Abstract
There are few studies on mercury content in hypersaline waters. Mercury content was studied in Bay Sivash (the Sea of Azov), the world’s largest hypersaline lagoon with a strong salinity gradient from 36 to 90 g l−1. The dissolved mercury compounds ranged from 120 to 250 ng l−1, Hg varied from 60 to 450 ng l−1 in the suspended matter, and total mercury in the water ranged from 200 to 600 ng l−1. Salinity and the total suspended matter had practically no effect on the amount of dissolved and suspended forms of mercury separately, but their growth significantly increased total mercury content in water. Only the concentration of dissolved forms of mercury in water significantly correlated with dissolved organic matter. The Hg concentration in the bottom sediments averaged 13.8 ng g−1 wet weight. Both high salinity and human activities on the Sivash drainage area are responsible for high Hg content in lagoon water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution of the biosphere is accelerated now. Their release into the atmosphere from human activity significantly exceeded that from natural sources, for individual metals this excess was from 2 to 335 times (Norton et al. 1990; Likens 1992; Pirrone et al. 2010; Mason 2013). Mercury (Hg) degassing from the World Ocean and soils are probably the most important natural contributions to the Hg global atmospheric content (Pirrone et al. 2010). Mercury compounds were widely used as fungicides, in the manufacture of paper, but now, in most agricultural and pharmaceutical products, the use of inorganic mercury has been discontinued in most countries around the world. It serves as catalysts in the production of plastics, etc., and so, their different forms fall into industrial effluents or the air (Norton et al. 1990; Schmidt 1991; Likens 1992; Pirrone et al. 2010; Eagles-Smith et al. 2016; Gębka et al. 2016). Currently, the estimated contribution from natural sources is only about 10% of a total annual emitted Hg to the atmosphere from all sources of 5500–8900 t (Zillioux 2015). Global and regional Hg emissions both lead to an increase of Hg content in the atmosphere and water bodies (Norton et al. 1990; Bank 2012; Eagles-Smith et al. 2016). Direct atmospheric fallout and income from watershed contribute to the pollution of water bodies by Hg (Norton et al. 1990; Van Metre and Fuller 2009; Eagles-Smith et al. 2016). Coastal erosion is also a source of Hg coming into water bodies, and there is a trend of its increase due to natural and anthropogenic causes (Zhang et al. 2004; Shadrin and Anufriieva 2013; Bełdowska et al. 2016). Analysis of the bottom sediment depth profiles in different water bodies showed a strong enrichment of mercury concentrations in the past decades (Arnason and Fletcher 2003; Díaz-Asencio et al. 2009).
Mercury can enter water bodies in a wide variety of forms, and in the aquatic environment, every form of mercury is converted to methyl mercury (MMHg), which is a highly toxic and persistent compound (Schmidt 1991; Canário et al. 2008; Johnson et al. 2015; Zillioux 2015; Boyd et al. 2017). Aquatic ecosystems are the most sensitive to MMHg pollution, as they are the main repositories of natural and anthropogenic mercury and the habitats for active populations of methylating mercury bacteria (Fitzgerald et al. 2007). Mercury was accumulated by planktonic organisms, and in the aquatic food chains, methylmercury concentration increases from lower trophic level organisms to higher link. In the top-level predators (fish, birds, humans), it may reach the highest concentration endangering health and livehood (Schmidt 1991; Sonesten 2003; Helgason et al. 2008; Eagles-Smith et al. 2016). So, every knowledge of Hg concentration and behavior in an aquatic environment is highly important to the sustainable use of water bodies. There are a lot of articles on the concentration of different Hg forms and their biological role in fresh and marine waters due to Hg health effects for humans and ecosystem disturbing (Schmidt 1991; Prato et al. 2006; Zillioux 2015; Boyd et al. 2017). However, there are only a few data on such issues in hypersaline waters despite that the highest concentration of Hg was recorded in some of them (Shumilin et al. 2002; Pietrelli and Biondi 2009; Johnson et al. 2015; Boyd et al. 2017).
Bay Sivash (the Sea of Azov) is the world’s largest hypersaline lagoon with a strong spatial salinity gradient from 30 to 35 to 100–110 g l−1 (Shadrin et al. 2018). In 2018, mercury content was studied in water and bottom sediments of the lagoon along with salinity, total suspended solids, and dissolved organic matter. The goals of this paper are to present new data, to discuss them comparing with published data for other hypersaline water bodies, and to prove or disprove a hypothesis that salinity may influence Hg concentration in the lagoon.
Material and methods
Study area
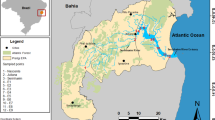
In Europe, Bay Sivash is the largest lagoon with its area of about 2600 km2 (Vorobyev 1940; Shadrin et al. 2018; Sergeeva et al. 2019). The narrow sand Arabat Spit of 112–116 km long separates the lagoon from the Sea of Azov, and the Crimean peninsula, largest in the Black Sea, is a lagoon west coast. Two narrow straits connect the Sea of Azov with the lagoon on the north (Fig. 1). Maximum depth does not exceed 2 m in the shallow semi-closed lagoon. Before the construction of the North Crimean Canal (from 1963 to 1975), Bay Sivash was a highly productive shallow hypersaline lagoon (average salinity of about 140 g l−1 and more than 200 g l−1 in the southern part); the small salt lakes and pools were in depressions surrounded it. The North Crimean Canal was built to improve water supply to the Crimean Peninsula by waters of the Dnieper River. Irrigated agriculture occupying 4000 km2, mainly rice cultivation, was developed on the Bay Sivash drainage area. A lot of different pesticides and fertilizers were used, especially, for rice cultivation. Drainage waters from agricultural lands began discharging into Bay Sivash in large volume, up to 1.1 × 106 m3 per year. As a result, in the lagoon, the salinity began to drop, and its average value had decreased up to 17–23 g l−1. The Dnieper River collects almost all Ukrainian industry and agriculture effluents, which are then supplied to Crimea through the North Crimean Canal. This led to an increase in the pollution level of many Crimean water bodies that were shown, as an example, for artificial radionuclides (Mirzoeva et al. 2020). In April 2014, the North Crimean Canal was blocked, and the supply of Dnieper water in Crimea was ceased. The discharge of drainage waters to the bay was also stopped. Salinity grew from 22 to 24 g l−1 up to 6–75 g l−1 in 2015, and up to 100 g l−1 in 2019 (Shadrin et al. 2019; Anufriieva and Shadrin 2020). The lagoon again became hypersaline. Changes occurred in ecosystem biotic structure leading also to changes in the environment. As an example, Cladophora siwaschensis (C.J. Meyer, 1922), filamentous green algae, again creates floating mats with high wet weight biomass up to 2.5–3.0 kg m−2 as before dropping salinity after the canal construction. Currently, they cover large areas leading often to hypoxic and even anoxic events below them near the bottom (Shadrin et al. 2018, 2019). There is a range of bottom sediment types in the bay—silt and silty-sand with fragments of mollusk shells.
Sampling and analyses
To analyze the mercury content, six water samples (stations 1, 3–7) were collected in May 2018 and seven samples of bottom sediments (stations 1–7) in November 2019 at different stations in Bay Sivash (Fig. 1, Table 1). To study the mercury content, 1-l water samples were taken at each station by bathometer. The upper layer of bottom sediments (4–5 cm) was taken by a manual grab with an area of 0.01 m2. In sampling stations, the concentrations of total suspended solids (TSS) and dissolved organic matter (DOM) were determined using the Condor complex (Akvastandart-Yug, Sevastopol, Russia) as described (Shadrin et al. 2019). Salinity was measured by a WZ212 refractometer (Kelilong Electron Co. Ltd, Fuan, Fujian, China), and temperature by РНН-830 electronic thermometer (OMEGA Engineering, INC., Norwalk, CT). To separate the forms of mercury, water samples, fixed by concentrated nitric acid (10 ml of HNO3 per 1 l of water), were filtered through pre-weighed nuclear 0.45-μm pore size filters.
The dissolved form of mercury was determined in the filtrate and the suspended form on the filters. Potassium permanganate was added to the filtered water sample for oxidation in a volume of 15–20 ml and then 5 ml ½ sulfuric acid, thereby destroying all dissolved forms of mercury to ions. Suspension and bottom sediments were acid burned (10 ml ½H2SO4 and 5 ml HNO3 per sample). Next, the samples were subjected to destruction at no more than 60 °C, and then cooled. A solution of potassium permanganate was added to the samples in an amount ensuring complete oxidation of the test sample (from 15 to 20 ml). After 10–15 min, the samples were filtered. For analysis on a device, sulfuric acid (1:1) in a volume of 5 ml was added to 100 ml of the filtered sample. A hydroxylamine solution (up to 5 ml) was poured into all samples to remove excess potassium permanganate. Before the determination, a reducing agent (SnCl2) was added in a volume of 10 ml. The prepared samples were analyzed by the flameless atomic absorption method on a Hiranuma-1 mercury analyzer (Hiranuma Sangyo Co. Ltd, Mito, Ibaraki, Japan). Mercury ions were reduced by tin dichloride (5 ml per sample) to gaseous form and then immediately introduced into the aerator of the device. The amount of mercury was determined on the scale of the device at a wavelength of 253.7 nm. To calibrate the Hiranuma-1 mercury analyzer, a standard sample of solutions of mercury (II) ions was used. First, a “blank calibration” was carried out (100 ml of distilled water + 5 ml of H2SO4 (1:1)), then calibration using a series of calibration solutions: 0.2; 0.4; 0.6; 0.8; 1 μg dm−3 (10 replicates for each concentration). The sensitivity of the Hiranuma-1 mercury analyzer is 0.01; the detection limit is 0.5 ng l−1 of mercury with high sensitivity 1/1000 of standard 0.5 ppb.
Coefficients of mercury accumulation by a suspended phase (also known as distribution coefficient) were calculated by the equation (Polikarpov and Egorov 1986):
where Kss – coefficients of mercury accumulation by TSS; Css – the concentration of mercury in TSS, ng g−1; Сw is the concentration of mercury in the dissolved phase, ng kg−1.
Data analysis
Statistical processing of the results was carried out in MS Excel 2007 and Statistica 6.0, where mean values, standard deviations (SD), coefficients of variability (CV), correlation (R), determination (R2), parameters of regression equations, and dendrograms were calculated. The significance of differences was assessed by Student’s t-test after normality tests (Thode 2002) were done. The confidence level (p) of the correlation coefficients was found (Muller et al. 1979).
Results
General hydrographic properties
In both periods of this study, a strong spatial gradient of salinity was expressed. Salinity increased from north to south, changing at sampling points in May from 36 to 82 g l−1, and from 38 to 90 g l−1 in November (Table 1). Changes in TSS and DOM were correlated with changes in salinity (Table 1). In May, TSS was determined by two methods, the average of these two values was used in further analysis. It varied from 2.6 to 18.2 mg l−1, on average 12.4 mg l−1 (SD = 6.5, CV = 0.52). Concentrations of TSS and DOM significantly positively correlated with salinity in May (Fig. 2a), but in November there were no such dependencies. In November, the concentration of TSS was 10% higher than in May (the differences were not significant), and the concentration of DOM was 2.6 times higher (the differences were significant, p = 0.001). There was a reliable positive correlation between TSS and DOM; its quantitative expression was different at different times. In May, it was best approximated by the equation (R = 0.918, p = 0.001):
and in November (R = 0.999, p = 0.0001):
Mercury in water
In May, concentration of dissolved mercury compounds ranged from 120 to 250 ng l−1, on average 170 ng l−1 (SD = 46.9, CV = 0.28), and the amount of mercury in TSS varied from 60 to 450 ng l−1, on average 165 ng l−1 (SD = 112, CV = 0.68) (Table 2). The mercury content in 1 g of suspension ranged from 3 × 103 to 50 × 103 ng g−1, on average of 23.4 × 103 ng g−1 (SD = 17.8 × 103, CV = 0.76), and this value significantly negatively correlated with TSS (R = − 0.754, p = 0.05):
where C’ss – Hg in TSS, ng g−1.
The total mercury content in the water varied from 200 to 600 ng l−1, on average of 335 ng l−1 (SD = 172, CV = 0.51). Salinity had practically no effect on the amount of dissolved and suspended forms of mercury separately, but significantly (p = 0.05) influenced its total content (Fig. 2b). Salinity also did not affect the ratio of the contents of suspended and dissolved forms. TSS significantly positively affected only the total amount of mercury (R = 0.740, p = 0.05), and only the Hg concentration in the dissolved phase significantly correlated with DOM (R = 0.755, p = 0.05). Coefficients of mercury accumulation by TSS varying from 1.5 × 104 to 41.7 × 104, averaging 16.7 × 104 (SD = 15.8 × 104, CV = 0.95), did not reliably depend on salinity and DOM. It significantly negatively correlated with TSS (R = − 0.771; p = 0.05):
where Kss – coefficients of mercury accumulation in TSS (eq. 1).
Cluster analysis using indicators of dissolved, suspended and total mercury contents showed that all stations fall into two main groups (Fig. 3a). Two stations located in the shallowest southernmost part were detached in a separate group. The content of dissolved forms of mercury practically did not differ in the two groups of stations, and the concentration of suspended matter in the group of shallow water stations was on average 5 times higher, while the total mercury content was 2 times higher. Cluster analysis was also carried out using salinity, TSS, and DOM, and it led to another grouping of stations (Fig. 3b).
Mercury in bottom sediments
There were a lot of empty shells of mollusks in the bottom sediments. From point to point, the concentration of mercury in the bottom sediments did not change much (Fig. 4), averaging 13.8 ng g−1 wet weight – Cww (SD = 3.1, CV = 0.23) or 19.8 ng g−1 dry weight – Cdw (SD = 6.3, CV = 0.32). The Cdw/Cww ratio averaged 1.4 (SD = 0.15, CV = 0.11).
No significant effect of salinity, TSS, and DOM on mercury content was found. It should be noted that during sampling of bottom sediments in November, the weather was very windy, while the direction of the wind often changed and resuspended bottom sediments and increased spatial homogeneity. The concentration of mercury in sediments in November was on average 1600 times less than in suspended matter in May (per g).
Discussion
The total Hg content in water of different Black Sea areas varied between 1 and 120 ng l−1 and averaged between 7.2 and 37.0 ng l−1 in different seasons and years (Kostova and Popovichev 2002; Stetsiuk and Egorov 2018). However, in a highly polluted Bay Sevastopolskaya (Black Sea), a total Hg content in the most part was not higher than 155 ng l−1 (Kostova et al. 2001) and episodically reached up to 460 ng l−1 in the most polluted sites (Stetsiuk, unpublished data). Hence, the total Hg concentrations in water were on average higher in Bay Sivash than in the Black Sea. There are also several hypersaline lakes nearby Bay Sivash with high Hg contents exceeding 500 ng l−1 (Mirzoyeva et al. 2015; Stetsiuk et al. 2018). It is worth comparing our data with those from the available literature. The total Hg concentrations vary from 0.14 to 15.1 ng l−1 in the freshwater lakes and from 2 to 45 ng l−1 in rivers (Petrisor 2006) and from < 1 to 78.0 ng l−1 in seawater (Fitzgerald et al. 2007; Mason 2013) with higher levels in some coastal heavy polluted areas (Mousavi et al. 2011). It may be even higher, up to 145 ng l−1, in polluted lagoons (Covelli et al. 2009). In hypersaline Great Salt Lake (Utah, USA), the total Hg content in water is high reaching up to 100 ng l−1 (Naftz et al. 2008) but 3–6 times lower compared to this study.
The contributions of different sources to high Hg content in Bay Sivash as well as the Crimean hypersaline lakes were not quantitatively assessed yet. In high salinity basins, the enhanced Hg transfer from atmosphere to water (Mason and Gill 2005) can be one of the causes since an increase of the total Hg content was found along the salinity gradient in Bay Sivash. The significant correlation of total Hg with salinity and TSS with salinity could be the result of the total Hg association with TSS. However, the human activities in the Sivash drainage area are probably more responsible. An economically developed zone, located in Krasnoperokopsk and Armyansk in the bay watershed, has a diverse chemical industry and their dangerous emissions include also different Hg species. Since the 1970s, the development of irrigated agriculture, mainly rice, was started with intensive use of various pesticides and fertilizers. Most of the drainage waters from the agricultural land were regularly discharged into Bay Sivash (Shadrin et al. 2018). Also, the water in the North Crimean Canal was initially high polluted by various pollutants including Hg (Bashkirtseva 2003; Mirzoyeva et al. 2015).
In coastal areas around Crimea, except Bay Sevastopolskaya, a Hg content in surface sediments averaged 70.4 ng g−1 wet weight (SD = 31.5, CV = 0.45) (Kostova et al. 2001; Raybushko et al. 2005). In highly polluted Bay Sevastopolskaya, it was significantly higher averaging 942 ng g−1 wet weight (SD = 457, CV = 0.50) for silty sediments and 96 ng g−1 wet weight (SD = 11.7, CV = 0.12) for sandy sediments (Kostova 2005). In the Bay Sivash sediments, there was a significantly lower Hg concentration (on average 13.8 ng g−1 wet weight) probably due to a high proportion of mollusk shells in sediments and the frequent anoxic near bottom conditions, as a result of the massive development of floating mats of filamentous green algae Cladophora (Shadrin et al. 2018, 2019, 2020), enhancing the Hg release from sediments (Koron and Faganeli 2012). The role of DOM, which most probably originates from the intense growth and decay of macrophytes, seems important in the complexation of dissolved Hg (Ravichandran 2004).
To make a comparison of Hg content in the water column and bottom deposits, we calculated the total content in both reservoirs. We assumed that the density of sediment is 2 g cm−3 and the average depth of Bay Sivash is 0.7 m. The calculation showed that there was on average 0.235 mg m−2 in the water column and 2.8 mg m−2 in the surface (10 cm) sediment layer. The total Hg reservoir in the surface sediment was, therefore, more than 100 times higher than in the water column. The resuspension of surface sediment also may contribute to high total Hg content in this shallow water column.
The food webs are the main pathway in the Hg flux in aquatic ecosystems (Jones and Wurtsbaugh 2014; Walters et al. 2020). The high Hg concentration leads to disruption of the normal functioning of aquatic ecosystems and their use by humans. Artemia cysts are the most valuable bioresource in Bay Sivash which can become the largest Artemia habitat in the world (Anufriieva and Shadrin 2020). Adult Artemia, as well cysts, can accumulate relatively high Hg up to 0.34 μg g−1 (Jones and Wurtsbaugh 2014; Stetsiuk et al. 2018). There are reports that salinity may affect the concentration and behavior of Hg in the aquatic environment as well as on toxicity of different Hg species on Artemia and other crustaceans (Jones 1973; Okasako and Siegel 1980; Ullrich et al. 2001; Jones and Wurtsbaugh 2014; Johnson et al. 2015; Boyd et al. 2017). Various Hg species, e.g., MeHg, may affect the processes and damaging the organism (Gebhardt 1976; Go et al. 1990; Sarabia et al. 1998; Jones and Wurtsbaugh 2014) and, for example, reducing the brine shrimp lifespan (Pandey and MacRae 1991). Since Artemia is a keystone species in the Bay Sivash ecosystem (Anufriieva and Shadrin 2020), and increased Hg concentration may disturb not only the normal functioning of the Artemia population but also the whole bay ecosystem. Since Artemia and other invertebrates are the main food source for birds, the high Hg content can adversely affect them (Schmidt 1991; Helgason et al. 2008; Pietrelli and Biondi 2009). Bay Sivash is an important area for many species of birds, which use it for nesting, wintering, and resting during transcontinental migrations (Havrylenko 2000; Verkuil et al. 2003). The bay is a crossroad of bird migratory routes between Europe, Asia, and Africa. Is the high content of Hg in Bay Sivash a real risk for all birds living there? Is this contamination also a significant risk for the Artemia cysts harvest and planned aquaculture development in the lagoon? The quantitative assessment of the impacts of this contamination on the ecosystem functioning, bird diversity, aquaculture perspectives, and Artemia cyst harvest is an urgent goal. Additional deeper studies in the bay are needed to plan the environmental management.
Conclusion
The concentration of the total mercury content is high in the water of Bay Sivash ranging from 200 to 600 ng l−1 due to its hypersalinity and heavy anthropogenic pollution. After the closing of the North Crimean Canal, the salinity growth significantly increased total mercury content in water. Currently, there are not enough data to quantitatively assess the salinity effects on the behavior of Hg in the aquatic environment as on the toxicity of different Hg forms for different aquatic organisms. New studies of these issues are very important for the development of general aquatic ecology to better understand the role of a salinity factor in geochemical and biological processes in their coupling. Bay Sivash is an interesting area to study due to a smooth spatial gradient of salinity from 30 to 150 g l−1 in the bay with higher salinity in some separated pools, up to 350 g l−1.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anufriieva E, Shadrin N (2020) The long-term changes in plankton composition: Is Bay Sivash transforming back into one of the world’s largest habitats of Artemia sp. (Crustacea, Anostraca)? Aquaculture Research 51:341–350
Arnason JG, Fletcher BA (2003) A 40+ year record of Cd, Hg, Pb, and U deposition in sediments of Patroon Reservoir, Albany County, NY, USA. Environmental Pollution 123:383–391
Bank MS (2012) Mercury in the environment: pattern and process. University of California Press, Berkeley, California
Bashkirtseva EV (2003) Assessment of the impact of an aquatic technogenic object on the components of the geosystem of coastal-marine territories. Ecological Safety of Coastal and Shelf Zones and Comprehensive Use of Shelf Resources 9:117–126 (In Russian)
Bełdowska M, Jędruch A, Łęczyński L, Saniewska D, Kwasigroch U (2016) Coastal erosion as a source of mercury into the marine environment along the Polish Baltic shore. Environmental Science and Pollution Research 23:16372–16382
Boyd ES, Yu RQ, Barkay T, Hamilton TL, Baxter BK, Naftz DL, Marvin-DiPasquale M (2017) Effect of salinity on mercury methylating benthic microbes and their activities in Great Salt Lake, Utah. Science of the Total Environment 581:495–506
Canário J, Poissant L, Nelson O, Ridal J, Delongchamp T, Pilote M, Constant P, Blais J, Lean D (2008) Mercury partitioning in surface sediments of the Upper St. Lawrence River (Canada): evidence of the importance of the sulphur chemistry. Water, Air, and Soil Pollution 187:219–231
Covelli S, Acquavita A, Piani R, Predonzani S, De Vittor C (2009) Recent contamination of mercury in an estuarine environment (Marano lagoon, Northern Adriatic, Italy). Estuarine, Coastal and Shelf Science 82:273–284
Díaz-Asencio M, Alonso-Hernández CM, Bolanos-Álvarez Y, Gómez-Batista M, Pinto V, Morabito R, Hernández-Albernas JI, Eriksson M, Sanchez-Cabeza JA (2009) One century sedimentary record of Hg and Pb pollution in the Sagua estuary (Cuba) derived from 210Pb and 137Cs chronology. Marine Pollution Bulletin 59:108–115
Eagles-Smith CA, Wiener JG, Eckley CS, Willacker JJ, Evers DC, Marvin-DiPasquale M, Obrist D, Fleck JA, Aiken GR, Lepak JM, Jackson AK (2016) Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Science of the Total Environment 568:1213–1226
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chemical Reviews 107:641–662
Gebhardt KA (1976) Effects of heavy metals (cadmium, copper, and mercury) on reproduction, growth, and survival of brine shrimp (Artemia salina) from the Great Salt Lake. Utah State University, Loganб United States, Master’s Thesis
Gębka K, Bełdowski J, Bełdowska M (2016) The impact of military activities on the concentration of mercury in soils of military training grounds and marine sediments. Environmental Science and Pollution Research 23:23103–23113
Go EC, Pandey AS, MacRae TH (1990) Effect of inorganic mercury on the emergence and hatching of the brine shrimp Artemia franciscana. Marine Biology 107:93–102
Havrylenko V (2000) The potential of ecological corridors for waterfowl migrating through the northern Black Sea. Acta Ornithologica 35:41–44
Helgason LB, Barrett R, Lie E, Polder A, Skaare JU, Gabrielsen GW (2008) Levels and temporal trends (1983–2003) of persistent organic pollutants (POPs) and mercury (Hg) in seabird eggs from Northern Norway. Environmental Pollution 155:190–198
Johnson WP, Swanson N, Black B, Rudd A, Carling G, Fernandez DP, Luft J, Van Leeuwen J, Marvin-DiPasquale M (2015) Total-and methyl-mercury concentrations and methylation rates across the freshwater to hypersaline continuum of the Great Salt Lake, Utah, USA. Science of the Total Environment 511:489–500
Jones EF, Wurtsbaugh WA (2014) The Great Salt Lake’s monimolimnion and its importance for mercury bioaccumulation in brine shrimp (Artemia franciscana). Limnology and Oceanography 59:141–155
Jones MB (1973) Influence of salinity and temperature on the toxicity of mercury to marine and brackish water isopods (Crustacea). Estuarine and Coastal Marine Science 1:425–431
Koron N, Faganeli J (2012) Benthic fluxes of mercury during redox changes in pristine coastal marine sediments from the Gulf of Trieste (northern Adriatic Sea). Journal of Soils and Sediments 12:1604–1614
Kostova SK (2005) Distribution of mercury in the surface layer of bottom sediments in Sevastopol Bay (the Black Sea). Ecological Safety of Coastal and Shelf Zones and Complex Use of Shelf Resources 12:273–279 (In Russian)
Kostova SK, Egorov VN, Popovichev VN (2001) The long-term investigation of the mercury pollution in the Sevastopol Bay (the Black Sea). Ekologia Morya 56:99–103 (In Russian)
Kostova SK, Popovichev VN (2002) Mercury distribution in the Black Sea waters near the Crimean coast. Ecological Safety of Coastal and Shelf Zones and Complex Use of Shelf Resources 6:118–127 (In Russian)
Likens GE (1992) The ecosystem approach: its use and abuse. Excellence in Ecology, Book 3. The Ecology Institute, Oldendorf-Luhe, Germany
Mason RP (2013) Trace metals in aquatic systems. Wiley-Blackwell, Chichester
Mason RP, Gill GA (2005) Mercury in the marine environment. In: Parsons MB, Percival JB (eds) Mercury: sources, measurements, cycles, and effects. Mineralogical Association of Canada, Ottawa, Ontario, pp 79–216
Mirzoyeva N, Gulina L, Gulin S, Plotitsina O, Stetsuk A, Arkhipova S, Korkishko N, Eremin O (2015) Radionuclides and mercury in the salt lakes of the Crimea. Chinese Journal of Oceanology and Limnology 33:1413–1425
Mirzoeva N, Shadrin N, Arkhipova S, Miroshnichenko O, Kravchenko N, Anufriieva E (2020) Does salinity affect the distribution of the artificial radionuclides 90Sr and 137Cs in water of the saline lakes? A case of the Crimean Peninsula. Water 12:349
Mousavi A, Chávez RD, Ali A-MS, Cabaniss SE (2011) Mercury in natural waters: A mini-review. Environmental Forensics 12:14–18
Müller PH, Neuman P, Storm R (1979) Tafeln der mathematischen statistik. VEB Fachbuchverlag, Leipzig
Naftz D, Angeroth C, Kenney T, Waddell B, Darnall N, Silva S, Perschon C, Whitehead J (2008) Anthropogenic influences on the input and biogeochemical cycling of nutrients and mercury in Great Salt Lake, Utah, USA. Applied Geochemistry 23:1731–1744
Norton SA, Dillon PJ, Evans RD, Mierle G, Kahl JS (1990) The history of atmospheric deposition of Cd, Hg, and Pb in North America: evidence from lake and peat bog sediments. In: Lindberg SE, Page AL, Norton SA (eds) Acidic precipitation: sources, deposition, and canopy interactions. Springer New York, New York, pp 73–102
Okasako J, Siegel S (1980) Mercury antagonists: effects of sodium chloride and sulfur group (VIa) compounds on excystment of the brine shrimp artemia. Water, Air, and Soil Pollution 14:235–240
Pandey AS, MacRae TH (1991) Toxicity of organic mercury compounds to the developing brine shrimp, Artemia. Ecotoxicology and Environmental Safety 21:68–79
Petrisor IG (2006) Mercury: hazards and forensic perspectives. Environmental Forensics 7:289–292
Pietrelli L, Biondi M (2009) Notes on Little Egret breeding biology and on mercury content in egg shells and feathers. Rendiconti Lincei 20:219–224
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher GB, Streets DG, Telmer K (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmospheric Chemistry and Physics 10:5951–5964
Polikarpov GG, Egorov VN (1986) Marine dynamic radiochemoecology. Energoatomizdat, Moscow (In Russian)
Prato E, Biandolino F, Scardicchio C (2006) Test for acute toxicity of copper, cadmium, and mercury in five marine species. Turkish Journal of Zoology 30:285–290
Ravichandran M (2004) Interactions between mercury and dissolved organic matter – a review. Chemosphere 55:319–331
Raybushko VI, Kozincev AF, Kostova SK, Parchevskaya DS, Shinkarenko VK (2005) Mercury concentration in the seawater, bottom sediments and mussel Mytilus galloprovincialis Lam. on the Crimean shelf (the Black Sea). Marine Ecological Journal 4:79–87 (In Russian)
Sarabia R, Torreblanca A, Del Ramo JJ, Dıaz-Mayans J (1998) Effects of low mercury concentration exposure on hatching, growth and survival in the Artemia strain La Mata parthenogenetic diploid. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 120:93–97
Schmidt D (1991) Eichler, Wolfdietrich: Umweltgifte in unserer Nahrung und überall. Ein Kompendium aktueller Umweltgefahren und Umweltsünden. Mit Ratgeber und Hinweisen, was der Verbraucher bei seinen Nahrungsmitteln wissen sollte und was er für seine Gesundheit tun kann. 2. Aufl. von ‘Gift in unserer Nahrung’. Kilda-Verlag, Greven, Germany
Sergeeva NG, Shadrin NV, Anufriieva EV (2019) Long-term changes (1979-2015) in the nematode fauna in Sivash Bay (Sea of Azov), Russia, worldwide the largest hypersaline lagoon, during salinity transformations. Nematology 21:337–347
Shadrin NV, Anufriieva EV (2013) Climate change impact on the marine lakes and their Crustaceans: the case of marine hypersaline Lake Bakalskoye (Ukraine). Turkish Journal of Fisheries and Aquatic Sciences 13:603–611
Shadrin NV, Anufriieva EV, Kipriyanova LM, Kolesnikova ЕА, Latushkin AA, Romanov RE, Sergeeva NG (2018) The political decision caused the drastic ecosystem shift of the Sivash Bay (the Sea of Azov). Quaternary International 475:4–10
Shadrin N, Kolesnikova E, Revkova T, Latushkin A, Chepyzhenko A, Dyakov N, Anufriieva E (2019) Macrostructure of benthos along a salinity gradient: the case of Sivash Bay (the Sea of Azov), the largest hypersaline lagoon worldwide. Journal of Sea Research 154:101811
Shadrin N, Mirzoeva N, Sidorov I, Korotkov A, Anufriieva E (2020) Natural radionuclides in bottom sediments of the saline lakes. What factors determine their concentration? Environmental Earth Sciences 79:1–12
Shumilin E, Grajeda-Muñoz M, Silverberg N, Sapozhnikov D (2002) Observations on trace element hypersaline geochemistry in surficial deposits of evaporation ponds of Exportadora de Sal, Guerrero Negro, Baja California Sur, Mexico. Marine Chemistry 79:133–153
Sonesten L (2003) Fish mercury levels in lakes—adjusting for Hg and fish-size covariation. Environmental Pollution 125:255–265
Stetsiuk AP, Egorov VN (2018) Marine suspensions ability to concentrate mercury depending on its contents in the shelf water area. Monitoring Systems of Environment 13:123–132 (In Russian)
Stetsiuk AP, Popovichev VN, Mirzoyeva NY, Rodionova NY, Bogdanova TA (2018) Mercury in abiotic and biotic components of the ecosystem of the salt lakes of the Crimea. Ekosistemy 16:47–57 (In Russian)
Thode HC (2002) Testing for normality. Marcel Dekker Inc, New York
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Critical Reviews in Environmental Science and Technology 31:241–293
Van Metre PC, Fuller CC (2009) Dual-core mass-balance approach for evaluating mercury and 210Pb atmospheric fallout and focusing to lakes. Environmental Science and Technology 43:26–32
Verkuil Y, van der Have TM, van der Winden J, Chernichko II (2003) Habitat use and diet selection of northward migrating waders in the Sivash (Ukraine): the use of brine shrimp Artemia salina in a variably saline lagoon complex. Ardea 91:71–83
Vorobyev VP (1940) Hydrobiological essay on the Eastern Sivash and its potential for fisheries. Proceedings of AzCherNIRO 12:69–164 (In Russian)
Walters DM, Cross WF, Kennedy TA, Baxter CV, Hall RO, Rosi EJ (2020) Food web controls on mercury fluxes and fate in the Colorado River, Grand Canyon. Science Advances 6:eaaz4880
Zhang K, Douglas BC, Leatherman SP (2004) Global warming and coastal erosion. Climatic Change 64:41–58
Zillioux EJ (2015) Mercury in fish: history, sources, pathways, effects, and indicator usage. In: Armon R, Hänninen O (eds) Environmental indicators. Springer, Dordrecht, pp 743–766
Acknowledgments
The authors thank Dr. Bindy Datson (Australia) for her help to improve English and anonymous reviewers for helpful suggestions to improve the manuscript.
Funding
Sampling in Bay Sivash, data analysis, and the writing of this manuscript was supported by the Russian Science Foundation (grant 18-16-00001 for Nickolai Shadrin and Elena Anufriieva); sample preparation and Hg determination were conducted in the framework of the state assignments of A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS (№ AAAA-A18-118020890090-2). TSS and DOM measurements were conducted in the framework of the state assignment of the Marine Hydrophysical Institute of RAS (№ 0827-2019-0002).
Author information
Authors and Affiliations
Contributions
Nickolai Shadrin: conceptualization, formal analysis, field investigation, methodology, writing—original draft and final text. Aleksandra Stetsiuk: Hg content assessment, formal analysis, writing—review and editing. Alexander Latushkin: field investigation, methodology, formal analysis, writing—review and editing. Elena Anufriieva: methodology, formal analysis, writing—review and editing.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
The authors declare consent to participate.
Consent to Publish
The authors declare consent to publish.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: V. V.S.S. Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shadrin, N., Stetsiuk, A., Latushkin, A. et al. Mercury in the world’s largest hypersaline lagoon Bay Sivash, the Sea of Azov. Environ Sci Pollut Res 28, 28704–28712 (2021). https://doi.org/10.1007/s11356-021-12745-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12745-9