allow the retained liquid to trickle, and the packing was removed and weighed. The biomass content was calculated by subtracting the packing weight.
The microbial activity was studied by respirometry. A biomass sample was taken from the packing, centrifuged, and resuspended twice in fresh MM. To evaluate the oxygen uptake rate (OUR), 200 μL of biomass and different MeOH concentrations from 35 to ~400 mM were analyzed at 25 °C in the respirometry chamber previously described by Arellano-García et al. (2010).
Biofilm analysis by magnetic resonance imaging
Analysis of MRI was made in one colonized cube of packing material removed from the reactor. The cube was extracted from the filter bed after draining the retained liquid; then, it was analyzed in a magnetic scanner (Varian, VNMR 7 T, USA). Gradient echo multislice (GEMS) sequences were acquired in a 50 per 50 mm field with a 512 × 512 pixel matrix giving a resolution of 97.5 μm. Sequences of 80 images were acquired from each cube taking a slice image every 0.5 mm registering the biofilm structure impregnated using gadolinium as contrast medium. The volume of the biofilm was calculated from 3D reconstructions made with the OsiriX imaging software (Arellano-García et al. 2015).
Microbial community analysis
Samples of biomass were taken from the BTF at different sections, before, during, and after O3 addition. The biomass samples were washed threefold in a saline solution centrifuging at 4000 rpm for 10 min, and finally the pellets were stored frozen at −80 °C until analysis. Total DNA was extracted with the PowerSoil® DNA Isolation Kit following the manufacturer’s instructions. The GC-338F and 907R primers were used to amplify the V3-V5 region of the bacterial 16S ribosomal RNA (rRNA) gene. The PCR reactions were performed with 50 ng DNA (final volume 25 μL) using Hot Start DNA Polymerase (Qiagen) as described by the manufacturer except that bovine serum albumin was added at a final concentration of 400 μg/mL. The PCR program consisted of initial denaturation at 95 °C for 15 min, 10 touchdown cycles of denaturation at 94 °C for 30 s, annealing at 56 °C (−0.5 °C per cycle) for 30 s, and extension at 72 °C for 60 s, followed by 25 cycles of 94 °C for 30 s, 51 °C for 30 s, and 72 °C for 1 min, and a final extension step of 72 °C for 10 min. The V1–V2 region of the fungal 18S rRNA gene was amplified with the NS1 and GCFung primers. PCR reactions were performed with 25 ng DNA (final volume 25 μL) using Hot Start DNA Polymerase (Qiagen) as described by the manufacturer except that bovine serum albumin was added at a final concentration of 400 μg/mL and MgCl2 was used at a final concentration of 2.5 mM. The PCR program consisted of initial denaturation at 95 °C for 15 min, 35 cycles of 94 °C for 30 s, 56 °C for 45 s, and 72 °C for 30 s, and a final extension step of 72 °C for 10 min. The acrylamide concentration in the denaturing gradient gel electrophoresis (DDGE) for bacteria was 6 % and the denaturing gradient was 40 and 60 %. An aliquot of 5 μL of the PCR product was run at 50 V for 16 h. For fungal DGGE gels, the acrylamide concentration was 6 % and the denaturing gradient was 30 and 60 %. An aliquot of 3 μL of the PCR product was run at 65 V for 19 h (DCode Bio-Rad equipment). The bands were visualized by staining the DGGE gels with silver nitrate. Selected bands were excised from the gel, reamplified, purified with the Kit Wizard® SV Gel and PCR Clean-Up System (Promega), and sequenced at the UAM-Iztapalapa Molecular Biology Laboratory (Mexico). The DNA sequences were analyzed using the biocomputing tools provided online by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). The comparison was made using the Basic Local Alignment Search Tool (BLAST). The nucleotide sequences in this study have been deposited in GenBank, and the corresponding accession numbers are shown Table 2.
Analytical methods
Gaseous MeOH concentrations were measured with a gas chromatograph Agilent Technologies 6890 with a capillary column (Alltech AT-FAME, 30 m × 250 μm × 0.25 μm), equipped with a flame ionization detector (FID), and helium was used as the carrier gas at a flow rate of 0.4 mL min−1. H2S was monitored with an OdaLog analyzer LL-H2S-1000. O3 detection in gas phase was made by the iodometric method (Rakness et al. 1996). Proteins were measured by the Lowry method, and carbohydrates were measured according to Dubois et al. (1956). Volatile solids (VS) were quantified in triplicate using standard methods (APHA 1998).
Results
Biotrickling filter performance
The BTF operated for 460 days; Table 1 shows the main operational conditions (inlet loads, pH, and addition of oxidants) and the resulting performance. H2S and MeOH were introduced simultaneously with initial inlet loads of 4.7 ± 0.2 and 13.4 ± 1.6 g m−3 h−1, respectively. While H2S was eliminated from the onset of the experiment, no detectable MeOH removal was observed during the first 26 days probably due to the low pH values (<1.0) induced by H2S oxidation to SO4 =. At day 24, the system was reinoculated and the pH was controlled in the 3.8–4.3 range. These conditions allowed increased MeOH uptake, and RE values close to 100 % were observed at day 29. The pH control was then gradually decreased to 2.5 and maintained thereof.
Biomass accumulation
MeOH consumption fostered biomass accumulation, and consequently, pressure drop increased as shown in Fig. 2. For almost 180 days of operation, the pressure drop was below 25 mmH2O m−1 maintaining steady H2S and MeOH removals. Accumulated biomass reduced mainly H2S removal, reaching RE values down to 50 % on day 236 where the packing material was extracted showing a wet biomass content of 236 gbiomass L−1 packing (Figs. 3 and S2). The colonized support cubes were reshuffled and packed again. Pressure drop values decreased down to 6.3 mmH2O m−1, and the system performance improved, but only for a few days, returning to the above conditions (Fig. 2, stage I). Increased growth and water accumulation was observed on some days over the filter bed by the presence of a biomass layer causing an obstruction in the system and fluctuations in the pressure drop (Fig. S5), and lower methanol removals followed down to 50 % RE on day 250.
Effect of the addition of oxidants on system performance
During the period of the first O3 addition with loads of 150.2 ± 16.7 g m−3 h−1 (Fig. 2, stage II), the pressure drop decreased and the performance recovered, obtaining the maximum EC found throughout this study of 587 g m−3 h−1 (81 % RE) and 15.8 g m−3 h−1 (97 % RE) for MeOH and H2S, respectively. Biomass content in this period was 333 gbiomass L−1 packing (Fig. 3). The stability in the RE for both pollutants was maintained for almost 30 days. A lower performance was observed thereafter, possibly by a negative effect of continuous O3 feed over microbial activity. Therefore, oxidant delivery was suspended observing a recovery in the RE in the subsequent 10 days coupled to fast biomass growth and increased pressure drop. The system operated under these conditions for more 100 days showed a notable decrease on MeOH removal (Fig. 2, stage III) at around day 370. To evaluate possible MeOH toxicity, the inlet loads were decreased on day 396 improving the global performance and reaching a 92 % RE (EC of 293 gMeOH m−3 h−1) but continued diminishing thereafter. During stage III on days 373 and 423, the filter bed was removed, weighed, and reintroduced in the reactor; the biomass on these days was 549 and 607 gbiomass L−1 packing, respectively (Fig. 3). MRI images of the support during this period (Fig. S3) showed a dense biofilm forming patches that almost completely obstructed sections of the packing, allowing only a small void volume for air and liquid distribution.
The pressure drop reached a maximum value of 344 mmH2O m−1 before O3 addition, decreasing down to less than 7 mmH2O m−1 during O3 treatment (MeOH inlet load was 622 ± 55.3 g m−3 h−1). After suspending the O3 feed, the pressure drop fluctuations increased up to a maximum value of 718 mmH2O m−1 on day 324 with similar MeOH loads. The mat of biomass was observed again on top of the lower section of the filter bed (Fig. S6).
A second O3 addition for 7 days from day 430 to 437 (pressure drop 156 and 231 mmH2Om−1, respectively) and with a lower load than the previous test (4.8 ± 0.1 g m−3 h−1) was performed to reduce the impact on activity. The biofilter did not improve the performance, and the RE continued to decrease reaching a value of 14 % of RE for MeOH and 44 % of RE for H2S. Hydrogen peroxide addition thereafter allowed a rapid response, and 88 % of RE and 70 % of RE for H2S and MeOH, respectively, were obtained in few days along with a reduction in the biomass content in the packing of around 40 % with a decrease of pressure drop of about 98 %, as shown in Fig. 3. A temporary spike in pressure drop was observed at around day 450 when 5 mg L−1 H2O2 addition provoked excess biomass detachment and transient clogging (Fig. 2 stage V). Interestingly, H2O2 addition reduced the density of the detached biomass which floated, in contrast to O3 addition where biomass debris settled, probably due to oxygen microbubbles formed from bacteria having catalase activity.
Content of substances in the liquid phase
Volatile solids in the recirculation liquid related to biomass detachment from the support increased during the addition of oxidants as shown in Fig. 4a. The values were around 80 % increase for the first O3 addition and 40 % for the second O3 addition. Soluble solids increased further (170 % with respect to the concentration before O3 addition) with the H2O2 treatment. Figure 4b presents the results of the protein and carbohydrate content in the recirculation liquid showing a global increase in the molecules when oxidants were added, which is consistent with biofilm detachment. Interestingly, the relative concentration varied with the oxidant use and higher protein values were found when the H2O2 treatment was applied.
Effect of the O3 on microbial activity
To evaluate the metabolic activity under O3 addition, the endogenous respiration was initially assessed yielding values of 61 ± 8 and 35.8 ± 2.4 μmol O2 g−1 protein min−1 for the biomass under normal operation and with O3 addition, respectively. The values for the activity at different MeOH concentrations depicted in Fig. 5 and corrected for the endogenous respiration show that O3 treatment reduced approximately by half the maximum OUR from 97 to 43 μmol O2 g−1 protein min−1. The Ks obtained by fitting a Monod-type model was 67 mmol L−1 without O3 exposure and was strongly reduced with O3 addition. The endogenous respiration was also reduced by around 40 % when ozone was supplied reflecting the viability reduction of the cells.
Microbial dynamics
The profile along the column reactor showed a decrease in the number of bands in the lanes corresponding to the samples taken in the lower section of the BTF (DII and DIII) after O3 addition, which can be attributed to the effect of this oxidant on the microbial populations in this section for both bacteria and fungi (Fig. S7 and Table 2). The fungal population profiles showed some bands before O3 addition in the lanes AI, BI, CI, and DI that could not be found in the other sections (Fig. S7b), i.e., bands AI1, BI1, CI1, and DI1 clearly disappeared after supplying the oxidant. Band AI-1 was identified as Ophiostoma sp. (Table 2) which did not seem to show resistance to O3. In general, the number and/or intensity of bands decreased after O3 treatment both for bacteria and fungi, which is consistent with the negative effect of O3 microbial activity previously presented. Concerning bacteria, O3 addition led to the appearance of new bands (Fig. S7a: AII, BII, CII, and DII) that disappeared later as O3 addition was stopped (bands AII-5, AII-6, BII-2, BII-3, CII-2, CII-3, DII-2, DII-3, DII-4, and DII-5). One strong band (AII-5, BII-2, CII-2, DII-2), corresponding to Acidiphilium sp. was found only during O3 treatment. After O3 treatment, one band (AIII-1, BIII-1, CIII-1, DIII-1), initially present in the consortium (AI-2, BI-2, CI-2, DI-2), was enriched. It corresponded to Acidithiobacillus sp. Dg-E11; the other Blast matches indicated that this isolate was affiliated to the A. thiooxidans genus. Concerning fungi, after O3 treatment, two bands were more intense (AIII-7, BIII-8, CIII-6, DIII-6 and AIII-10, BIII-11, CIII-9, DIII-8). Band DIII-6, AII-10, and AI-14 matched with uncultured fungi related to Pezizomycotina (See Table 2 and Fig. S7).
Discussion
Reactor startup showed that acidification due to H2S oxidation to SO4 = impeded initial MeOH removal which was detected only after pH was controlled and the system reinoculated (Fig. 2, stage I). The maximum ECs for MeOH and H2S for this experimental period were 565 g m−3 h−1 (97 % RE) and 13 g m−3 h−1 (100 % RE), respectively. Microscopic observation showed an abundant yeast presence favored by the acidic conditions. These results are consistent with those reported in the literature where a higher eukaryote population was found in the presence of MeOH in a system without pH control (Barcón et al. 2012). Regarding the performance, the results are comparable with those at acid pH previously reporting ECmax of 236 gCH3OH m−3 h−1 (inlet load of ~250 g m−3 h−1) and 6.4 gH2S m−3 h−1 (RE = 100 %) to pH = 2.0 (Jin et al. (2007).
Biomass increased in the BTF was mainly due to heterotrophic growth on MeOH which allowed higher biomass yields for fungi (Egli and Fiechter 1981) compared to autotrophic bacteria (Alcántara et al. 2004). Excessive growth impaired H2S and MeOH removals. Similar problems in the treatment of H2S and MeOH were reported by Jin et al. (2007), where accumulation of biomass and elemental sulfur, from partial H2S oxidation, caused a pressure drop increase and RE declined to 15 % compared with the value when only H2S was treated. In our study, elemental sulfur accumulation was not observed in the filter bed and sulfate concentrations were close to the stoichiometric values (results not shown); these findings are consistent with those generally observed with low sulfide loads (Alcántara et al. 2004). Reshuffling the packing material on day 236 decreased pressure drop and allowed an increase in RE (Fig. 2, stage I). This step allowed reducing the macroscopic heterogeneity in the bed by limiting channeling and the zones where excess growth limited both air and liquid flow, thus recovering the microbial activity (Auria et al. 2000). Furthermore, channeling forms pockets of biomass that may be subject to drying with the concomitant activity reduction (Cercado et al. 2012). Several methods to remove excess biomass, and hence decrease the pressure drop, such as increasing the liquid flow and using alkaline chemical solutions, may not be sufficient as they affect biomass activity or may have short-term effectiveness (Flores-Valle et al. 2011; Wang et al. 2014) so other control strategies may be necessary.
Attached biomass content was controlled during the period of O3 addition. This oxidant affects the biofilm structure by attacking the EPS, thus inducing biomass detachment, improving gas flow distribution, lowering the pressure drop, and improving performance. The oxidizing potential of O3 increases at low pH (pH <4) reacting primarily with double bonds and aromatic groups and slightly with carbohydrates (Adams and Gorg 2002; Meng et al. 2016; Roncero et al. 2003), which may explain the sustained activity despite the high loads used. The results are consistent with previous studies (Xi et al. 2014) where the bed pressure drop remained below to 5.7 mmH2O m−1 (superficial velocity 17.7 m h−1) throughout the operating period with O3 inlet load in a range from 10.5 to 18.8 g m−3 h−1 in a conventional biofilter. After O3 addition was halted, the system operated for 100 days showing strong biomass growth (up to 607 gbiomass L−1 packing) with high variations in the pressure drop and in the RE for MeOH and H2S (Fig. 2, stage III). The strong fluctuations in pressure drop before and after the O3 addition resulted from heterogeneous biomass growth which was visible by the formation of gelatinous pockets in the support and mats over the parts exposed to air. MRI showed a total volume compaction of around 20 %; this value is higher than expected and was probably due to a reduction in the actual volume of the cubes caused by the weight from cubes positioned above and possibly to a decrease in the resilience of the PUF caused by O3. Numerical estimation of the occupied volume yields a value of 85 % (Fig. S3). The compaction was also observed in both sections of the BTF where the bed height reduced to almost 13 %.
The second O3 addition reduced slightly the pressure drop but without improving the performance as compared with the first addition which may be attributed to the lower O3 load used in this period. Furthermore, biomass growth was extensive and may have hindered O3 distribution, biofilm diffusion, and oxidative activity. The subsequent H2O2 addition that, in contrast to O3, oxidizes some polysaccharides at low pH (Christensen et al. 1990; Miller 1986) allowed further cell detachment, lower pressure drop, and improved system performance. The effectiveness of H2O2 can be partly attributed to a synergistic effect with O3, (Tachikawa and Yamanaka 2014) and also to the H2O2 interaction with the Fe2+ present in the mineral medium producing •OH radicals that could react with the biofilm and favor biomass detachment (Gosselin et al. 2013).
Biofilm detachment by the action of the oxidants increased solids in the recirculation liquid which was consistent with those reported in the literature where the thickness of biofilm was reduced along to an increase of the microbial activity (García-Pérez et al. 2013; Wang et al. 2009). The action of the oxidants on the detached biofilms may as well favor depolymerization and uptake by the suspended and attached cells which may explain the variations in soluble and suspended protein and carbohydrates (Meng et al. 2016). The addition of O3 also affected the microbial activity as shown by the change in endogenous respiration and the activity. This alteration in the metabolic activity reflects the structural changes of the population caused by the variable sensitivities of the different species (Wei et al. 2013). The negative effect of O3 was also reported by Xi et al. (2014), where the deterioration of toluene removal was attributed to a decrease in activity by microorganisms susceptible to higher O3 concentration.
An unexpected consequence of the addition of O3 for an extended period was the damage observed in the packing material, particularly in the bottom section (Fig. 1), where there was a direct contact with the incoming feed. This corresponded to the reduction of the number of bands of the DGGE analyses described before. Small pieces of the material were also observed in the liquid phase, which explain the degradation shown in the Fig. S4. The double bonds present in the polyurethane chemical structure could be the main reason of its deterioration due to higher reactivity of O3 toward unsaturated compounds (Adams and Gorg 2002). It has been reported that O3 can change the chemical and physical properties of polyurethane foam increasing the porosity and reducing its hydrophobicity (Bertoldi et al. 2015).
The relevant effect of the addition of oxidants was evident as the changes on microbial populations were more noticeable through the different operating conditions implemented than throughout the different sections of the filter bed. The bands sequenced from fungal DGGE profiles were all related to the Pezizomycotina, a subphylum of the Ascomycota. Comparative phylogenetic analyses have shown that MeOH utilization genes are conserved in several Pezizomycotina lineages, indicating their potential capability to use MeOH as a carbon and energy source (Ravin et al. 2013). Ophiostoma sp. was apparently eliminated after O3 addition; this fungus had already been reported in a BF used for the degradation of gas-phase mixtures of MeOH, α-pinene, and H2S (López et al. 2013). The bands remaining after O3 treatment corresponded to uncultured fungi. The higher number of bands in the fungal DGGE profiles compared to bacteria (Fig. S7b) could be explained as follows. Firstly, fungal biomass is more abundant in the BTF due to growth on MeOH, compared to bacteria which only grow autotrophically with H2S which was fed in lower concentrations. Thus, MeOH consumption by fungi may promote the formation of a dense biofilm. In addition, the faster growth of MeOH consumers could limit the growth of autotrophic microorganisms, which would explain the lower number of bands for bacteria in all stages (Fig. S7a). Secondly, the resistance of fungi to adverse conditions such as O3 presence and acidic pH exposure could help to preserve their abundance (Dong et al. 2015; Mara and Horan 2003; Prenafeta-Boldú et al. 2012 ). Furthermore, suspended and soluble material from the biofilm may also promote the development of heterotrophic microorganisms which are not necessarily H2S consumers.
The bacterial band detected under O3 treatment (AII5: Acidiphilium sp.) suggests that the growth of this microorganism may be favored by high ozone tolerance (Wei et al. 2013) and possibly to the presence of some molecules enhanced by the ozone oxidation. It has been reported that some strains of this microorganism are capable to oxidize elemental sulfur to sulfate as a final metabolite and consume a high variety of organic substrates (Arellano-García et al. 2015; Zhang et al. 2013). In our study, Acidithiobacillus sp. could not have grown in high O3 concentration, as indicated in the DGGE analyses; however, it remained viable, possibly protected by the dense biofilms, reappearing once the conditions were again favorable.
In conclusion, both O3 and H2O2 were effective to remove excess biomass accumulation in trickle bed biofilters and offset the reduction in performance caused by excess biomass. From a practical point of view, it has been reported that continuous ozone addition may entail significant investment and operation costs (Xi et al. 2014), but the results found in the present study suggest that the use of H2O2 could be an interesting alternative. Oxidants altered the established population although this proved to be reversible when oxidant addition was stopped. The continuous use of the oxidants may have a deleterious effect on the support which requires further study if this strategy is adopted for the biomass control.
References
Adams CD, Gorg S (2002) Effect of pH and gas-phase ozone concentration on the decolorization of common textile dyes. J Environ Eng 128:293–298. doi:10.1061/(ASCE)0733-9372~2002!128:3(293)
Alcántara S, Velasco A, Revah S (2004) Sulfur formation by steady-state continuous cultures of a sulfoxidizing consortium and Thiobacillus thioparus ATCC 23645. Environm Technol 25:1151–1157. doi:10.1080/09593330.2004.9619409
APHA, Standards Methods for the Examination of Water of Wastewater (1998) 20th ed. American Public Health Association-American Water Works. Association-Water Pollution Control Federation (AWWA-WPCF), Washington, DC
Arellano-García L, González-Sánchez A, Baquerizo G, Hernández-Jimenez S, Revah S (2010) Treatment of carbon disulfide and ethanethiol vapors in alkaline biotrickling filters using an alkaliphilic sulfo-oxidizing bacterial consortium. J Chem Technol Biotechnol 85:328–335. doi:10.1002/jctb.2328
Arellano-García L, Dorado AD, Morales-Guadarrama A, Sacristan E, Gamisans X, Revah S (2015) Modelling the effects of biomass accumulation on the performance of a biotrickling filter packed with PUF support for the alkaline biotreatment of dimethyl disulfide vapors in air. Appl Microbiol Biotechnol 99:97–107. doi:10.1007/s00253-014-5929-7
Auria R, Frere G, Morales M, Acuña ME, Revah S (2000) Influence of mixing and water addition on the removal rate of toluene vapors in a biofilter. Biotechnol Bioeng 68:448–455. doi:10.1002/(SICI)1097-0290(20000520)68:4<448::AID-BIT10>3.0.CO;2-2
Barcón T, Alonso-Gutierrez J, Omil F (2012) Molecular and physiological approaches to understand the ecology of methanol degradation during the biofiltration of air streams. Chemosphere 87:1179–1185. doi:10.1016/j.chemosphere.2011.12.039
Bertoldi S, Farè S, Haugen HJ, Tanzi MC (2015) Exploiting novel sterilization techniques for porous polyurethane scaffolds. J Mater Sci Mater Med 26:182. doi:10.1007/s10856-015-5509-0
Cercado B, Auria R, Cardenas B, Revah S (2012) Characterization of artificially dried biofilms for air biofiltration studies. J Environ Sci Health A Tox Hazard Subst Environ Eng 47:940–948. doi:10.1080/10934529.2012.667292
Christensen BE, Naper T, Vollan K, Smidsrod O, Bake R (1990) Biofilm removal by low concentration of hydrogen peroxide. Biofouling 2:165–175. doi:10.1080/08927019009378142
Chung YC (2007) Evaluation of gas removal and bacterial community diversity in a biofilter developed to treat composting exhaust gases. J Hazard Mater 144:377–385. doi:10.1016/j.jhazmat.2006.10.045
Ding Y, Das KC, Whitman WB, Kastner JR (2006) Enhanced biofiltration of hydrogen sulfide in the presence of methanol and resultant bacterial diversity. Trans ASABE 49:2051–2059. doi:10.13031/2013.22266
Dong T, Zhang Y, Islam MDS, Liu Y, El-Din MG (2015) The impact of various ozone pretreatment doses on the performance of endogenous microbial communities for the remediation of oil sands process-affected water. Int Biodeter Biodegr 100:17–28. doi:10.1016/j.ibiod.2015.01.014
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. doi:10.1021/ac60111a017
Egli T, Fiechter A (1981) Theoretical analysis of media used in the growth of yeasts on methanol. J Gen Microbiol 123:365–369. doi:10.1099/00221287-123-2-365
Flores-Valle SO, Ríos-Bernÿ O, Chanona-Pérez J, Fregoso-Aguilar T, Morales-González JA, Prado-Rubianes OJ, Herrera-Bucio R, López-Albarán P, Morales-González A, Garibay-Febles V, Domínguez EG, Kennes C, Veiga-Barbazán MC, Mendoza-Pérez JA (2011) A comparative study of physical and chemical processes for removal of biomass in biofilters. Molecules 16:6927–6949. doi:10.3390/molecules16086927
García-Pérez T, Aizpuru A, Arriaga S (2013) By-passing acidification limitations during the biofiltration of high formaldehyde loads via the application of ozone pulses. J Hazard Mater 262:732–740. doi:10.1016/j.jhazmat.2013.09.053
González-Sánchez A, Revah S, Deshusses MA (2008) Alkaline biofiltration of H2S odors. Environ Sci Technol 42:7398–7404. doi:10.1021/es800437f
Gosselin F, Madeira LM, Juhna T, Block JC (2013) Drinking water and biofilm disinfection by Fenton-like reaction. Water Res 47:5631–5638. doi:10.1016/j.watres.2013.06.036
Hu QY, Wang C, Huang KX (2015) Biofiltration performance and characteristics of high-temperature gaseous benzene, hexane and toluene. Chem Eng J 27:689–695. doi:10.1016/j.cej.2015.05.019
Jin Y, Veiga MC, Kennes C (2007) Co-treatment of hydrogen sulfide and methanol in a single-stage biotrickling filter under acidic conditions. Chemosphere 68:1186–1193. doi:10.1016/j.chemosphere.2007.01.069
Kong X, Wang C, Ji M (2013) Analysis of microbial metabolic characteristics in mesophilic and thermophilic biofilters using Biolog plate technique. Chem Eng J 230:415–421. doi:10.1016/j.cej.2013.06.073
Lauderdale C, Chadik P, Kirisits MJ, Brown AJ (2012) Engineered biofiltration: enhanced biofilter performance through nutrient and peroxide addition. J Am Water Works ASS 104:E298–E309. doi:10.5942/jawwa.2012.104.0073
López ME, Rene ER, Malhautier L, Rocher J, Bayle S, Veiga MC, Kennes C (2013) One-stage biotrickling filter for the removal of a mixture of volatile pollutants from air: performance and microbial community analysis. Bioresour Technol 138:245–252. doi:10.1016/j.biortech.2013.03.136
Mara D, Horan NJ (2003) Handbook of water and wastewater microbiology. Academic press, 832. ISBN9780124701007
Meng L, Xi J, Yeung M (2016) Degradation of extracellular polymeric substances (EPS) extracted from activated sludge by low-concentration ozonation. Chemosphere 147:248–255. doi:10.1016/j.chemosphere.2015.12.060
Miller AR (1986) Oxidation of cell wall polysaccharides by hydrogen peroxide: a potential mechanism for cell wall breakdown in plants. Biochem Biophys Res Commun 141:238–244. doi:10.1016/S0006-291X(86)80359-X
Moussavi G, Mohseni M (2007) Using UV pretreatment to enhance biofiltration of mixtures of aromatic VOCs. J Hazard Mater 144:59–66. doi:10.1016/j.jhazmat.2006.09.086
Mudliar S, Giri B, Padoley K, Satpute D, Dixit R, Bhatt P, Pandey R, Juwarkar A, Vaidya A (2010) Bioreactors for treatment of VOCs and odours—a review. J Environ Manag 91:1039–1054. doi:10.1016/j.jenvman.2010.01.006
Prenafeta-Boldú FX, Guivernau M, Gallastegui G, Viñas M, Sybren de Hoog G, Elías A (2012) Fungal/bacterial interactions during the biodegradation of TEX hydrocarbons (toluene, ethylbenzene and p-xylene) in gas biofilters operated under xerophilic conditions. FEMS Microbiol Ecol 80:722–734. doi:10.1111/j.1574-6941.2012.01344.x
Rakness K, Gordon G, Langlais B, Masschelein W, Matsumoto N, Richard Y, Michael RC, Somiya I (1996) Guideline for measurement of ozone concentration in the process gas from an ozone generator. Ozone Sci Eng 18:209–229. doi:10.1080/01919519608547327
Ravin NV, Eldarov MA, Kadnikov VV, Beletsky AV, Schneider J, Mardanova ES, Elena M, Smekalova EM, Zvereva MI, Dontsova OA, Mardanov AV, Skryabin KG (2013) Genome sequence and analysis of methylotrophic yeast Hansenula polymorpha DL1. BMC Genomics 14:837. doi:10.1186/1471-2164-14-837
Rene ER, López ME, Veiga MC, Kennes C (2010) Steady- and transient-state operation of a two-stage bioreactor for the treatment of a gaseous mixture of hydrogen sulphide, methanol and α-pinene. J Chem Technol Biotechnol 85:336–348. doi:10.1002/jctb.2343
Revah S, Morgan-Sagastume JM (2005) Methods of odor and VOC control. In: Biotechnology for odor and air pollution control. Springer Berlin Heidelberg, pp 29–63
Roncero MB, Queral MA, Colom JF, Vidal T (2003) Why acid pH increases the selectivity of the ozone bleaching processes. Ozone-Sci Eng 25:523–534. doi:10.1080/01919510390481838
Sempere F, Gabaldón C, Martínez-Soria V, Marzal P, Penya-roja JM, Álvarez-Hornos FJ (2008) Performance evaluation of a biotrickling filter treating a mixture of oxygenated VOCs during intermittent loading. Chemosphere 73:1533–1539. doi:10.1016/j.chemosphere.2008.08.037
Sologar VS, Lu Z, Allen DG (2003) Biofiltration of concentrated mixtures of hydrogen sulfide and methanol. Environ Prog 22:129–136. doi:10.1002/ep.670220215
Tachikawa M, Yamanaka K (2014) Synergistic disinfection and removal of biofilms by a sequential two-step treatment with ozone followed by hydrogen peroxide. Water Res 64:94–101. doi:10.1016/j.watres.2014.06.047
Wang C, Xi JY, Hu HY, Yao Y (2009) Stimulative effects of ozone on biofilter treating gaseous Chlorobenzene. Environ Sci Technol 43:9407–9412. doi:10.1021/es9019035
Wang L, Yang C, Cheng Y, Huang J, Yang H, Zeng G, Lu L, He S (2014) Enhanced removal of ethylbenzene from gas streams in biotrickling filters by Tween-20 and Zn (II). J Environ Sci 26:2500–2507. doi:10.1016/j.jes.2014.04.011
Wei ZS, Li HQ, He JC, Ye HQ, Huang QR, Luo YW (2013) Removal of dimethyl sulfide by the combination of non-thermal plasma and biological process. Bioresour Technol 146:451–456. doi:10.1080/10934529709376619
Xi J, Saingam P, Gu F, Hu YH, Zhao X (2014) Effect of continuous ozone injection on performance and biomass accumulation of biofilters treating gaseous toluene. Appl Microbiol Biotechnol 98:9437–9446. doi:10.1007/s00253-014-5888-z
Yang C, Chen H, Zeng G, Yu G, Lou S (2010) Biomass accumulation and control strategies in gas biofiltration. Biotechnol Adv 28:531–540. doi:10.1016/j.biotechadv.2010.04.002
Zehraoui A, Hassan AA, Sorial GA (2013) Biological treatment of n-hexane and methanol in trickle bed air biofilters under acidic conditions. Biochem Eng J 77:129–135. doi:10.1016/j.bej.2013.06.001
Zhang Y, Peng A, Yang Y, Liu J, Qiu G (2013) Isolation, characterization of Acidiphilium sp. DX1-1 and ore bioleaching by this acidophilic mixotrophic organism. Trans Nonferrous Met Soc China 23:1774–1782. doi:10.1016/S1003-6326(13)62660-6
Acknowledgments
The authors thank Universidad Autónoma Metropolitana and CONACYT for the scholarship (372404), as well as the CI3M Center by conducting analyses of MRI. Special thanks to Dra. Angélica López Moreno for their help and collaboration in the analysis of molecular biology, as well as to Dra. Alejandra Serrato Díaz and to the Laboratory of Molecular Biology of the UAM-Iztapalapa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 449 kb)
Rights and permissions
About this article
Cite this article
García-Pérez, T., Le Borgne, S. & Revah, S. Ozone and hydrogen peroxide as strategies to control biomass in a trickling filter to treat methanol and hydrogen sulfide under acidic conditions. Appl Microbiol Biotechnol 100, 10637–10647 (2016). https://doi.org/10.1007/s00253-016-7861-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7861-5