Abstract
This study evaluated the combined effects of biochar and straw on N2O flux and the community compositions of nitrifiers and denitrifiers in the maize season in an intensively farmed area in northern China. The experiment consisted of four treatments: (1) CK (only chemical fertilizer application); (2) C (biochar application); (3) SR (straw application to the field); and (4) C+SR (the application of both biochar and straw). The results indicated that during the maize growing season, N2O flux decreased by 30.3% in the C treatment and increased by 13.2% and 37.0% in the SR and C+SR treatments compared with CK, respectively. NO3−-N, NH4+-N, and microbial biomass carbon (MBC) were the main soil factors affecting N2O flux, and they were positively correlated with NO3−-N and negatively correlated with MBC in the C treatment and positively correlated with NH4+-N in the SR and C+SR treatments. Both biochar addition and straw return shifted the community compositions of nitrifiers and denitrifiers. N2O production was mainly reduced by promoting the ammonia-oxidizing bacteria (AOB) gene abundance and inhibiting the nirK gene abundance in the C treatment but promoted by inhibiting the AOB and nosZ gene abundances in the SR and C+SR treatments. Nitrosospira (AOB) and Rhizobium (nirK) were the main contributors among the treatments. NO3−-N, NH4+-N, and MBC were the main soil factors affecting the denitrifier communities. The predominant species associated with the nirK, nirS, and nosZ genes were positively correlated with NO3−-N and MBC and negatively correlated with NH4+-N. These results provide valuable information on the mechanism of N2O production and reduction in biochar- and straw-amended soil under field conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is one of the most important greenhouse gases, and agricultural soil is the dominant emission source. Many studies have confirmed that more than 70% of N2O emissions are derived from arable land where excessive nitrogen (N) fertilizer is applied (Yao et al. 2017; Tian et al. 2018; Song et al. 2020). The North China Plain is one of the main intensively farmed agricultural areas in China (Zhou et al. 2017a, b; Tan et al. 2017; Xu et al. 2019), and annual N fertilizer application can reach up to 600 kg N ha−1 year−1 in the winter wheat and summer maize rotation system in this region (Tan et al. 2017; Liu et al. 2019). This high level of N input will inevitably lead to high N2O emissions. Earlier in situ field studies observed that the cumulative N2O emissions in the maize season accounted for 75.2–90.0% of the annual total emissions due to the high temperature and moisture in the maize season (Liu et al. 2019; Shi et al. 2019; Xu et al. 2019). Based on this background, it is imperative to apply practical strategies to reduce N2O emissions and better understand the mitigation mechanism in the maize season in intensively farmed agricultural areas.
Nitrification and denitrification, dominated by microorganisms, are the main pathways of N2O emission (Hu et al. 2015). Nitrification is the oxidation of ammonium nitrogen to nitrite and then nitrate. Ammonia oxidation catalyzed by ammonia monooxygenase is the rate-limiting step of nitrification, and it is mainly driven by ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB). N2O is the byproduct of incomplete NH2OH oxidation to NO2− or the final product of AOB denitrification (Spokas et al. 2012; Hu et al. 2015; Caranto et al. 2016). Denitrification refers to the process by which NO3− or NO2− is gradually reduced to NO, N2O and N2 under the action of enzymes. The reduction of NO2− to NO is considered to be the rate-limiting step of denitrification, and nitrite reductase is encoded by the nirK and nirS genes, which respond differently to the soil environment (Zhang et al. 2015). The reduction step of N2O to N2 is the known biological sink of N2O, and the nosZ gene plays an important role in coding N2O reductase, which determines whether N2O can be completely reduced (Yuan et al. 2019). A number of studies have shown that N2O emissions were significantly correlated with nirK, nirS, and nosZ (Yao et al. 2012; Ai et al. 2013; Harter et al. 2016; Hink et al. 2018; Wang et al. 2020). But there is no established evidence of a quantitative relationship between N2O fluxes and the denitrifiers of nirK, nirS, and nosZ, and which are the key drivers in the N2O production and reduction.
Previous studies have shown that the application of biochar can change soil physical and chemical properties by absorbing soil NH4+-N and NO3−-N or by increasing soil carbon storage, C/N ratio, soil pH, and water holding capacity, therefore improving soil fertility (Zhang et al. 2012; Ribas et al. 2019). Based on 16S rRNA gene sequencing technology, it was found that biochar addition significantly affected the diversity of soil microorganisms involved in nitrogen conversion processes, such as nitrogen fixation, nitrification and denitrification (Chen et al. 2013; Wu et al. 2019). The activity and abundance of AOA and AOB genes were increased significantly by biochar addition (Yao et al. 2012). Lin et al. (2017) found that AOA copy numbers increased and N2O emissions decreased after adding biochar to paddy soil. Shi et al. (2019) also found that adding biochar could significantly increase the copy numbers of AOA and AOB and that AOB was more sensitive than AOA to biochar.
Biochar significantly changes the denitrifier community composition. Previous studies have shown that biochar addition significantly changed the abundance and activity of the nirS and nirK genes, which significantly reduced N2O emissions (Harter et al. 2014; Krause et al. 2018). Harter et al. (2016) also found that biochar improved the activity and abundance of the nosZ gene, thus promoting complete denitrification and reducing N2O emissions. However, there is no clear causal relationship between soil properties and reductions in N2O emissions in biochar-amended soils. Therefore, the effects of biochar addition on N2O production and reduction (consumption) processes and the mechanisms are still unclear.
Straw return, as an important way of reusing agricultural waste, is strongly recommended by the Chinese government (Zhou et al. 2017a, b). Straw return is generally believed to have positive effects on the ability of soil microorganisms to hold carbon and nitrogen, increasing the total soil microbial biomass and improving the metabolic capacity and functional diversity of the soil microbial community (Peng et al. 2016; Li et al. 2018; Ma et al. 2019). However, there are different opinions on the impact of straw return on N2O emissions. Some studies found that straw return can promote N2O emissions (Ju et al. 2011; Cui et al. 2012; Liu et al. 2014a, b; Ma et al. 2019), while other studies confirmed that straw return can reduce N2O emissions (Yao et al. 2017; Lin et al. 2017; Wang et al. 2020). In addition, there are few studies on the effect of straw on nitrification and denitrification microorganisms. Zhao et al. (2017) found that straw increased the copy numbers of AOA and AOB and shifted the denitrifier community composition, thus affecting N2O generation. Wang et al. (2020) indicated that upon adding straw, the abundances of nirK and nirS decreased, and the abundance of the nosZ gene increased, thus reducing N2O emissions. Therefore, it is urgent to clarify the effect of straw return on nitrification- and denitrification-mediated N2O emissions and the associated microbial communities in agricultural soil.
In this study, through high-throughput sequencing, we investigated the N2O emissions and the response mechanism of nitrification and denitrification in intensively farmed land following 2 years of biochar addition and straw return. The objectives of our study were (1) to compare the effects of biochar, straw, and a combination of biochar and straw on N2O emissions; (2) to clarify the effects of biochar and straw on the community composition of nitrifiers and denitrifiers; and (3) to explore the response mechanism of N2O emissions to biochar and straw amendment in intensively farmed land in northern China.
Material and methods
Location description
The field experiment was located at the ecology and sustainability research station (36° 58′ N, 117° 59′ E, 17 m a.s.l) in Huantai County, Shandong Province, China. This site has a warm, temperate, continental monsoon climate with a mean annual temperature of 11.8–12.9 °C. The mean annual precipitation is approximately 550 mm, with the majority (70%) falling from June through September (the summer maize growing season). Winter wheat/summer maize rotation is the most important cropping system in this region. Winter wheat is usually sown in early October and harvested in early June of the next year, and maize is sown in early June and matures in late September. The fluvo-aquic soil is a sandy loam with a bulk density of 1.50 g cm−3. The soil pH was 8.1, the soil organic carbon (SOC) content was 10.8 g kg−1, the total N content was 0.7 g kg−1, the available N content was 48.0 mg kg−1, the available P content was 11.5 mg kg−1, and the available K content was 210.0 mg kg−1.
Biochar characterization
The biochar was purchased from Shandong Mingchen Sanitation Equipment Co., Ltd. It was made from cotton straw by slow pyrolysis at 800 °C under an oxygen-free atmosphere. The density of the biochar was 0.30 g cm−3, the pH value was 8.6, the C content was 68.7%, the N content was 0.33%, the available P was 0.12%, the available K was 1.60%, the ash content was 25.4%, and the specific surface area and pore volume of it were 12.5 m2 g−1 and 1.9 mL g−1.
Field experiment
The field experiment started in the winter wheat season (October 2017). Four treatments with three replications were established, and each plot (6 m × 6 m) was randomly distributed. The four treatments were as follows: (1) CK (only chemical fertilizer); (2) C (biochar, 9.0 t ha−1 year−1); (3) SR (all straw returned to the field); and (4) C+SR (biochar plus straw). All treatments received the same amounts of N (200 kg ha−1 year−1), P2O5 (55.0 kg ha−1 year−1), and K2O (40.0 kg ha−1 year−1), for which the N fertilizer was urea, the P fertilizer was superphosphate, and the K fertilizer was potassium sulfate. The biochar and fertilizer were distributed equally between the wheat and maize. Half of the urea and all of the P and K fertilizers were uniformly applied as the base fertilizer, and the other half of the urea was used as topdressing in the wheat and maize seasons. Biochar and the base fertilizers were broadcasted onto the soil surface by hand and then immediately incorporated into the soil by rotary tillage. In the SR treatment, all the wheat straw or maize straw produced in the plot was mechanically chopped into 5–10 cm pieces and incorporated into the soil by rotary tillage in the following growth season. The supplemental urea fertilizer was applied as topdressing and washed into the soil with flood irrigation to prevent NH3 volatilization.
N2O flux measurements
Field samples were collected from June 24 to September 26, 2019, throughout the maize season due to the high N2O emissions in this period. N2O flux was measured by the static chamber gas chromatograph (GC) method once a week, as well as once a day for approximately one week after fertilization, irrigation or rainfall. A total of 17 samples were collected during the maize growing season. The sampling chamber consisted of a top chamber (0.4 m width × 0.4 m length × 0.5 m height) and a stainless steel square base. An additional chamber (0.5 or 1.0 m height) was added to the top if the height of the crops exceeded 50 cm. Before sampling, a 15 cm deep base was placed on the soil surface and kept for sampling in each plot. The upper edge of the base had a groove (5 cm deep) for water filling to seal the edge of the chamber with the horizontal surface. Distilled water was injected into the groove to seal the entire system during sampling. The top chamber was equipped with a circulating fan to ensure gas uniformity and was wrapped with a layer of sponge and aluminum foil to reduce the influence of solar radiation on the inner air temperature.
Gas sampling was carried out from 9:00 to 11:00 am; four gas samples were extracted from the top of the chamber at 0, 8, 16, and 24 min with a three-way stopcock using a 60 mL airtight syringe after enclosure, and 60 mL gas was pumped into pre-evacuated gas bags for analysis. During gas sampling, the atmospheric temperature, soil temperature, and internal air temperature of the chamber were simultaneously measured.
The N2O concentration was analyzed on the sampling day by an Agilent 7890A gas chromatograph (Agilent, USA, 2007) equipped with an electron capture detector (ECD). The carrier gas was argon-methane (5%), the flow rate was 40 mL min−1, and the column temperature was 40 °C. Compressed air was used as a reference gas with a N2O concentration of 313 ppbv. N2O concentration was calculated by comparing the peak areas of the samples with that of the reference gas.
N2O flux was calculated using the following equation:
where F is the N2O flux (μg m−2 h−1); ρ is the density of N2O under standard conditions, which is 1.977 g L−1; h is the height of the sampling chamber (m); dc/dt is the rate of N2O emission (μg h−1); and T is the average temperature in the chamber (°C).
The cumulative N2O emissions (EN2O, kg ha−1) were calculated as follows:
where M is the cumulative N2O emissions (μg m−2); F is the N2O emission flux (μg m−2 h−1); i is the number of samples; and (ti+1 − ti) is the number of days between samplings.
Soil sampling and analysis
Soil samples were collected synchronously with gas sampling at a depth of 0–20 cm in the soil layer with an auger. Five soil samples were taken randomly in each plot and mixed thoroughly. After removing all of the plant roots by sieving (sieve mesh 2 mm), the mixed soil was divided into three subsamples. One subsample was immediately frozen in liquid nitrogen and kept at − 80 °C for DNA and microbial community composition analysis. The other subsample was maintained fresh at 4 °C for the determination of SWC, NH4+-N and NO3−-N concentrations, as well as soil microbial biomass carbon and nitrogen (MBC and MBN). The SWC was determined by the oven-drying method at 105 °C for 24 h. The soil pH was determined in a soil-water suspension (1:2.5 w/v) using a PH100 ExStick pH meter (Extech Instruments Corp., Nashua, NH, USA). The soil NH4+-N and NO3−-N were analyzed by a flow injection autoanalyzer (Braun and Lübbe, Norderstedt, Germany) after extraction of the soil samples with 0.01 M CaCl2 solution. The soil MBC and MBN were determined using the chloroform fumigation method and were quantified by a multi N/C 2100/2100S TOC Analyzer (Jena, Germany).
DNA extraction and sequencing
DNA extraction was performed from soil samples taken on August 21, 2019, during the key period of nitrogen nutrient regulation, by which time the microorganisms had had the opportunity to adapt to the changes in environmental conditions caused by the application of biochar and straw return (Shi et al. 2019). The total DNA from 1 g of fresh soil was extracted directly from membranes using a Power Soil DNA Isolation Kit (Axygen, USA) according to the manufacturer’s instructions. The quality of the DNA was examined by 1.0% agarose gel electrophoresis, and the DNA was stored at − 20 °C for further use. The electrophoresis bands were bright, and the samples were not luminous, which met the requirements for PCR amplification.
The V3-4 hypervariable region of bacterial 16S rRNA gene were amplified with the primers 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT) (Munyaka et al. 2015). High-throughput sequencing of the AOA-amoA, AOB-amoA, nirK, nirS, and nosZ genes were performed for each soil sample, 10-digit barcode sequence was added to the 5′ end of the forward and reverse primers (provided by Allwegene Company, Beijing). The PCR was carried out on a Mastercycler Gradient (Eppendorf, Germany) using 25 μl reaction volumes, containing 12.5 μl KAPA 2G Robust Hot Start Ready Mix, 1 μl forward primer (5 μM), 1 μl reverse primer (5 μM), 5 μl DNA (total template quantity is 30 ng), and 5.5 μl H2O. Cycling parameters were 95 °C for 5 min, followed by 28 cycles of 95 °C for 45 s, 55 °C for 50 s, and 72 °C for 45 s with a final extension at 72 °C for 10 min. Three PCR products per sample were pooled to mitigate reaction-level PCR biases. The PCR products were purified using a QIAquick Gel Extraction Kit (QIAGEN, Germany), quantified using real-time PCR, the primers and reaction conditions for PCR amplification are shown in Table 1. The PCR products were separated by 2% agarose gel electrophoresis, and the desired fragment was recovered using an AxyPrep DNA Gel Extraction Kit (Axygen, USA). Sequencing was performed using an Illumina MiSeq PE300 sequencing platform (Illumina, Inc., CA, USA) according to the manufacturer’s instructions.
Raw sequencing data were processed by Beijing Allwegene Technology Inc. (Beijing, China) using the QIIME pipeline tools (Wood et al. 2015; Chen et al. 2016). The raw data were first screened and sequences were removed from consideration if they were shorter than 200 bp, had a low-quality score (≤ 20), contained ambiguous bases or did not exactly match to primer sequences and barcode tags. Qualified reads were separated using the sample-specific barcode sequences and trimmed with Illumina Analysis Pipeline Version 2.6. And then, the dataset was analyzed using QIIME. The sequences were clustered into operational taxonomic units (OTUs) at a similarity level of 97% (Munyaka et al. 2015), to generate rarefaction curves and to calculate the richness and diversity indices. The Ribosomal Database Project (RDP) Classifier tool was used to classify all sequences into different taxonomic groups (Edgar 2013).
To examine the similarity between different samples, clustering analyses and PCA were used based on the OTU information from each sample using R (Cole et al. 2014). The evolution distances between microbial communities from each sample were calculated using the tayc coefficient and represented as an unweighted pair group method with arithmetic mean (UPGMA) clustering tree describing the dissimilarity between multiple samples (Wang et al. 2012).
Statistical analysis
The N2O flux and soil physicochemical property data were processed by Microsoft Office Excel 2010 (Microsoft Corporation, USA), and figures were generated by Origin Pro 8.5 (Origin Lab, USA). The least significant difference (LSD) method of one-way analysis of variance (ANOVA) was used to test the differences in N2O flux, soil physicochemical properties, and the relative abundances of nitrifiers and denitrifiers. Pearson’s correlation analysis was used to test the correlation between N2O flux and soil properties and the abundances of nitrifiers and denitrifiers at the 0.05 level. QIIME1 (v1.8.0) software was used for alpha diversity index analysis. Redundancy discrimination analysis (RDA) was used to explore the correlations between the nitrifiers, denitrifiers and the soil physicochemical properties in R (R, Version 3.6.2).
Results
N2O flux
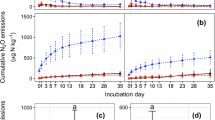
The variation in N2O flux in each treatment was similar in the maize growing season (Fig. 1a). The N2O flux decreased by 40.4% in the C treatment, while it increased by 29.0% in the C+SR treatment compared with the CK treatment from June 24 to July 5 (P < 0.05). Then, the N2O flux increased slightly due to rainfall on July 12 and then decreased to a low level. The maximum N2O emission peak appeared on August 3 after topdressing and irrigation. From July 28 to August 14, the N2O flux decreased by 11.6% in the C treatment and increased by 53.5% and 123.9% in the SR and C+SR treatments compared with CK, respectively (P < 0.05). After August 14, there was no significant difference in N2O flux among treatments, and N2O flux remained at a low level.
N2O flux (a) and the cumulative N2O emissions (b) of the CK, C, SR, and C+SR treatments during the maize growing season. Data points and error bars represent means and standard errors (n = 3), respectively. Abbreviations: CK, control treatment; C, biochar treatment; SR, straw return treatment; C+SR, biochar plus straw treatment
The cumulative N2O emissions were between 1.46 and 2.83 kg hm−2 in each treatment during the maize growing season (Fig. 1b). The cumulative N2O emissions in the C treatment decreased by 30.3% compared with the CK treatment and increased by 13.2% and 37.0% in the SR and C+SR treatments, respectively (P < 0.05).
Soil physical and chemical properties
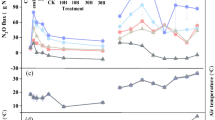
The variations in soil temperature at the 5 cm soil layer in each treatment during the maize season are shown in Fig. 2a. There was no significant difference among treatments. Two peaks of soil temperature appeared on July 5 (36.6 °C) and July 22 (35.1 °C). The average soil temperature of each treatment during the maize season was 28.8 °C. The soil pH ranged from 7.4 to 7.9 in all the treatments (Fig. 2b). The pH of the C treatment increased by an average of 0.09 units compared with the CK treatment. The pH values of the SR and C+SR treatments significantly decreased by 0.4 and 0.2 units, respectively. The variations in SWC of all treatments were similar in the same period, and they were not significantly influenced by biochar, straw or combined biochar and straw (Fig. 2c). The average SWC was between 17.6% and 19.2% in all treatments, with the highest value of 26.6% in the SR treatment.
Soil temperature (a), pH (b), SWC (c), NH4+-N (d), NO3−-N (e), MBC (f), MBN (g), and MBC/MBN(h) of the CK, C, SR, and C+SR treatments during the maize growing season. Data points and error bars represent means and standard errors (n = 3), respectively. Abbreviations: CK, control treatment; C, biochar treatment; SR, straw return treatment; C+SR, biochar plus straw treatment
The soil NH4+-N content did not significantly change in the C, SR and C+SR treatments compared with the CK treatment (Fig. 2d). However, it decreased by 15.6% in the SR treatment and by 6.9% in the C+SR treatment on August 4 (P < 0.05). The soil NO3−-N content of the C, SR, and C+SR treatments were higher than that of CK and increased by 46.8%, 33.7%, and 21.5% on average, respectively (P < 0.05, Fig. 2e). On August 4th, the soil NO3−-N content reached the highest peak during the growing season; the NO3−-N content of the C+SR treatment was significantly higher than that of the other treatments, and NO3−-N was significantly decreased in the SR treatment compared with the CK treatment.
Soil microbial biomass carbon and nitrogen
The variations in MBC content in the CK, C, SR, and C+SR treatments were basically the same (Fig. 2f). The MBC content increased significantly and reached the peak value on August 4 after fertilization. The MBC contents in each treatment were C+SR (504.2 μg g−1) > SR (495.2 μg g−1) > C (485.1 μg g−1) > CK (295.1 μg g−1), and the MBC contents of the C, SR, and C+SR treatments increased by 64.39%, 67.81% and 70.86%, respectively, compared with CK (P < 0.05). During the maize growing season, the average MBC content of each treatment was C+SR (401.5 μg g−1) > SR (351.7 μg g−1) > C (313.5 μg g−1) > CK (224.9 μg g−1), corresponding to increases of 39.4%, 56.3%, 78.5%, and 0% compared with the CK treatment, respectively (P < 0.05).
The variations in MBN content in each treatment present an “M”-shaped bimodal curve (Fig. 2g). The MBN contents reached peak values on August 5, in the order C+SR (272.7 μg g−1) > C (267.8 μg g−1) > SR (245.3 μg g−1) > CK (158.3 μg g−1). The MBN contents of the C, SR, and C+SR treatments increased by 69.1%, 55.0%, and 72.2%, respectively, compared with the CK treatment (P < 0.05). The average MBN contents of each treatment followed the order C+SR (132.9 μg g−1) > C (115.7 μg g−1) > SR (112.9 μg g−1) > CK (62.4 μg g−1). The average MBN content of the C, SR, and C+SR treatments increased by 85.4%, 81.1%, and 113.1%, respectively, compared with the CK treatment (P < 0.05).
The variations in MBC/MBN in each treatment were basically the same before August 5 (Fig. 2h). The MBC/MBN of the SR and C+SR treatments decreased by 3.8% and 5.9%, respectively, and that of the C treatment increased by 18.78% compared with the CK treatment (P < 0.05).
Copy numbers of nitrifying and denitrifying genes
The copy numbers of AOA (2.8 × 106–5.0 × 106 g−1 dry soil) were significantly higher than those of AOB (6.2 × 105–9.2 × 105 g−1 dry soil) (P < 0.05, Fig. 3). Compared with the CK treatment, the copy number of AOA in the C treatment decreased by 40.6% (P < 0.05), and that of AOB increased by 33.9% (P < 0.05). There was no significant difference with respect to the CK treatment in either AOA or AOB numbers for the SR and C+SR treatments.
Copy numbers of nitrification and denitrification functional genes in the CK, C, SR, and C+SR treatments during the experimental period. Data points and error bars represent means and standard errors (n = 3), respectively. Abbreviations: CK, control treatment; C, biochar treatment; SR, straw return treatment; C+SR, biochar plus straw treatment
Regarding denitrifier genes, the copy number of the nirK gene decreased by 43.1% in the C treatment, increased by 57.2% in the SR treatment, and decreased by 35.0% in the C+SR treatment (P < 0.05). The copy number of the nirS gene increased by 42.4% and 31.2% in the C and SR treatments, respectively (P < 0.05). The copy number of the nosZ gene increased by 35.1% in the C treatment but decreased by 53.9% and 13.6% in the SR and C+SR treatments, respectively (P < 0.05).
Correlation analysis of N2O emissions with soil factors and functional genes
The correlation analysis between N2O flux and the soil physicochemical properties and the copy number of nitrifiers and denitrifiers are shown in Table 2. N2O flux was significantly positively correlated with NO3−-N and negatively correlated with MBC in the CK and C treatments (P < 0.05). While N2O flux was significantly positively correlated with NH4+-N in the SR and C+SR treatments (P < 0.05). There was no significant correlation between N2O flux and SWC, pH, MBN, and soil temperature.
N2O flux was negatively correlated with nosZ gene copies in the CK treatment and negatively correlated with AOB, nirS, and nosZ gene copies in the C treatment (P < 0.05). N2O flux was significantly positively correlated with the nirK gene in the SR treatment and positively correlated with the nosZ in the C+SR treatment (P < 0.05). N2O flux was significantly negatively correlated with AOB copies (P < 0.05). Overall, N2O flux was negatively correlated with AOB, nirS, and nosZ gene copies but positively correlated with AOA and nirK gene copies.
The correlations between soil environmental factors and the copy numbers of nitrifiers and denitrifiers in each treatment are shown in Table 3. Soil pH was significantly negatively correlated with SWC (P < 0.01), MBN content and soil temperature (P < 0.05). SWC was significantly positively correlated with soil temperature (P < 0.01) and positively correlated with NO3−-N and NH4+-N content (P < 0.05). NO3−-N content was significantly positively correlated with NH4+-N and MBC content (P < 0.05). NH4+-N content was significantly positively correlated with MBN content (P < 0.05). There was a significant positive correlation between MBC and MBN content (P < 0.01).
There was no significant correlation between gene copy numbers and soil environmental factors in the experiment. There were significant negative correlations between AOA, AOB, and nirS copies (P < 0.01), but these factors were significantly negatively correlated with nosZ (P < 0.05). The number of nirK copies was negatively correlated with nosZ and nirS copies (P < 0.01). There was a significant positive correlation between nirS and nosZ copies (P < 0.05).
Alpha diversity of AOA, AOB, nirK, nirS, and nosZ
Biochar addition significantly increased the OTUs and Chao1 index of AOA by an average of 62.1% and 73.3%, respectively (P < 0.05, Table 4). Biochar addition and straw return increased both the OTUs and Chao1 index of AOB (P < 0.05); OTUs increased by 91.8 and 90.0% in the C and SR treatments, respectively, and the Chao1 index increased by 99.6% and 95.2%. Straw return significantly increased the OTUs and Chao1 index of the nirK gene compared with CK, with average increases of 58.5% and 32.5%, respectively (P < 0.05). The C+SR treatment significantly decreased the OTUs and Chao1 index of the nosZ gene compared with CK (P < 0.05), with average decreases of 18.1% and 18.4%, respectively. However, there was no significant difference among the OTUs and Chao1 index of the nirS gene in each treatment.
Community compositions of nitrifiers and denitrifiers
The community compositions of nitrifiers and denitrifiers at the genus level are shown in Fig. 4. The relative abundance of unidentified genera of AOA reached 99% in the CK, C, SR, and C+SR treatments, so AOA are not further discussed. Among the AOB sequences, Nitrosospira and Nitrosovibrio were the dominant genera, accounting for more than 74% of the total bacterial community, and Nitrosospira was the dominant genus in each treatment. The relative abundance of Nitrosospira exceeded 60% in all treatments, with the order of C+SR > CK > C > SR. Therefore, the application of biochar and straw return had a significant effect on the community composition of AOB but not AOA at the genus level.
Agrobacterium, Rhizobium, Bradyrhizobium, Ensifer, Chelatococcus, Mesorhizobium, and Xanthomonas were the dominant genera associated with the nirK gene, with relative abundances of more than 30%. The relative abundance of Agrobacterium increased significantly in the C and C+SR treatments (P < 0.05) but decreased in the SR treatment compared with CK. The relative abundance of Rhizobium decreased significantly in the C treatment but increased in the SR and C+SR treatments, which was consistent with the effect of different treatments on N2O flux. The relative abundance of Xanthomonas increased significantly in the SR and C+SR treatments (P < 0.05), while there were no significant changes in the C treatment.
There were 4 dominant genera with a relative abundance of ≥1% associated with the nirS gene: Rhodanobacter, Magnetospirillum, Azospirillum, and Sulfurifustis. Compared with CK, the genus Rhodanobacter increased significantly in the SR and C+SR treatments (P < 0.05). The relative abundances of the genus Azospirillum in the C and C+SR treatments were significantly higher than that in the SR treatment (P < 0.05).
There were 9 dominant genera with a relative abundance of ≥1% associated with the nosZ gene: Azospirillum, Microvirga, Chelatococcus, Ramibacter, Mesorhizobium, Paracoccus, Sinorhizobium, Achromobacter, and Pseudomonas. The relative abundance of Azospirillum, the dominant genus in each treatment, was more than 10%, and the order was SR > C > CK > C. The relative abundance of Sinorhizobium was significantly increased in the C treatment, while the relative abundance of Pseudomonas was significantly increased in the SR treatment compared with CK (P < 0.05).
RDA analysis of soil denitrifying bacteria and environmental factors
The RDA analysis results for soil denitrifying bacteria and environmental factors are shown in Fig. 5. Because the number of dominant species of AOA and AOB was less than the number of environmental factors, AOA and AOB are not further discussed. The red arrows represent soil environmental factors, such as MBC, MBN, NH4+-N, NO3−-N, pH, and SWC. The blue arrows represent the dominant genera associated with denitrification genes. There were positive correlations between MBC, MBN, and NO3−-N content. NH4+-N content was negatively correlated with MBC, MBN, and NO3−-N content. Soil pH was positively correlated with SWC. With respect to the nirK gene, the genera Rhizobium, Xanthomonas, and Mesorhizobium were positively correlated with MBC, MBN, and NO3−-N and negatively correlated with NH4+-N. The genus Agrobacterium was positively correlated with pH and SWC. The genera Chelatococcus and Mesorhizobium were positively correlated with NH4+-N. With respect to the nirS gene, the genera Azospirillum and Sulfurifustis were positively correlated with MBN and NO3−-N and negatively correlated with NH4+-N, while Rhodonobacter and Magnetospirillum had a negative correlation with pH and SWC. With respect to the nosZ gene, the genera Pseudomonas and Sinorhizobium were positively correlated with pH and SWC and negatively correlated with NO3−-N.
Discussion
Effects of biochar on N2O flux
During the whole maize growing season, the addition of biochar significantly reduced the cumulative N2O emissions by 30.3% (Fig. 1b). Previous studies have also confirmed this conclusion (Harter et al. 2016; Krause et al. 2018; Zaw et al. 2018; Ribas et al. 2019; Song et al. 2019). In this study, soil NO3−-N and MBC contents were the main factors affecting N2O emissions in the biochar treatment (Table 2). Biochar addition decreased the soil NO3−-N content, which was consistent with the results of Song et al. (2019). Biochar can affect the process of nitrogen conversion by adsorbing NO3− ions, thus reducing N2O emissions from soil (Yi et al. 2017; He et al. 2019). The MBC content increased, which might be due to an increase in soil organic carbon content (Song et al. 2019), and promoted the carbon and nitrogen cycle (Huang et al. 2017a, b), thus affecting N2O emissions. In addition, the addition of biochar increased soil pH (Fig. 2b) and promoted the conversion of N2O to N2, therefore reducing N2O emissions (Zhou et al. 2017a, b; Yuan et al. 2019). In this study, we found that biochar had no significant effect on soil temperature and SWC and the changes in SWC were mainly due to irrigation and rainfall. Therefore, the inhibitory effect of biochar on N2O emissions could not be attributed to changes in temperature and SWC.
AOA and AOB play important roles in N2O production (Caranto et al. 2016). We found that with biochar addition, the AOA copy numbers decreased, and the AOB copy numbers increased (Fig. 3); additionally, N2O flux was positively correlated with AOA abundance and negatively correlated with AOB abundance. Liu et al. (2014a, b) also found that biochar addition reduced AOA abundance, thus reducing N2O emissions caused by nitrification. Harter et al. (2016) found that adding biochar could increase AOB copy numbers in saline-alkali soil, which was consistent with our results. For the biochar treatment, N2O flux was significantly negatively correlated with AOB abundance but not correlated with AOA abundance. There was a negative correlation between AOA and AOB, indicating that AOA and AOB may have an antagonistic relationship with N2O emissions, and AOB was more sensitive to N2O emissions than AOA (Shi et al. 2019). According to the analysis of community compositions at the genus level (Fig. 4), the AOB genus Nitrosospira was the main contributor to N2O emissions.
For the denitrifiers, we found that N2O flux was negatively correlated with nirS and nosZ copies. Biochar addition increased the nirS and nosZ numbers and accelerated the process of N2O reduction to N2, thus reducing N2O emissions. Ji et al. (2020) found that biochar significantly increased the abundance of the nosZ gene and reduced N2O emissions regardless of soil type, which was in line with our results. For the nirK gene, Van Zwieten et al. (2014) found a positive correlation between N2O flux and nirK gene abundance. In this study, we found that the copy number of the nirK gene in the C treatment was significantly reduced, and RDA analysis revealed that the NH4+-N and NO3−-N contents were the most important factors affecting the nirK gene (Fig. 5). After adding biochar, the NH4+-N content increased and the NO3−-N content decreased in the soil, thus affecting the community richness of the dominant species of the nirK gene, which reduced N2O emissions. This finding was consistent with the results of Krause et al. (2018), who also found that biochar reduced soil N2O emissions by affecting denitrifier community composition under field conditions. In addition, the relative abundance of Agrobacterium (nirK) increased significantly in the C and C+SR treatments but decreased slightly in the SR treatment. This result might be due to the promotion effects of biochar on the Agrobacterium community. RDA analysis also indicated that the relative abundance of Agrobacterium was positively correlated with the MBC and MBN contents (Fig. 5a). The addition of biochar increased the soil organic carbon content; therefore, the promotion effects of biochar on the Agrobacterium community are well explained. The genus Rhizobium decreased significantly in the C treatment but increased in the SR and C+SR treatments, which was consistent with the effect of different treatments on N2O flux. Shi et al. (2019) also found a decreased abundance of Rhizobium in biochar-amended soil. Hidalgo-García et al. (2019) proved that Rhizobium could assimilate nitrate as a substrate and produce N2O through denitrification. We also found that Rhizobium was positively correlated with NO3−-N in the RDA analysis (Fig. 5a), which helps to elucidate the relationship between N2O emissions and the abundance of the nirK gene.
Effects of straw return on N2O flux
Numerous studies have shown that straw return can increase N2O emissions from farmland soils (Liu et al. 2011; Wu et al. 2018; Liu et al. 2019), and the same conclusion was reached in this study. The results showed that NH4+-N content was the most important environmental controlling factor of N2O emissions (Table 2). After straw returning, the contents of NH4+-N and NO3−-N in the soil increased significantly, and they were significantly higher than those in the biochar treatment (Fig. 2d, e). Higher NH4+-N and NO3−-N contents can increase the chemical reaction of substrates for microbial nitrification and denitrification; therefore, soil N2O emission was promoted (Wu et al. 2017). Mitchell et al. (2013) indicated that substrate carbon concentration was one of the main driving factors affecting N2O emissions and that straw decomposition provided a carbon source for soil microorganisms and increased the soil MBC content (Zhou et al. 2017a, b). Exogenous organic carbon increases the soil C/N ratio, provides a suitable environment for soil denitrification, and results in a large amount of N2O emissions (Cui et al. 2016; Huang et al. 2017a, b). In addition, straw return decreases the soil pH to a certain extent, and lower pH inhibits the activity of N2O reductase (Liu et al. 2011), which might be another reason for the increase in N2O emissions in straw-amended soil.
After straw return, the copy number of the AOA gene increased slightly, and that of AOB decreased slightly (Fig. 3), but straw had a small effect on the community composition of AOA and AOB (Fig. 4). For denitrifiers, straw addition increased the copy number of the nirK gene, and the OTUs and Chao1 index of the nirK gene increased significantly (Table 4), which increased the relative abundance of the genus Rhizobium. N2O flux had a positive correlation with nirK gene abundance in this research. Wang et al. (2020) also found that with straw return in paddy soil, a decrease in nirK gene abundance inhibited N2O emission. Therefore, the change in nirK gene abundance should be one of the main reasons for stimulating N2O emission. Straw return also reduced the copy numbers of nirS and nosZ genes, which was consistent with the results of Liu et al. (2019). Moreover, the abundance of the nirK gene was significantly negatively correlated with the abundance of the nirS and nosZ genes, illustrating that there might be an antagonistic relationship among genes involved in denitrification, which jointly affected denitrification-mediated N2O emissions. We also found that the relative abundance of Rhizobium increased in the SR and C+SR treatments due to the increased MBC, MBN, and NO3−-N in the straw-amended soil (Fig. 2e). Therefore, Rhizobium could assimilate nitrate substrates and produce N2O through denitrification (Hidalgo-García et al. 2019). The relative abundances of Azospirillum (nosZ) and Rhodanobacter (nirS) increased in the SR and C+SR treatments compared with CK, which was in accordance with the results of Shi et al. (2019). Previous studies have shown that the genus Azospirillum can drive the reduction of NO3− with no accumulation of NO2− during aerobic assimilation (Nelson and Knowles 1978). Therefore, we could infer that straw promoted N2O emissions mainly by affecting the community composition of the nirK, nirS, and nosZ genes.
Effects of the combination of biochar and straw on N2O flux
Contrary to our expectations, the combination of biochar and straw increased soil N2O emissions by 37.0% in this experiment (Fig. 1b), resulting in a value even higher than that in the SR treatment. The results showed that the contents of NH4+-N and NO3−-N in the C+SR treatment were higher than those in the C and SR treatments (Fig. 2d, e). We found that NH4+-N content was the most important influencing factor of N2O emissions in the C+SR treatment (Table 2). The microorganisms participating in the N cycle obtained sufficient substrate, improving the nitrification rate, and thus produced a large amount of N2O (Zhou et al. 2017a, b). Liu et al. (2017) also found that N2O and NO emissions were strongly affected by soil mineral N and that total N2O and NO emissions were significantly positively correlated with soil NH4+-N and NO3−-N contents. Biochar addition and straw return provided sufficient carbon sources for soil, increased soil MBC and MBN (Fig. 2f, g), and promoted N2O emissions caused by denitrification (Zhang et al. 2012; Liu et al. 2014a, b). In addition, the soil pH of the C+SR treatments decreased by 0.2 units compared with CK, which might be one of the reasons for the higher N2O flux. Overall, the soil NH4+-N and NO3−-N contents were the main environmental factors affecting N2O emissions from the C+SR treatment.
In this experiment, the copy number of the AOB gene in the C+SR treatment was significantly reduced compared with that in the CK treatment, and N2O flux was negatively correlated with AOB gene abundance (Table 2), which stimulated N2O emission from the soil (Harter et al. 2016). The genus Nitrosospira, the dominant genus associated with the AOB gene, had the highest relative abundance in the C+SR treatment, which may be one of the main reasons why the AOB gene inhibited N2O emission. However, for denitrifying genes, the copy numbers of nirS and nosZ in the C+SR treatment showed an obvious downward trend, while in the C treatment, there was an upward trend (Fig. 3), which illustrated that the influence of straw on the nirS and nosZ gene communities was greater than that of biochar. The OTUs and Chao1 index of the C+SR treatment were significantly reduced, while there was no significant difference in the nirS gene among treatments (Table 4), which indicated that straw mainly affected the denitrification pathway of microorganisms by reducing the abundance of the nosZ gene, thus promoting N2O emission. However, the nirK gene copy number in the C+SR and C treatments significantly decreased, while it increased significantly in the SR treatment, and Agrobacterium associated with the nirK gene increased significantly in the C+SR treatment but had the lowest abundance in the SR treatment (Fig. 4). The results showed that the effect of biochar on the nirK gene was greater than that of straw. The increase in the relative abundance of Rhizobium might affect N2O emissions. The abundance of Rhizobium was positively correlated with NO3−-N content and negatively correlated with NH4+-N content in the RDA analysis (Fig. 5), which supported the conclusion that Rhizobium could use nitrate as a substrate for denitrification (Hidalgo-García et al. 2019). In conclusion, straw inhibited the abundance of AOB and nosZ genes, which stimulated N2O emission.
Conclusions
This study indicated that biochar addition reduced soil N2O emissions by 30.3%, while straw return and biochar plus straw increased soil N2O emissions by 13.2% and 37.0%, respectively, during the maize season in the northern China. Biochar addition and straw return shifted the community composition of nitrifiers and denitrifiers. N2O production was mainly reduced by promoting AOB and inhibiting nirK gene abundance in the biochar-amended soil, and it was mainly promoted by inhibiting AOB and nosZ gene abundances in the straw and biochar plus straw-amended soil. NO3−-N, NH4+-N, and MBC were the main factors. Based on our results, the application of biochar to cropland is an effective option to mitigate greenhouse gases, whereas direct straw return to the field may not be an effective strategy. We suggest that future work should focus on the effect of straw return with different maturities on N2O production and reduction.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ai C, Liang G, Sun J, Wang X, He P, Zhou W (2013) Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol Biochem 57:30–42
Caranto JD, Vilbert AC, Lancaster KM (2016) Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proc Natl Acad Sci 113:14704–14709
Chen CR, Phillips IR, Condron LM, Goloran J, Xu ZH, Chan KY (2013) Impacts of greenwaste biochar on ammonia volatilisation from bauxite processing residue sand. Plant Soil 367(1-2):301–312
Chen X, Yang J, Zhu X, Liang X, Lei Y, He C (2016) N-fixing trees in wetland restoration plantings: effects on nitrogen supply and soil microbial communities. Environ Sci Pollut Res 23:24749–24757
Cole JR, Wang Q, Fish JA, Chai BL, Mcgarrell DM, Sun YN, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642
Cui F, Yan G, Zhou Z, Zheng X, Deng J (2012) Annual emissions of nitrous oxide and nitric oxide from a wheat–maize cropping system on a silt loam calcareous soil in the North China Plain. Soil Biol Biochem 48:10–19
Cui P, Fan F, Yin C, Song A, Huang P, Tang Y, Liang Y (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol Biochem 93:131–141
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998
Harter J, Krause HM, Schuettler S, Ruser R, Fromme M, Scholten T, Kappler A, Behrens S (2014) Linking N2O emissions from biochar–amended soil to the structure and function of the N–cycling microbial community. ISME J 8:660–674
Harter J, Weigold P, El-Hadidi M, Huson DH, Kappler A, Behrens S (2016) Soil biochar amendment shapes the composition of N2O-reducing microbial communities. Sci Total Environ 562:379–390
He L, Zhong H, Liu G, Dai Z, Brookes PC, Xu J (2019) Remediation of heavy metal contaminated soils by biochar: mechanisms, potential risks and applications in China. Environ Pollut 252:846–855
Henry S, Baudoin E, López-Gutiérrez JC, Martin-Laurent F, Brauman A, Philippot L (2004) Quantification of denitrifying bacteria in soils by nirK gene targeted real–time PCR. J. Microbiol Meth 59:327–335
Hidalgo-García A, Torres MJ, Salas A, Bedmar EJ, Girard L, Delgado MJ (2019) Rhizobium etli produces nitrous oxide by coupling the assimilatory and denitrification pathways. Front Microbiol 10:980
Hink L, Gubry-Rangin C, Nicol GW, Prosser JI (2018) The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J 12:1084–1093
Hu H, Chen D, He J (2015) Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiol Rev 39:729–749
Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, Wan J (2017a) The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 174:545–553
Huang T, Yang H, Huang C, Ju X (2017b) Effect of fertilizer N rates and straw management on yield-scaled nitrous oxide emissions in a maize-wheat double cropping system. Field Crop Res 204:1–11
Ji C, Li S, Geng Y, Miao Y, Ding Y, Liu S, Zou J (2020) Differential responses of soil N2O to biochar depend on the predominant microbial pathway. Appl Soil Ecol 145:103348
Ju X, Lu X, Gao Z, Chen X, Su F, Kogge M, Zhang F (2011) Processes and factors controlling N2O production in an intensively managed low carbon calcareous soil under sub-humid monsoon conditions. Environ Pollut 159:1007–1016
Krause HM, Hüppi R, Leifeld J, El-Hadidi M, Harter J, Kappler A, Gattinger A (2018) Biochar affects community composition of nitrous oxide reducers in a field experiment. Soil Biol Biochem 119:143–151
Li H, Dai M, Dai S, Dong X (2018) Current status and environment impact of direct straw return in China’s cropland–a review. Ecotox Environ Safe 159:293–300
Lin Y, Ding W, Liu D, He T, Yoo G, Yuan J, Fan J (2017) Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol Biochem 113:89–98
Liu C, Wang K, Meng S, Zheng X, Zhou Z, Han S, Yang Z (2011) Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emissions from a wheat–maize rotation field in northern China. Agric Ecosyst Environ 140:226–233
Liu C, Lu M, Cui J, Li B, Fang C (2014a) Effects of straw carbon input on carbon dynamics in agricultural soils: a meta-analysis. Glob Chang Biol 20:1366–1381
Liu L, Shen G, Sun M, Cao X, Shang G, Chen P (2014b) Effect of biochar on nitrous oxide emission and its potential mechanisms. J. Air Waste Manag 64:894–902
Liu S, Lin F, Wu S, Ji C, Sun Y, Jin Y, Li S, Li Z, Zou J (2017) A meta-analysis of fertilizer-induced soil NO and combined NO+N2O emissions. Glob Chang Biol 23:2520–2532
Liu X, Ren J, Zhang Q, Liu C (2019) Long-term effects of biochar addition and straw return on N2O fluxes and the related functional gene abundances under wheat-maize rotation system in the North China Plain. Appl Soil Ecol 135:44–55
Ma Y, Liu D, Schwenke G, Yang B (2019) The global warming potential of straw-return can be reduced by application of straw-decomposing microbial inoculants and biochar in rice-wheat production systems. Environ Pollut 252:835–845
Mitchell DC, Castellano MJ, Sawyer JE, Pantoja J (2013) Cover crop effects on nitrous oxide emissions: role of mineralizable carbon. Soil Sci Soc Am J 77:1765–1773
Munyaka PM, Eissa N, Bernstein CN, Khafipour E, Ghia JE (2015) Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: a role of the gut microbiota. PLoS One 10(11):e0142536
Nelson LM, Knowles R (1978) Effect of oxygen and nitrate on nitrogen fixation and denitrification by Azospirillum brasilense grown in continuous culture. Can J Microbiol 24:1395–1403
Park SJ, Park BJ, Rhee SK (2008) Comparative analysis of archaeal 16S rRNA and amoA genes to estimate the abundance and diversity of ammonia-oxidizing archaea in marine sediments. Extremophiles 12:605–615
Peng X, Zhu Q, Xie Z, Darboux F, Holden NM (2016) The impact of manure, straw and biochar amendments on aggregation and erosion in a hillslope Ultisol. Catena 138:30–37
Ribas A, Mattana S, Llurba R, Debouk H, Sebastià MT, Domene X (2019) Biochar application and summer temperatures reduce N2O and enhance CH4 emissions in a Mediterranean agroecosystem: role of biologically-induced anoxic microsites. Sci Total Environ 685:1075–1086
Scala DJ, Kerkhof LJ (1998) Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol Lett 162:61–68
Shi Y, Liu X, Zhang Q (2019) Effects of combined biochar and organic fertilizer on nitrous oxide fluxes and the related nitrifier and denitrifier communities in a saline-alkali soil. Sci Total Environ 686:199–211
Song Y, Li Y, Cai Y, Fu S, Luo Y, Wang H, Chang SX (2019) Biochar decreases soil N2O emissions in Moso bamboo plantations through decreasing labile N concentrations, N-cycling enzyme activities and nitrification/denitrification rates. Geoderma 348:135–145
Song H, Wang J, Zhang K, Zhang M, Hui R, Sui T, Yang L, Du W, Dong Z (2020) A 4-year field measurement of N2O emissions from a maize-wheat rotation system as influenced by partial organic substitution for synthetic fertilizer. J Environ Manag 263:110384
Spokas KA, Novak JM, Venterea RT (2012) Biochar’s role as an alternative N-fertilizer: ammonia capture. Plant Soil 350:35–42
Tan Y, Xu C, Liu D, Wu W, Lal R, Meng F (2017) Effects of optimized N fertilization on greenhouse gas emission and crop production in the North China Plain. Field Crop Res 205:135–146
Throbäck IN, Enwall K, Jarvis Å, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417
Tian H, Yang J, Xu R, Lu C, Canadell JG, Davidson EA, Gerber S (2018) Global soil nitrous oxide emissions since the pre-industrial era estimated by an ensemble of terrestrial biosphere models: magnitude, attribution and uncertainty. Glob Chang Biol 25:640–659
Van Zwieten L, Singh BP, Kimber SWL, Murphy DV, Macdonald LM, Rust J, Morris S (2014) An incubation study investigating the mechanisms that impact N2O flux from soil following biochar application. Agric Ecosyst Environ 191:53–62
Wang Y, Sheng H, He Y, Wu J, Jiang Y, Tam NFY, Zhou H (2012) Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of Illumina tags. Appl Environ Microbiol 78:8264–8271
Wang N, Luo J, Juhasz AL, Li H, Yu J (2020) Straw decreased N2O emissions from flooded paddy soils via altering denitrifying bacterial community compositions and soil organic carbon fractions. FEMS Microbiol Ecol 96:fiaa046
Wood SA, Almaraz M, Bradford MA, McGuire KL, Naeem S, Neill C, Palm CA, Tully KL, Zhou JZ (2015) Farm management, not soil microbial diversity, controls nutrient loss from smallholder tropical agriculture. Front Microbiol 6:90
Wu D, Senbayram M, Well R, Brüggemann N, Pfeiffer B, Loick N, Stempfhuber B, Dittert K, Bol R (2017) Nitrification inhibitors mitigate N2O emissions more effectively under straw-induced conditions favoring denitrification. Soil Biol Biochem 104:197–207
Wu D, Wei Z, Well R, Shan J, Yan X, Bol R, Senbayram M (2018) Straw amendment with nitrate-N decreased N2O/(N2O+N2) ratio but increased soil N2O emission: a case study of direct soil-born N2 measurements. Soil Biol Biochem 127:301–304
Wu X, Wang W, Xie K, Yin C, Hou H, Xie X (2019) Combined effects of straw and water management on CH4 emissions from rice fields. J Environ Manag 231:1257–1262
Xu C, Han X, Ru S, Cardenas L, Rees RM, Wu D, Wu W, Meng F (2019) Crop straw incorporation interacts with N fertilizer on N2O emissions in an intensively cropped farmland. Geoderma 341:129–137
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89:1467–1471
Yao Z, Yan G, Zheng X, Wang R, Liu C, Butterbach-Bahl K (2017) Straw return reduces yield-scaled N2O plus NO emissions from annual winter wheat-based cropping systems in the North China Plain. Sci Total Environ 590:174–185
Yi Q, Tang S, Fan X, Zhang M, Pang Y, Huang X, Huang Q (2017) Effects of nitrogen application rate, nitrogen synergist and biochar on nitrous oxide emissions from vegetable field in south China. PLoS One 12:e0175325
Yuan H, Zhang Z, Li M, Clough T, Wrage-Mönnig N, Qin S, Zhou S (2019) Biochar’s role as an electron shuttle for mediating soil N2O emissions. Soil Biol Biochem 133:94–96
Zaw OA, Shigeto S, Hiroko A, Thuzar WK, Akira S, Akinori Y, Tomohito S, Yuhei H (2018) Effect of dolomite and biochar addition on N2O and CO2 emissions from acidic tea field soil. PLoS One 13:e0192235
Zhang A, Liu Y, Pan G, Hussain Q, Li L, Zheng J, Zhang X (2012) Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 351:263–275
Zhang L, Zeng G, Zhang J, Chen Y, Yu M, Lu L, Li H, Zhu Y, Yuan Y, Huang A, He L (2015) Response of denitrifying genes coding for nitrite (nirK or nirS) and nitrous oxide (nosZ) reductases to different physico-chemical parameters during agricultural waste composting. Appl Microbiol Biotechnol 99:4059–4070
Zhao J, Ni T, Xun W, Huang X, Huang Q, Ran W, Shen Q (2017) Influence of straw incorporation with and without straw decomposer on soil bacterial community structure and function in a rice-wheat cropping system. Appl Microbiol Biotechnol 101:4761–4773
Zhou Y, Zhang Y, Tian D, Mu Y (2017a) The influence of straw returning on N2O emissions from a maize-wheat field in the North China Plain. Sci Total Environ 584:935–941
Zhou M, Zhu B, Wang S, Zhu X, Vereecken H, Brüggemann N (2017b) Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: a global meta-analysis. Glob Chang Biol 23:4068–4083
Funding
The research was financially supported by the National Natural Science Foundation of China (No. 41773090).
Author information
Authors and Affiliations
Contributions
LXR planned and designed research; TZM and ZQW conducted experiments; TZM and KWD conducted chemical analysis; LXR and TZM conducted statistical analysis and wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Tang, Z., Zhang, Q. et al. The contrasting effects of biochar and straw on N2O emissions in the maize season in intensively farmed soil. Environ Sci Pollut Res 28, 29806–29819 (2021). https://doi.org/10.1007/s11356-021-12722-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12722-2