Abstract
There are some key processes that regulate cadmium (Cd) accumulation in rice. Understanding the characteristics and mechanisms of Cd accumulation in high Cd-accumulating rice lines benefits for excavating relevant genes. Cd accumulation and distribution in roots of Lu527-8, a high Cd-accumulating rice line, were investigated by a hydroponic experiment, with a control of a normal rice line (Lu527-4). Lu527-8 showed significantly higher Cd concentrations in roots than Lu527-4. More than 81% of Cd in roots of two rice lines is distributed in soluble fraction and cell wall. In soluble fraction, there were more organic acids, amino acids, and phytochelatins in Lu527-8, benefiting Cd accumulation. Pectin and hemicellulose 1 (HC1), especially pectin, were main polysaccharides in cell wall. Lu527-8 showed more pectin and HC1 along with higher pectin methylesterase (PME) activity compared with Lu527-4, promoting Cd accumulation. Besides, Lu527-8 showed higher Cd translocation from root to shoot due to more amounts of ethanol-extractable Cd in roots than Lu527-4. In conclusion, specific characteristics of Cd chemical forms and subcellular distribution in roots of high Cd-accumulating rice line are important for Cd accumulation and translocation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) has been considered a global toxic trace pollutant, which is readily absorbed and accumulated in plants, thus influencing a series of physiological processes, such as nutrient element assimilation, enzyme activity, respiration, and photosynthesis (Uraguchi and Fujiwara, 2013; Feng et al. 2017). To alleviate Cd toxicity, some plants, especially phytoextractors, have developed complex mechanisms for Cd detoxification and translocation (Clemens et al. 2013; He et al. 2015; He et al. 2019). Those plants pose a strong potential for Cd accumulation and transportation by mediating Cd distribution and chemical forms (Wang et al. 2008; Fu et al. 2011). Some indica rice varieties are considered potential phytoextractors for Cd-contaminated paddy fields because of their high biomass and Cd accumulation in shoots (Ibaraki et al. 2009). An understanding of Cd chemical form and subcellular distribution in a high Cd-accumulating rice line is beneficial to clarify the mechanism of Cd detoxification and accumulation.
The subcellular distribution of Cd is closely related to Cd accumulation and detoxification in plants (Wu and Wang 2011; Su et al. 2014; Wang et al. 2015). Plant accumulates Cd in cell wall or soluble fraction to achieve Cd detoxification (Su et al. 2017; Zhou et al. 2017). As the first barrier, cell wall poses a critical capacity of protecting Cd from being transported into the protoplast (Krzesłowska, 2011). The major components of cell wall are polysaccharides, including pectin and hemicellulose (Xiong et al. 2009). Both of them contain abundant functional groups, such as carboxyl, hydroxide radical, and sulfhydryl, which can efficiently bind metal cations (Li et al. 2015). Under Cd stress, cell wall components are actively modified to enhance Cd binding capacity (Krzesłowska, 2011). With the demethylation catalysis of pectin methylesterase (PME), more and more exposure of free carboxyl groups is observed in pectin, resulting in an increase for Cd binding capacity (Krzesłowska, 2011; Li et al. 2015). Meanwhile, there are amounts of ligands, such as polypeptides and organic acids, which can chelate Cd ions in soluble fraction. Cd complexes are then transported into vacuoles to compartment, resulting in Cd detoxification and reducing Cd transport (Li et al. 2016; Mwamba et al. 2016; Xin et al. 2017).
Chemical forms have attracted great attention because of their importance in ecotoxicology and translocation (Lai, 2015; Wang et al. 2016). Cd uptake by plants is usually with different chemical forms, thereby influencing Cd tolerance and translocation (Lai et al. 2015; Li et al. 2016). Cd in water-soluble and ethanol-extractable forms shows more mobility and poison to plant cell, compared with Cd in protein-extractable and pectate-extractable forms, undissolved phosphate forms, and oxalate forms (Zhan et al. 2015; Guan et al. 2018). Moreover, Cd chemical forms usually vary greatly among plant species or genotypes, suggesting different capacities of Cd mobilization and immobilization (Li et al. 2016; Yang et al. 2018).
A rice line designated Lu527-8 was selected as a high Cd-accumulating rice line due to great Cd uptake and translocation to shoots as well as greater Cd tolerance (Tang et al. 2016; Fu et al. 2019). Root is an important tissue for Cd uptake and translocation, while little information available on the characteristics and mechanisms of Cd accumulation and distribution in root of Lu527-8. Therefore, this study would comprehensively elucidate the physiological mechanisms of Cd accumulation and detoxification by clarifying Cd chemical forms and Cd subcellular distribution in the roots of Lu527-8.
Materials and methods
Plant material
A high Cd-accumulating rice line (Oryza sativa L.) designated Lu527-8 was used in this study, and a normal rice line designated Lu527-4 was used as a control. Both of them are indica varieties with similar growth period.
Hydroponic culture and plant sampling
A hydroponic experiment was conducted with the following treatments: 0 (CK), 2, and 5 mg L−1 Cd. Cd was supplied as a CdCl2·2.5H2O solution. The experiment was conducted in an entirely random permutation with five replications. The formulation recommended by the International Rice Research Institute was used as the basal nutrient medium (Ponnamperuma, 1977).
The seeds were sterilized in 30% H2O2 for 30 min and then with 0.1% sodium hypochlorite for 24 h and then sowed in watered perlite at 25 °C and 60% of relative humidity until germination, during which period 1/4 nutrient solution was used. At the third-leaf stage, uniform seedlings were selected and transplanted into basal nutrient solution (pH 5.5) treated with different concentrations of Cd as designed. The seedlings were cultivated in a growth room under natural light at 30 °C/25 °C (day/night temperature). The nutrient solution was renewed every 5 d.
The samples were collected at tillering stage. The roots were soaked in 20 mmol·L−1 Na2-EDTA for 0.5 h and rinsed with deionized water to remove surface-adsorbed Cd. Then the samples were divided into roots and shoots. Half of the samples were immediately frozen by liquid nitrogen for the analysis of fresh sample, and the others were oven-dried at 75 °C for Cd analysis.
Determination of Cd concentrations in roots and shoots
About 250-mg dry samples were digested with HNO3/HClO4 (5:1, v/v) for 8 h and diluted with deionized water to a total volume of 50 mL. Flame atomic absorption spectrophotometer (AA900T, PerkinElmer, America) was used to analyze Cd concentrations in digested solutions.
Cd subcellular fraction
Subcellular fractions were processed according to previous method with minor modifications (Fu et al. 2011; Zhu et al. 2012). The samples were divided into four fractions: cell wall, organelle, membrane, and soluble fractions. About 1-g fresh root samples were homogenized with 75% ethanol for 20 min in an ice bath. The homogenates were centrifuged at 4579×g, 4 °C for 10 min. The sediments were gradually homogenized with 5-mL acetone, methanol:chloroform (1:1), and methanol for 20 min in an ice bath and centrifugated at 4579×g, 4 °C for 10 min to obtain the residue as “cell wall fraction.” Meanwhile, the supernatants were centrifuged at 10000×g, 4 °C for 30 min and the residues were identified as “organelle fraction.” The supernatants were then centrifuged at 100000×g, 4 °C for 30 min and the residues were identified as “membrane fraction.” The final supernatants were considered “soluble fraction,” including vacuoles and cytoplasm. The four fractions were dried at 75 °C and then digested with HNO3/HClO4 (5:1, v/v). The digested solutions were then diluted with deionized water up to a total volume of 10 mL. Cd concentrations in the digested solutions were analyzed by graphite furnace atomic absorption spectrophotometer (AA900T, PerkinElmer, America).
Determination of organic acid and amino acid concentrations
About 0.5-g fresh root samples were ground and transferred into 2 ml 0.5 mol L−1 HCl at 60 °C for 1 h. The solutions were centrifuged at 8000×g for 10 min and filtered with a 0.45-μm millipore filter to obtain organic acids. The organic acids concentrations were determined by HPLC (Shimadzu, Japan) with the procedure described by Fu et al. (2018).
About 0.3-g fresh root samples were ground and transferred to 30 ml 0.1 mol L−1 HCl for 24 h. The solutions were mixed with 10% sulfonylsalicylic acid solution (1:3, v/v) and centrifuged at 14000×g for 20 min and filtered with a 0.45-μm millipore filter to obtain amino acids. The amino acid concentrations were determined by L-8800 amino acid analyzer (Hitachi, Japan) with the procedure described by Fu et al. (2018).
Cell wall preparation and polysaccharides extraction
Cell wall preparation referred to the method of Sharifi et al. (2016). The fresh root samples were ground to powder and further homogenized with ice-cold 75% ethanol in an ice bath for 20 min. Then the homogenates were centrifuged at 5000×g for 10 min. The residues were washed with the following solutions: ice-cold acetone (1:5, w/v), methanol-chloroform mixture (1:1, v/v), and methanol three times. The final residues were obtained as crude cell walls and freeze-dried and stored at 4 °C for subsequent extraction.
The extraction of cell wall polysaccharides was carried out according to Yang et al. (2008) with minor modifications. Cell wall was divided into three parts: pectin, hemicellulose 1 (HC1), and hemicellulose 2 (HC2). About 10-mg cell wall was extracted thrice with 5-mL deionized water at 100 °C for 1 h to obtain pectin. After centrifugation at 16800×g for 10 min, the three supernatants were pooled and diluted with deionized water to a total volume of 15 mL. Then, the residues were extracted twice with 5-mL solutions containing 4% (w/v) KOH and 0.1% (w/v) NaBH4 for 12 h and centrifuged at 16800×g for 10 min to obtain HC1, followed by a similar extraction procedure with 24% (w/v) KOH and 0.1% NaBH4 to obtain HC2. Three hundred microliter of the above extracted solutions were digested with HNO3/HClO4 (5:1, v/v). Cd concentrations in the solutions were determined by graphite furnace atomic absorption spectrophotometer (AA900T, PerkinElmer, America).
Determination of uronic acid and total sugar concentrations in cell wall polysaccharides
Uronic acid concentration in polysaccharides was determined according to Zhu et al. (2012) with galacturonic acid (Sigma) as a standard. One-milliliter pectin solutions were incubated with 5 mL 98% H2SO4 (containing 0.0125 mol L−1 Na2B4O7·10H2O) at 100 °C for 5 min. One hundred microliter 0.15% M-hydro-dipheny was added after the solution chilled. Subsequently, the solutions were incubated at indoor temperature for 20 min. Spectrophotometer was used to determine the absorbance at 520 nm. Total sugar concentration in hemicellulose was determined according to phenol sulfuric acid method and expressed as glucose equivalents. Five-milliliter 98% H2SO4 and 10 μL 80% phenol were added into 1-mL HC1 and HC2 extracting solutions and incubated at indoor temperature for 15 min and then at 100 °C for 15 min, respectively. Spectrophotometer was used to determine the absorbance at 490 nm.
Determination of PME activity
PME activity was determined according to Zhu et al. (2012). Ten-milligram freeze-dried cell walls were extracted with 1 mL 1 mol L−1 NaCl solution in an ice bath for 20 min and mixed every 10 min. Extracts were centrifuged at 13000×g for 10 min to obtain the supernatants containing PME. Two hundred fifty microliter PME extracts were added into 50-μL alcohol oxidase (pH 7.5) and 500 μL 0.64 mg mL−1 pectin (Sigma) and incubated at 30 °C for 10 min. Subsequently, 1-mL solutions containing 5 mg mL−1 purpald and 0.5 mol L−1 NaOH were added and incubated at 30 °C for 30 min. Then, the solutions were diluted with deionized water to a total volume of 5 mL and their absorbance was measured at 550 nm by spectrophotometer.
The extraction of Cd chemical forms
Cd chemical forms were determined according to Zhao et al. (2015) with minor modifications. Cd was extracted with the following extraction solutions in sequence: 80% (v/v) ethanol (FE), deionized water (FW), 1 mol L−1 NaCl (FNaC), 2% (v/v) acetic acid (FHAc), and 0.6 mol L−1 HCl (FHCl). About 0.3-g fresh samples were ground and transferred into extraction solutions. Corresponding supernatants were obtained after being suspended with different extraction solutions and then shaken at 25 °C for 22 h and centrifuged at 5000×g for 10 min. The above steps were repeated, and then all the supernatants were collected. Cd concentrations in the supernatant solutions were determined as described in the “Cd subcellular fraction” section.
Statistical analysis
Translocation factor (TF) was calculated according to Zhang et al. (2012) as follows:
Data were analyzed with DPS 11.0. The least significant difference (LSD) test was used to determine differences among the treatments or rice lines at 0.05 level. Graphical work was exported by OriginPro 9.0 and Excel 2013.
Results
Cd subcellular distribution in roots
Cd concentrations of subcellular fractions in roots of the two rice lines markedly increased with increasing Cd doses (Table 1). Cd mainly distributed in soluble fraction and cell wall fraction in roots. A small amount of Cd distributed in organelle and membrane fractions. There were 47–48% and 37–39%, 42–45%, and 39% of Cd in soluble fraction and cell wall in Lu527-8 and Lu527-4, respectively. Cd concentrations in soluble fraction and cell wall of Lu527-8 were 1.37–1.52 times and 1.28–1.34 times higher than those of Lu527-4, respectively.
Organic acid and amino acid concentrations in roots
There were eight organic acids detected in roots of the two rice lines (Table 2). Oxalate, tartaric, malate, citrate, and acetic acid accounted for the most among organic acids. The concentrations of five organic acids significantly decreased with increasing Cd doses. The concentrations of tartaric, malate, citrate, and acetic acid in Lu527-8 were much more than those in Lu527-4 under Cd treatments.
There were nine amino acids detected in roots of the two rice lines (Table 3). The concentrations of proline, phenylalanine, histidine, lysine, and methionine in roots of the two rice lines markedly increased with increasing Cd doses. The concentrations of glutamic acid, proline, phenylalanine, glycine, histidine, lysine, and methionine in Lu527-8 were markedly higher than those in Lu527-4 under Cd treatments.
Cd accumulation in cell wall polysaccharides
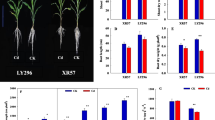
The concentrations of cell wall polysaccharides, including pectin, HC1, and HC2, markedly increased with increasing Cd doses (Fig. 1). Pectin concentrations in Lu527-8 were markedly higher than Lu527-4 when exposed to 2 mg L−1 Cd. HC1 and HC2 concentrations in Lu527-8 were markedly higher than those in Lu527-4 under different Cd treatments, up to 1.11 times and 1.51 times, respectively.
Pectin, hemicellulose 1 (HC1), and hemicellulose 2 (HC2) concentrations in the root cell wall of high Cd-accumulating rice line (Lu527-8) and normal rice line (Lu527-4) grown in different Cd treatments. The data mean the average of three replicates ± standard deviations (SDs). Different letters above the bars indicate significant level at p < 0.05 among the Cd treatments, * indicates significant level at p < 0.05 among the rice lines
As for Cd concentrations in cell wall polysaccharides, Cd concentrations in pectin were the highest, followed by HC1 and HC2 (Fig. 2). Cd concentrations in the three cell wall fractions increased by 117–229% with increasing Cd doses. Cd concentrations in the three cell wall fractions of Lu527-8 was 1.25–2.42 times higher than Lu527-4.
Cd concentrations in different cell wall fractions extracted from the roots of high Cd-accumulating rice line (Lu527-8) and normal rice line (Lu527-4) grown in different Cd treatments. The data mean the average of three replicates ± standard deviations (SDs). Different letters above the bars indicate significant level at p < 0.05 among the Cd treatments, * indicates significant level at p < 0.05 among the rice lines
PME activity
PME activity significantly increased in roots of the two rice lines with increasing Cd doses (Fig. 3). Compared with CK, PME activity increased 69 and 36% in Lu527-8 and Lu527-4, respectively, when exposed to 5 mg L−1 Cd. The activity in Lu527-8 was 1.25–1.27 times higher than Lu527-4 under different treatments.
Pectin methylesterase activity in the root cell wall of high Cd-accumulating rice line (Lu527-8) and normal rice line (Lu527-4) grown in different Cd treatments. The data mean the average of three replicates ± standard deviations (SDs). Different letters above the bars indicate significant level at p < 0.05 among the Cd treatments, * indicates significant level at p < 0.05 among the rice lines
Cd chemical forms in roots
Cd concentrations in all chemical forms in the two rice lines enhanced with increasing Cd doses (Table 4). FE was the predominant chemical form in the two rice lines, accounting for 53–55% in Lu527-8 and 44–48% in Lu527-4, respectively, followed by FNaC (26% in Lu527-8 and 27–31% in Lu527-4, respectively) and FHAc (15–18% in Lu527-8 and 20–21% in Lu527-4, respectively). Cd concentrations in the other chemical forms were much lower. In general, Cd concentrations in all chemical forms in the roots of Lu527-8 were greater than those in Lu527-4 under different Cd treatments.
Discussion
Subcellular distribution involved in Cd accumulation and detoxification in roots
As reported, 49–79% of Cd ions are retained in rice roots which are the primary tissues of Cd absorption, thus influencing xylem-mediated Cd transport from root to shoot and eventually Cd accumulation in shoot (Uraguchi et al. 2009; Nocito et al. 2011; Ricachenevsky et al. 2018). In this study, Lu527-8 accumulated more Cd in roots than Lu527-4 with more Cd translocation to shoots (Table S1), indicating that root shows a strong ability of Cd accumulation and translocation in Lu527-8. However, the mechanisms of Cd accumulation and distribution in the roots of Lu527-8 remain unclear.
Once into cells, large amounts of Cd ions are bound to cell walls, with many binding sites that show high affinity to metals, or transferred into soluble fraction (Nocito et al. 2011; Zhao et al. 2015). Cell wall and soluble fraction are the major binding sites for Cd in plants, thus being vital for Cd accumulation and detoxification (Xin et al. 2013; Zhang et al. 2015; Mwamba et al. 2016). In this study, soluble fraction and cell wall were the primary fractions for Cd accumulation in roots of the two rice lines. Cd concentrations in the soluble fraction and cell wall of Lu527-8 were much higher than Lu527-4 (Table 1). It suggests that soluble fraction and cell wall contributed greatly to high Cd accumulation in the roots of Lu527-8. In soluble fraction, there are amounts of phytochelatins, organic acids and amino acids, benefiting Cd detoxification and accumulation (Degola et al. 2014; Osmolovskaya et al. 2018; Zhu et al. 2018). Phytochelatins are rich in thiol moieties which have positive effect on Cd chelation (Degola et al. 2014; Hazama et al. 2015). Similarly, organic acids and amino acids can form strong bond with Cd through their functional groups, such as carboxyl groups (Osmolovskaya et al. 2018; Zhu et al. 2018). Our previous study proved that soluble fraction played an important role in Cd accumulation in the roots of Lu527-8 due to being with more phytochelatins, glutathione and nonprotein thiols than Lu527-4 (Tang, 2016). Organic acids and amino acids in the roots of Lu527-8 were also detected in this study. There were more amino acids, especially glutamic acid, proline, and henylalanine, in Lu527-8 under Cd stress (Table 3). Glutamic acid, cysteine, and glycine are precursors of glutathione and phytochelatins (Nocito et al. 2008). Proline plays an important role in protecting plants from heavy metal stress through scavenging of free radicals, stabilizing protein synthesis, and regulating cytosolic acidity (Choudhary et al. 2007). Meanwhile, the concentrations of tartaric, malate, citrate, and acetic acid were significantly higher in Lu527-8 compared with Lu527-4 (Table 2). All of these were conducive to Cd detoxification and Cd accumulation in the soluble fraction of Lu527-8. Besides, cell wall was the subdominant domain for Cd accumulation in the roots of Lu527-8. It may attribute to its abundant polysaccharides with functional groups, and the underlying mechanism was further explored.
Cell wall polysaccharides involved in cd accumulation in roots
Cell wall is rich in polysaccharides (Gallego et al. 2012; Chen et al. 2013). Pectin and hemicellulose are the major components of cell wall and play an important role in binding Cd (Zhu et al. 2013; Li et al. 2015). There are amounts of negative charge functional groups with highly effective ability of Cd binding in pectin and hemicellulose (Li et al. 2015; Loix et al. 2017). Thus, more specific polysaccharides show greater potentiality to Cd binding. In this study, Cd induced more polysaccharides in cell walls of the two rice lines, and Lu527-8 showed more polysaccharides in cell walls than Lu527-4 (Fig. 1). Thus, there were more Cd bound to cell wall polysaccharides in the roots of Lu527-8 (Fig. 2). Meanwhile, Cd concentrations in pectin and HC1 showed positive linear correlations with Cd concentrations in roots of the two rice lines (Fig. S1), indicating that pectin and HC1 were important for Cd binding in root cell wall of the two rice lines. Particularly, Cd accumulation in pectin accounted for more than 60% of the total Cd in cell wall polysaccharides, suggesting that pectin plays a dominant role in binding Cd in the root cell wall of Lu527-8. Cd stress commonly induced some modifications to pectin in root cell wall (Krzesłowska et al. 2016). Pectin undergoes demethylation with the catalysis by PME, leading to an increase for free carboxyl groups available to bind Cd ions (Li et al. 2015; Chebli and Geitmann, 2017; Gutsch et al. 2019). In this study, Cd stress induced an increase for PME activity in root cell wall of Lu527-8, which shows higher PME activity than Lu527-4 (Fig. 3). Therefore, Lu527-8 presented more Cd accumulation in root cell walls than Lu527-4 when exposed to Cd. It could be demonstrated that abundant polysaccharides, especially pectin, and high PME activity in root cell walls contributed greatly to high Cd accumulation in the roots of Lu527-8.
Cd chemical forms in roots and their role in Cd translocation
Cd is usually present in chemical forms with different bioavailability and mobility in plants (Wang et al. 2015; Li et al. 2016; Zhao et al. 2015). Among Cd chemical forms, water-soluble and ethanol-extractable Cd showed the highest mobility and thus can be easily transported to aboveground tissues, followed by NaCl-extractable, HAC-extractable, and HCl-extractable Cd (Lu et al. 2017). In this study, Cd mainly presented as ethanol-extractable form, followed by NaCl-extractable and HAC-extractable forms in two rice lines (Table 4). About 53–55% of the total Cd was present in ethanol-extractable form in the roots of Lu527-8, which was more than that of Lu527-4. This fraction is some Cd bound to nitrate/nitrite, chloride, and aminophenol, showing high mobility to transport to shoot (Wang et al. 2009; Su et al. 2014; Lai et al. 2015; Xin et al. 2017). NaCl-extractable Cd refers to Cd bound to pectate and protein, playing an important part in Cd tolerance (Lu et al. 2019; Xue et al. 2019). The chemical forms of Cd in the roots of Lu527-8, especially those with high mobility, were markedly more than those in Lu527-4, thus making a great contribution to high Cd translocation to shoots in Lu5278. In addition, Cd in the shoots of Lu527-8, especially in leaves, was mainly in NaCl-extractable and HAc-extractable forms (Table S2). More than 70% of the total Cd were NaCl-extractable and HAc-extractable Cd in leaves of Lu527-8, which were more than those of Lu527-4, showing great benefit to Cd accumulation as well as Cd detoxification in the shoots of Lu527-8. It suggests that most of Cd in the roots of Lu527-8 shows high mobility, resulting in Cd translocation to shoot. Meanwhile, there are amounts of Cd ions with high immobility in the shoots, which is beneficial to high Cd tolerance and accumulation.
Conclusion
The roots of high Cd-accumulating rice line Lu527-8 show a strong ability of Cd accumulation and translocation due to its specific characteristic of Cd subcellular distribution and chemical forms. Cd was mainly distributed in soluble fraction and cell wall in the roots of Lu527-8, benefiting Cd accumulation. In soluble fraction, organic acids and amino acids as well as thiols played important roles in Cd detoxification and accumulation. Meanwhile, there were amounts of polysaccharides, especially pectin, in the roots of Lu527-8. Under the catalysis of PME, pectin posed a strong potential for binding Cd, resulting in alleviating Cd toxicity and promoting Cd accumulation in the root cell wall of Lu527-8. Furthermore, a large proportion of Cd in the roots of Lu527-8 were present in water-soluble and ethanol-extractable form with high mobility, contributing to high Cd translocation to the shoots. Therefore, Lu527-8 accumulates amounts of Cd not only in roots but also in shoots, which is beneficial to excavate relevant genes.
Data availability
The data and materials will be available on request.
Abbreviations
- Cd:
-
cadmium
- PME:
-
pectin methylesterase
- HC1:
-
hemicellulose 1
- HC2:
-
hemicellulose 2
- TF:
-
translocation factor
References
Chebli Y, Geitmann A (2017) Cellular growth in plants requires regulation of cell wall biochemistry. Curr Opin Cell Biol 44:28–35
Chen GC, Liu YQ, Wang RM, Zhang JF, Owens G (2013) Cadmium adsorption by willow root: the role of cell walls and their subfractions. Environ Sci Pollut Res 20:5665–5672
Choudhary M, Jetley UK, Khan MA, Zutshi S, Fatma T (2007) Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol Environ Saf 66:204–209
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18:92–99
Degola F, De Benedictis M, Petraglia A, Massimi A, Fattorini L, Sorbo S, Basile A, di Toppi LS (2014) A Cd/Fe/Zn-responsive phytochelatin synthase is constitutively present in the ancient liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol 55:1884–1891
Feng JJ, Jia WT, Lv SL, Bao HXGDL, Miao FF, Zhang X, Wang JH, Li JH, Li DS, Zhu C, Li SZ, Li YX (2017) Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol J 16:558–571
Fu HJ, Yu HY, Li TX, Wu Y (2019) Effect of cadmium stress on inorganic and organic components in xylem sap of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol Environ Saf 168:330–337
Fu HJ, Yu HY, Li TX, Zhang XZ (2018) Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol Environ Saf 150:168–175
Fu X, Dou C, Chen Y, Chen X, Shi J, Yu M, Xu T (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J Hazard Mater 186:103–107
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unraveling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Guan MY, Zhang HH, Pan W, Jin CW, Lin XY (2018) Sulfide alleviates cadmium toxicity in Arabidopsis plants by altering the chemical form and the subcellular distribution of cadmium. Sci Total Environ 627:663–670
Gutsch A, Sergeant K, Keunen E, Prinsen E, Guerriero G, Renaut J, Hausman JF, Cuypers A (2019) Does long-term cadmium exposure influence the composition of pectic polysaccharides in the cell wall of Medicago sativa stems? BMC Plant Biol 19:271
Hazama K, Nagata S, Fujimori T, Yanagisawa S, Yoneyama T (2015) Concentrations of metals and potential metal-binding compounds and speciation of Cd, Zn and Cu in phloem and xylem saps from castor bean plants (Ricinus communis) treated with four levels of cadmium. Physiol Plant 154:243–255
He SY, He ZL, Yang XE, Stoffella PJ, Baligar VC (2015) Chapter Four - Soil biogeochemistry, plant physiology, and phytoremediation of cadmium-contaminated soils. Adv Agron 134:135–225
He X, Zhang J, Ren Y, Sun C, Deng X, Qian M, Hu Z, Li R, Chen Y, Shen Z, Xia Y (2019) Polyaspartate and liquid amino acid fertilizer are appropriate alternatives for promoting the phytoextraction of cadmium and lead in Solanum nigrum L. Chemosphere 237:124,483
Ibaraki T, Kuroyanagi N, Murakami M (2009) Practical phytoextraction in cadmium-polluted paddy fields using a high cadmium accumulating rice plant cultured by early drainage of irrigation water. Soil Sci Plant Nutr 55:421–427
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33:35–51
Krzesłowska M, Rabęda I, Basińska A, Lewandowski M, Mellerowicz EJ, Napieralska A, Samardakiewicz S, Woźny A (2016) Pectinous cell wall thickenings formation - A common defense strategy of plants to cope with Pb. Environ Pollut 214:354–361
Lai HY (2015) Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to its phytoextraction potential. Chemosphere 138:370–376
Li H, Luo N, Zhang LJ, Zhao HM, Li YW, Cai QY, Wong MH, Mo CH (2016) Do arbuscular mycorrhizal fungi affect cadmium uptake kinetics, subcellular distribution and chemical forms in rice? Sci Total Environ 571:1183–1190
Li T, Tao Q, Shohag MJI, Yang X, Sparks DL, Liang Y (2015) Root cell wall polysaccharides are involved in cadmium hyperaccumulation in Sedum alfredii. Plant Soil 389:387–399
Loix C, Huybrechts M, Vangronsveld J, Gielen M, Keunen E, Cuypers A (2017) Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front Plant Sci 8:1867
Lu HP, Li ZA, Wu JT, Shen Y, Li YW, Zou B, Tang YT, Zhuang P (2017) Influences of calcium silicate on chemical forms and subcellular distribution of cadmium in Amaranthus hypochondriacus L. Sci Rep 7:40,583
Lu Q, Xu Z, Xu X, Liu L, Liang L, Chen Z, Dong X, Li C, Wang Y, Qiu G (2019) Cadmium contamination in a soil-rice system and the associated health risk: an addressing concern caused by barium mining. Ecotoxicol Environ Saf 183:109,590
Mwamba TM, Li L, Gill RA, Islam F, Nawaz A, Ali B, Farooq MA, Lwalaba JL, Zhou W (2016) Differential subcellular distribution and chemical forms of cadmium and copper in Brassica napus. Ecotoxicol Environ Saf 134:239–249
Nocito FF, Lancilli C, Dendena B, Lucchini G, Sacchi GA (2011) Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell Environ 34:994–1008
Nocito FF, Espen L, Crema B, Cocucci M, Sacchi GA (2008) Cadmium induces acidosis in maize root cells. New Phytol 179:700–711
Osmolovskaya N, Dung VV, Kuchaeva L (2018) The role of organic acids in heavy metal tolerance in plants. Bio Comm 63:9–16
Ponnamperuma FN (1977) Screening rice for tolerance to mineral stresses. Int Rice Res Inst (IRRI) 25:67–72
Ricachenevsky FK, de Araújo Junior AT, Fett JP, Sperotto RA (2018) You shall not pass: root vacuoles as a symplastic checkpoint for metal translocation to shoots and possible application to grain nutritional quality. Front Plant Sci 9:412
Sharifi M, Khoshgoftarmanesh AH, Hadadzadeh H (2016) Changes in the chemical properties and swelling coefficient of alfalfa root cell walls in the presence of toluene as a toxic agent. Environ Sci Pollut Res 23:7022–7031
Su CL, Jiang YJ, Li FF, Xu QS (2017) Investigation of subcellular distribution, physiological, and biochemical changes in Spirodela polyrhiza as a function of cadmium exposure. Environ Exp Bot 142:24–33
Su Y, Liu JL, Lu ZW, Wang XM, Zhang Z, Shi GR (2014) Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ Exp Bot 97:40–48
Tang H (2016) Cadmium accumulation and tolerance characteristics in high cadmium accumulating rice. Sichuan agricultural university (In Chinese)
Tang H, Li TX, Yu HY, Zhang XZ (2016) Cadmium accumulation characteristics and removal potentials of high cadmium accumulating rice line grown in cadmium contaminated soils. Environ Sci Pollut Res 23:15,351–15,357
Uraguchi S, Fujiwara T (2013) Rice breaks ground for cadmium-free cereals. Curr Opin Cell Biol 16:328–334
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Wang J, Yuan J, Yang Z, Huang B, Zhou Y, Xin J, Gong Y, Yu H (2009) Variation in cadmium accumulation among 30 cultivars and cadmium subcellular distribution in 2 selected cultivars of water spinach (Ipomoea aquatica Forsk.). J Agric Food Chem 57:8942–8949
Wang JB, Su LY, Yang JZ, Yuan JG, Yin AG, Qiu Q, Zhang K, Yang ZY (2015) Comparisons of cadmium subcellular distribution and chemical forms between low-Cd and high-Cd accumulation genotypes of watercress (Nasturtium officinale L. R. Br.). Plant Soil 396:325–337
Wang QY, Liu JS, Hu B (2016) Integration of copper subcellular distribution and chemical forms to understand copper toxicity in apple trees. Environ Exp Bot 123:125–131
Wang X, Liu YG, Zeng GM, Chai LY, Song XC, Min ZY, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62:389–395
Wu Y, Wang WX (2011) Accumulation, subcellular distribution and toxicity of inorganic mercury and methylmercury in marine phytoplankton. Environ Pollut 159:3097–3105
Xin J, Huang B, Yang Z, Yuan J, Zhang Y (2013) Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) cultivars. Plant Soil 372:431–444
Xin J, Zhao XH, Tan QL, Sun XC, Hu CX (2017) Comparison of cadmium absorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L.). Ecotoxicol Environ Saf 145:258–265
Xiong J, An LY, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xue WJ, Zhang CB, Wang PP, Wang CR, Huang YC, Zhang X, Liu ZQ (2019) Rice vegetative organs alleviate cadmium toxicity by altering the chemical forms of cadmium and increasing the ratio of calcium to manganese. Ecotoxicol Environ Saf 184:109640
Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146:602–611
Yang LP, Zhu J, Wang P, Zeng J, Tan R, Yang YZ, Liu ZM (2018) Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol Environ Saf 160:10–18
Zhang HZ, Guo QJ, Yang JX, Shen JX, Chen TB, Zhu GX, Chen H, Shao CY (2015) Subcellular cadmium distribution and antioxidant enzymatic activities in the leaves of two castor (Ricinus communis L.) cultivars exhibit differences in Cd accumulation. Ecotoxicol Environ Saf 120:184–192
Zhang SJ, Li TX, Huang HG, Zou TJ, Zhang XZ, Yu HY, Zheng ZC, Wang YD (2012) Cd accumulation and phytostabilization potential of dominant plants surrounding mining tailings. Environ Sci Pollut Res 19:3879–3888
Zhao YF, Wu JF, Shang DR, Ning JS, Zhai YX, Sheng XF, Ding HY (2015) Subcellular distribution and chemical forms of cadmium in the edible seaweed, Porphyra yezoensis. Food Chem Toxicol 168:48–54
Zhou JT, Wan HX, He JL, Lyu D, Li HF (2017) Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front Plant Sci 8:1–16
Zhu G, Xiao H, Guo Q, Zhang Z, Zhao J, Yang D (2018) Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol Environ Saf 158:300–308
Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ (2012) Cell wall polysaccharides are involved in P-deficiency induced Cd exclusion in Arabidopsis thaliana. Planta 236:989–997
Zhu XF, Wang ZW, Dong F, Lei GJ, Shi YZ, Li GX, Zheng SJ (2013) Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J Hazard Mater 263:398–403
Funding
This work was supported by Major Science and Technology Project of Sichuan Province (2018SZDZX0029), National Key Research and Development Program of China (2018YFC1802605), and National Natural Science Foundation of China (41807147).
Author information
Authors and Affiliations
Contributions
Haiying Yu has contributed to conceptualization and writing (reviewing and editing). Keji Wang has contributed to formal analysis, visualization, and writing (original draft). Huagang Huang has contributed to methodology and software. Xizhou Zhang has contributed to supervision and validation. Tingxuan Li has contributed to supervision, resources, and writing (reviewing and editing). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A.Supplementary information
ESM 1
(DOCX 80 kb)
Rights and permissions
About this article
Cite this article
Yu, H., Wang, K., Huang, H. et al. The regulatory role of root in cadmium accumulation in a high cadmium-accumulating rice line (Oryza sativa L.). Environ Sci Pollut Res 28, 25432–25441 (2021). https://doi.org/10.1007/s11356-021-12373-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12373-3