Abstract
The present study explores the antioxidant, anti-microbial, and hepatoprotective potentials of flavonoid-rich fractions from Fumaria officinalis against permethrin-induced liver damage ex vivo/in vivo in rat. However, HPLC-DAD analysis revealed the richness of 6 components in ethyl acetate fraction (EAF) where ferulic acid, rosmarinic acid, and myricetin are the most abundant. The in vitro assays showed that EAFs have impressive antioxidant and anti-microbial properties. Ex vivo, permethrin (PER) (100 μM) induced a decrease of hepatic AST and ALT activities and 25-OH vitamin D and vitamin C levels and an increase of ALP and LDH activities, TBARS, and ϒ-GT levels with a disturbance of oxidative status. The hepatoprotective effect of EAF (1 mg/mL) against PER was confirmed by the amelioration of oxidative stress profile. In vivo, permethrin was found to increase absolute and relative liver weights, plasma transaminase activities, lactate-to-pyruvate ratio, hepatic and mitochondrial lipid peroxidation, and protein oxidation levels. This pesticide triggered a decrease of Ca2+ and Mg2+-ATPases and mitochondrial enzyme activities. The co-treatment with EAF reestablished the hepatic and mitochondrial function, which could be attributed to its richness in phenolic compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver is a vital organ in the processes of drugs and xenobiotic metabolism and detoxification (Sahreen et al. 2011). Sometimes, toxic substances are converted during these processes to active metabolites that can cause hepatic damage (Cullen 2005). The risk of liver injury has significantly been associated with the metabolic dysfunctions that can result in various disorders ranging from an ephemeral elevation of liver enzymes to life-threatening hepatocellular carcinoma (HCC). HCC is one of the most frequent tumors representing the fifth commonest malignancy worldwide and the third cause of mortality from cancer accounting for > 60% (Yan et al. 2009; Yang et al. 2010). Biological research has specifically revealed that a high-amount oxidative stress is a biochemical condition that occurs and produces various types of reactive species such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Adams et al. 2015). The high amount of oxidative stress is cytotoxic to the biological cell and inhibits proliferation by affecting apoptosis and/or necrosis, while a low concentration is stimulatory to the cell division in the promotion stage and initiates the promotion of tumor growth (Dreher and Junod 1996).
During the progression of carcinogenesis, tumor cells stimulate in various interactions with the tumor microenvironment involving the components of extracellular matrices (ECMs) dramatically influenced by the behavior of stromal cells, including endothelial cells, immune cells, and fibroblasts (Nikitovic et al. 2013; Cirri and Chiarugi 2012). This tumor initiation can be affected by a DNA damage event perpetrated by ROS production (Loft and Poulsen 1996). ROS is one of the pathological mechanisms that contribute to liver damage, including a variety of mechanisms such as the inactivation of antioxidant enzymes, depletion of reduced glutathione, and alteration of the breakdown of fat molecules as well as lipid peroxidation (Dubey et al. 2013). Pesticides have been proven to be one of the chief reasons behind the increase in ROS (Lushchak et al. 2018). For a population to be exposed to a large range of pesticides is a serious global problem that can cause many health hazards as well as environmental pollution. The last European Union monitoring program has revealed that 28.3% of the analyzed samples has pesticides, with 7.5% of them in baby food samples, exceeding the maximum residue levels (Docea et al. 2018). This exposure has been proven by many researchers to be linked to several difficulties, namely electrocardiogram abnormalities, functional remodeling, histopathological threat to many organs such as liver (vacuole formation, hemorrhage, indicators of apoptosis and degeneration), and biochemical variations (Georgiadis et al. 2018; Fountoucidou et al. 2019). Moreover, the most involved mechanism is the inhibition of cytochrome c oxidase in cells mitochondria, leading to the reduction of the ATP production and induction of oxidative stress (Asghari et al. 2017). Permethrin (PER) is a commonly used insecticide in diverse forms. Indeed, it is used not only to control pests in residential areas, namely the textile industry and agricultural settings, but also to treat head lice and scabies in humans and fleas in pets, for public health vector control and for disinfecting commercial aircrafts thanks to its effective insecticide activity (Wang et al. 2016; Rosita et al. 2015). This insecticide might have a variety of toxic effects on animals and humans such as hepatotoxicity (Gabbianelli et al. 2013). Different methodologies have proven that antioxidant enzymes, such as redox biomarkers, are time-, dose-, tissue-, and gender-sensitive and may be considered a good indicator of the toxic effects induced by permethrin (Wang et al. 2016). Aiming to decrease the oxidative damage, antioxidant consumption is deemed essential (Goutzourelas et al. 2015). Actually, to prevent chronic diseases (atherosclerotic-based cardio-vascular disease, hypertension, type 2 diabetes mellitus, malignancies, hepatic disorders, cancer, etc.), the search for novel functional ingredients from natural products has become among the challenges in food science and technology (Costa et al. 2017; Margină et al. 2015; Mantle et al. 2000; Medina-Holguín et al. 2008). These natural products have been proven to be an important source of phenolic acids, polyphenols, monoterpenes, flavonoids, sesquiterpenes, and phenylpropanoids. These compounds can be used incorporated into the cosmetic, pharmaceutical, and food industry owing to their potential biological properties, such as anti-microbial, anti-inflammatory, antioxidant, anti-hepatotoxicity, anti-lipoperoxidant, anti-tumoral, anti-allergic, and antimutagenic effects (Veskoukis et al. 2020; Doshi et al. 2015; Jung et al. 2017; El Arem et al. 2014; Sibanda et al. 2004).
The chief mechanism involved is accredited either to the direct chelation of toxic substance or through the redox signaling pathways and the expression of genes coding for antioxidant enzymes (Veskoukis et al. 2012; Margaritis et al. 2020). Specifically, flavonoid-rich plant has currently affected the cancer cell blockage in vitro and other diseases via several mechanisms such as anti-inflammatory, anti-angiogenetic, and anti-metastatic activity and in vivo by the inhibition of metastatization in transgenic adenocarcinoma (Cipolla et al. 2015). These effects could be ascribed to the richness of plant in bioactive molecules, namely catechin, apigenin, quercetin, naringenin, rutin, and venoruto (Tapas et al. 2008). Fumaria officinalis was a medicinal plant that can be used to treat hepatic diseases (Uday et al. 2012). However, to the best of our knowledge, no research work has been dedicated to the extraction of flavonoids from Fumaria officinalis.
The extraction method is the important step to isolate essential bioactive components derived from plant. Maceration is established as a useful and traditional method of the complete extraction of flavonoids isolated in plant (Ćujić et al. 2016).
In this study, we determined the antioxidant and anti-microbial properties as well as the hepatoprotective effects of flavonoid-rich fractions extracted from Fumaria officinalis against permethrin-induced liver damage ex vivo and in vivo studies in rat.
Materials and methods
Chemicals and plant materials
The aerial part of Fumaria officinalis (stem and leaf) was purchased from Parachimic Laboratory (Tunisia) (ref. HEMF001003) for flavonoid extraction. Permethrin was purchased from Protagri Company, Sfax, Tunisia. All purchased chemicals and solvents from Sigma Chemical Co. (St. Louis, MO, USA) were of analytical grade.
Extraction and fractionation process of total flavonoids
The total flavonoids were extracted from Fumaria officinalis using maceration method according to Ćujić et al. (2016). In fact, the powder was homogenized with ethanol-water ratio of 25 mL g−1 and 60% ethanol concentration for 75 min. The combination and filtering of the obtained homogenate were realized, and the solvent was removed by means of rotary evaporation at 50 °C. The total flavonoids were fractionated successively with petroleum ether to eliminate the lipophilic compounds, ethyl acetate, and n-butanol. Each partitioned fraction’s solution was concentrated using a rotary evaporation at 50 °C.
Determination of total flavonoid contents
The total flavonoid contents were determined according to Zhishen et al. (1999) with some modification. The sample was mixed with 1 mL of distilled water adding 75 μL of 5% NaNO2. The mixture was allowed to react for 5 min then 75 μL of 10% ALCL3 solution was added and mixed well. After 6 min, 500 μL of 1 M NaOH was combined and the total was made to 2.5 mL with distilled water. The absorbance was measured at 510 nm using a spectrophotometer and the results were expressed as milligrams of quercetin equivalents (QEs) per gram of extract (mg QE/g).
Determination of total polyphenol contents
The total polyphenol contents were determined according to Stintzing et al. (2005). The quantity of 1 mL of Folin Ciocalteu reagent diluted 10 times was mixed with 200 μL of sample for 3 min. The amount of 0.8 mL 7.5% Na2CO3 was added in a test tube and the contents were mixed and allowed to stand for 30 min. Absorption at 760 nm was read in a Shimadzu 300 UV-vis spectrometer (Shimadzu UV-1601, Norcross, GA) against blank sample. The results were expressed in milligrams equivalent of gallic acid (GAE).
HPLC-DAD analysis
The phenolic compounds of F. officinalis ethanolic extract were identified by HPLC system equipped with a reversed phase C18 analytical column of 4.6 × 100 mm and 3.5-μm particle size (Zorbax Eclipse XDB C18). The DAD detector was set to a scanning range of 200–400 nm. The column temperature was incubated at 25 °C. The injected sample volume was 2 μL and the flow rate of mobile phase was 0.4 mL/min. Mobile phase B was milli-Q water consisting of 0.1% formic acid, and mobile phase A was methanol. The optimized gradient elution was illustrated as follows: 0–5 min, 10–20% A; 5–10 min, 20–30% A; 10–15 min, 30–50% A; 15–20 min, 50–70% A; 20–25 min, 70–90% A; 25–30 min, 90–50% A; 30–35 min, return to initial conditions. For the quantitative analysis, a calibration curve was obtained by plotting the peak area against different concentrations for each identified compound at 280 nm.
Fourier transform infrared spectroscopy spectra analysis
The Fourier transform infrared spectroscopy (FT-IR) analysis of the flavonoid-rich fractions was investigated at room temperature on a Universal ATR Sampling Accessory infrared spectrophotometer. The FT-IR analysis was carried out on the liquid extracts and scanned with a scan range from 550 to 4000 cm−1 at a resolution of 4 cm−1.
Antioxidant activities
Radical scavenging activity on DPPH determination
This assay was determined according to Hou et al. (2002). The amount of 1 mL of various sample concentrations (0 to 1 mg/mL) was added in 1 mL of 0.025 g/mL DPPH for 30 min at room temperature. Then, the absorbance was measured at 517 nm against blank. Vitamin C was used as a positive control at the same condition as before.
Radical scavenging activity on ABTS determination
The ABTS radical scavenging activity was analyzed by the method reported by Ozgen et al. (2006). When combined with a potassium persulfate (2.45 mM), ABTS (7 mM in 20 mM sodium acetate buffer, pH 4.5) was reacted to give a stable radical at blue-green solution following incubation in the dark at 4 °C for 12–16 h. Next, the solution was diluted to an absorbance of 0.7 ± 0.01 at 734 nm. This reaction containing 20 μL of sample and 3 mL of reagent was placed in a water bath at 30 °C for 30 min. As unpaired electrons were sequestrated by antioxidants in the sample, the reaction turns colorless and the absorbance was reduced at 734 nm.
Ferric reducing power determination
The ferric reducing power (FRAP) activity was carried out according to Oyaizu (1986). Various concentrations of the sample (0 to 1 mg/mL) were mixed with 2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 mL 1% potassium ferric cyanide. This mixture was placed at 50 °C for 20 min. After the incubation period, 2.5 mL 10% TCA was added following the vortex. Afterwards, this content was centrifugated at 3000 rpm for 10 min to obtain the supernatant that was used in this mixture with 2.5 mL of distilled water and 500 μL of 0.1% FeCL3. The absorbance was recorded at 700 nm against the blank.
Screening of anti-microbial activity
Microbial strains
Flavonoid-rich fractions of F. officinalis was assessed against a panel of microorganisms including two bacterial strains: Escherichia coli (ATCC 8739) and Bacillus amyloliquefaciens (FZB 425), one yeast strain Candida albicans (ATCC 90028), and one fungal strain such as Fusarium oxysporum. All the tested strains were provided by the Microbiology Department, Faculty of Science (Sfax, Tunisia). Bacterial strains were cultured for 24 h at 37 °C in Muller-Hinton agar (MHA) and the yeast and fungal strains were cultured in Sabouraud Chloramphenicol agar (SCA) for 48 h at 30 °C.
Disk diffusion method
For the purpose of the anti-microbial activity, the disk diffusion method was used as descripted by Nilsson (1978). Briefly, 150 μL of each bacterial suspension (106 colony-forming units (CFU mL−1)) was spread on the surface of MHA solid media plates. Sterilized filter paper disks (5 mm in diameter) were impregnated with 10 μL of each extract (1 mg mL−1) dissolved in the used solvent and placed on the inoculated plates. The antibacterial activity was estimated by measuring the diameters of inhibition zone and comparing it to ampicillin and amphotericin (10 μg per disk) as positive control against bacteria, yeast, and fungi. After being stored at 4 °C for 2 h, the plates were placed at 37 °C for 24 h for bacteria and at 30 °C for 48 h for yeast and fungi.
Animals
Male albino Wistar rats (body weight 180 ± 6.069 g) were obtained from the Society of Pharmaceutical Industries of Tunisia (SIPHAT, Ben Arous, Tunisia). The animals were housed under controlled conditions (12:12 h light-dark cycle, room temperature set at 25 ± 2 °C with a relative humidity of 50–60%). The animals had free access to commercial food and water ad libitum. The animal experiments were conducted according to the Ethical Committee Guidelines for the care and use of Research and Animal Experimentation of the Faculty of Science, Sfax University, Tunisia (ethics approval number: 1204).
Design of the ex vivo experiment
Preparation and incubation of liver
Liver tissues were quickly removed, minced, cut into small pieces, and placed in 10 volumes of Krebs Ringer Bicarbonate Buffer (KRBJ) (10 Mm d-glucose, 0.49 mM MgCl2, 119.78 mM NaCl, 4.56 mM KCl, 0.7 mM Na2HPO4, 1.3 mM NaH2PO4, and 14.99 mM NaHCO3, pH 7.3) following dry form 15 min at 37 °C. Liver suspensions (10% W/V) were randomly divided into eight groups.
-
Control group (C): Liver was incubated with KRBJ buffer.
-
Permethrin group (PER): Liver was incubated with 100 μM permethrin dissolved in KRBJ buffer (El-Shenawy 2010).
-
Ethyl acetate fraction group (EAF): Liver was incubated with 1 mg/mL ethyl acetate fraction in KRBJ buffer (Lata and Mittal 2017).
-
N-butanol fraction group (NBF): Liver was incubated with 1 mg/mL n-butanol fraction dissolved in KRBJ buffer (Lata and Mittal 2017).
-
Water fraction group (WF): Liver was incubated with 1 mg/mL water fraction dissolved in KRBJ buffer (Lata and Mittal 2017).
-
PER+EAF group: Liver was incubated with permethrin associated with ethyl acetate fraction (1 mg/mL) (Lata and Mittal 2017).
-
PER+NBF group: Liver was incubated with permethrin associated with n-butanol fraction group (1 mg/mL) (Lata and Mittal 2017).
-
PER+WF group: Liver was incubated with permethrin associated with water fraction (1 mg/mL) (Lata and Mittal 2017).
After 60 and 90 min of incubation at 37 °C, all samples were currently placed in ice-cold water and liver cells were used for experimental analysis.
Biochemical evaluation
Aspartate aminotransferase (7D81-21,304410/R1), alanine aminotransaminase (7D56, 304663/R02), alkaline phosphatase (7D55,304752/R02), lactate dehydrogenase (2p56-21), 25-OH vitamin D (B3L522), and ϒ-GT analyses were assessed by spectrophotometer method using the commercial diagnostics kits according to the manufacturer’s recommendations (Abbott Park, IL, USA) Architect/Aeroset).
Protein estimation in the liver
Protein content was measured using Folin’s reagent and bovine serum albumin as reference following the method described by Lowry et al. (1951).
Thiobarbituric acid reactive substance measurement
Thiobarbituric acid reactive substance (TBARS) levels were assayed calorimetrically at 532 nm using the method of Esterbauer and Cheeseman (1990). The results are expressed as nanomoles/milligram of protein.
Advanced oxidation protein product measurement
Advanced oxidation protein product (AOPP) levels were evaluated spectrophotometrically at 340 nm according to the method of Witko et al. method (1992). The obtained results are expressed as micromoles/milligram of protein.
Vitamin C levels measurement
Vitamin C levels in the liver and mitochondria were determined spectrophotometrically by means of the method described by Jagota and Dani (1982). The obtained results are expressed as nanograms/milligram of protein.
Antioxidant enzyme activities in rat liver
Superoxide dismutase (SOD) activity was assayed spectrophotometrically at 580 nm according to the method of Asada et al. method (1974). SOD activity is expressed as units/milligram of protein.
Catalase (CAT) activity was determined calorimetrically at 240 nm using Aebi’s method (1984). CAT activity is expressed as micromoles of H2O2 consumed/milligram of protein.
Glutathione peroxidase (GPx) activity was determined using the method described by Flohé and Günzler (1984). The obtained results are expressed as micromoles of reduced GSH/milligram of protein.
GSH level estimation in the liver
GSH level was assayed spectrophotometrically at 412 nm using the method described by Ellman (1959) modified by Jollow et al. (1974). Data are expressed as micromoles/milligram of protein.
Design of the in vivo experiment
The protocol of treatment and the particular dose of permethrin were used according to that of Cantalamessa (1993). Considering that “the No Observed Adverse Effect Level” (NOAEL) for PER is 25 mg/kg/day, we administrated a dose of 34.05 mg/kg/day corresponding to 1/50 of LD50. This dose was conducted following an acute reference dose (RfD) of 0.25 mg/kg/day in humans for both acute and chronic dietary exposures to permethrin (U.S. EPA 2009). Rats were equally divided into four groups containing six rats as follows:
-
Group C: served as the control negative group, rats received every day corn oil by gavage at a dose of 2 mL/kg BW.
-
Group EAF: served as control positive group, rats received every day by gavage ethyl acetate fraction extract from Fumaria officinalis at a dose of 200 mg/kg BW and corn oil at a dose of 2 mL/kg BW (Verma 2011).
-
Group PER: rats received every day a dose of 34.05 mg/kg BW of permethrin by gavage (corresponding to1/50 of DL50) dissolved in corn oil.
-
Group PER+EAF: rats received every day permethrin by gavage at the same dose of that of group PER and administrated after ethyl acetate fraction extract at a similar dose to that of EAF group.
All animals of all groups received pellet diet and drinking water ad libitum. Body and food intake were monitored daily for 7 days. At the end of the experiment, rats were sacrificed by cervical decapitation to avoid any stress.
Blood samples were collected and allowed to coagulate at room temperature then centrifuged at 3000g for 15 min. The clear non-hemolyzed supernatant was quickly removed and kept at − 20 °C for biochemical analysis. Liver tissues were quickly ground into a small piece of freshly excised tissue in 9% NaCl. The amount of 1 g of hepatic tissue was used for mitochondrion isolation, 1 g was homogenized using an Ultra Turrax homogenizer (T25, Germany) with Tris-buffered saline solution (TBS, pH 7.4), and the same liver slices were used for histological examinations.
Liver mitochondria was isolated from the differential centrifugation procedure as described by Hoppel et al. (1979). Briefly, homogenate was obtained by grinding a small piece using a Potter-Elvehjem homogenized with a loose-fitting Teflon pestle. Nuclei and cell debris were removed by centrifugation at 600×g (10 min, 4 °C). Mitochondria were obtained by the centrifugation of supernatant at 7000g (10 min, 4 °C). The pellet was washed with 2 mL of isolation buffer (100 mM saccharose, 50 mM KCl, 50 mM Tris, and 5 mM EDTA, pH 7.4) and centrifugated (3500×g, 10 min, 40 °C). Then, the obtained mitochondria were stored at − 20 °C until biochemical analysis.
Biochemical evaluation
Aspartate aminotransferase (AST) and alanine aminotransaminase (ALT) activities and C-reactive protein (CRP), gamma-GT, lactate, and pyruvate levels were measured using commercial kits according to the manufacturer’s directions (Abbott Park, IL, USA) (Architect/Aeroset). Hepatic and mitochondrial protein contents were determined as described by Lowry et al. (1951); lipid peroxidation was analyzed by measuring thiobarbituric acid reactive substances (TBARSs) according to Esterbauer and Cheeseman (1990); AOPP level was determined using the spectrophotometrical method of Witko et al. (1992); protein carbonyl (PCO) levels were measured spectrophotometrically at 370 nm as described by Resnik and Packer (1994); non-protein-bound (NPSH) group was measured at 540 nm using the method described by Sedlak and Lindsay (1968); nitrite oxide (NO) levels in the liver and mitochondria were measured spectrophotometrically using the method of Green et al. (1982), and ascorbic acid (vitamin C) level determination was measured according to Jagota and Dani (1982).
Liver homogenates were also used for the estimation of superoxide dismutase (SOD) activity (Asada et al. 1974), catalase (CAT) activity (Aebi 1984), glutathione-peroxide (GPx) activity (Flohé and Günzler 1984), and glutathione (GSH) level (Ellman 1959).
Mitochondrial enzyme activity determination
NADH ubiquinone reductase (complex I) activity was analyzed spectrophotometrically at 37 °C in hepatic mitochondria by monitoring the oxidation of NADH at 340 nm using the method of Malgat et al. (1999). Ubiquinol-cytochrome c reductase (complex III) activity was determined spectrophotometrically at 37 °C by reducing cytochrome c at 550 nm as described by Malgat et al. (1999). Cytochrome c oxidase (complex IV) activity was analyzed by monitoring the reduction of cytochrome c in 550 nm in hepatic mitochondria according to the method of Malgat et al. (1999).
Ca2+ and Mg2+-ATPase activity determination
Hepatic and mitochondrial Ca2+ and Mg2+-ATPase activities were evaluated spectrophotometrically using the method described by Ohinishi et al. (1982) and Hjerten and Pan (1983).
Histological examination
Paraffin portions of liver tissues were cut into small sections of 5 μm, then stained with hematoxylin-eosin solution (H&E) and examined under light microscopy at × 40 magnifications.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). The obtained results were analyzed statistically by ANOVA method using multiple comparisons between groups. Their significant levels were tested at p < 0.05.
Results
Amounts of total flavonoids and polyphenols in flavonoid-rich fractions
The total flavonoid content ranged from 30.80 ± 4.51 to 71.80 ± 9.23 mg QE/g DW (Table 1). The total amounts of flavonoids of each solvent fraction showed a different trend which decreased in the following order: NBF > EAF > WF. As can be seen, the WF concentrates most polyphenol compounds compared to other fractions.
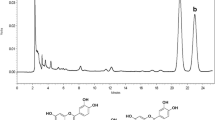
HPLC-DAD analysis of flavonoid-rich fractions
Figure 1 and Table 2 show the HPLC identification of the major phenolic compounds of flavonoid-rich fractions from F. officinalis. A total of 6 monomeric phenolics of EAF were identified, including 2 flavonols (quercetin and kaempferol), 2 flavanones (naringenin and myricetin), and 2 hydroxycinnamic (rosmarinic acid and ferulic acid). NBF contained 2 flavonols catechin and amentoflavone. Two flavonols (quercetin and catechin) and 1 hydroxycinnamic (caffeic acid) were identified in WF.
HPLC-DAD chromatographic profiles acquired at 280 nm representing the polyphenols in flavonoid-rich fractions from F. officinlais : (a) standard, (b) ethyl acetate fraction; (1) quercetin, (2) kaempferol, (3) ferulic acid, (4) rosmarinic acid, (5) naringenin, and (6) myricetin; (c) n-butanol fraction; (1) catechin and (2) amentoflavone; and (d) water fraction; (1) quercetin, (2) catechin, and (3) caffeic acid. The identification was performed according to a home-generateddatabase containing 10 phenolics compounds
FT-IR spectral data analysis of flavonoid-rich fractions
FT-IR spectral analysis for different flavonoid-rich fractions from F. officinalis revealed the existence of multiple functional groups. Spectral features and absorption bands identified in each fraction of the different fractions are shown in Table 3.
Antioxidant activities of flavonoid-rich fractions
The antioxidant activities of flavonoid-rich fractions from Fumaria officinalis were evaluated by measuring their scavenging capacity against DPPH and ABTS free radical and FRAP power (Fig. 2). The DPPH, ABTS, and FRAP power activities revealed that EAF showed the highest antioxidant activity compared to those of NBF and WF.
Antioxidants activity of flavonoid-rich fractions from F. officinalis: (a) DPPH radical scavenging activity, (b) ABTS radical scavenging activity, and (c) ferric reducing power (FRAP). Data are presented as mean ± SD of 3 determinations. EAF—ethyl acetate fraction. NBF—n-butanol fraction. WF—water fraction
Anti-microbial activity of flavonoid-rich fractions
The anti-microbial activities of flavonoid-rich fractions were performed using agar-well diffusion assay (Table 4). In the seeds, EAF and NBF were more effective against the gram-negative strain E. coli with an inhibition zone diameter of 12.00 ± 0.00 and 14.00 ± 0.00 mm, respectively, while the WF was not active. Similarly, the antibacterial activity against gram-positive bacteria B. amyloliquefaciens revealed that all fractions were not active, when compared with antibiotics standards. For the antifungal activity, EAF and NBF showed an interesting activity by a whole inhibition of 14.00 ± 0.10 and 17.00 ± 0.10 mm for F. oxysporum, respectively, while the WF revealed moderate activity (9.00 ± 0.100 mm). Furthermore, C. albicans seem to be the most sensitive microorganisms for WF that EAF and NBF by 20.00 ± 1.40, 15.00 ± 1.40, and 14.00 ± 1.40 mm, respectively, when compared with antibiotics standards.
Hepatoprotective activity ex vivo
Protective effects of flavonoid-rich fractions on permethrin-induced hepatic injury
AST, ALT, ALP, and LDH activities after 60 and 90 min in the liver incubated with permethrin are displayed in Table 5. As can be seen, permethrin induced a significant elevation of ALP and LDH activities associated with a decrease of AST and ALT activities (by − 42% and − 48% at 60 min and − 59% and − 52% at 90 min, respectively) when compared with the control group (C). The co-incubation of liver with F. officinalis flavonoid-rich fractions linked to insecticide restored significantly these activities. Our results showed that EAF had the best hepatoprotective effect.
Table 6 shows that TBARS levels in the liver were increased in the PER group in comparison to (C) group. There was a statistically significant decrease of TBARS rates in the PER+EAF, PER+NBF, and PER+WF groups when compared to the PER group.
As can be seen from Table 6, liver incubated with PER during 60 and 90 min revealed an increase of AOPP level. In the PER+EAF, PER+NBF, and PER+WF groups, we observed a significant decrease of AOPP rates by 11.03%, 9.81%, and 9.07% at 60 min, respectively, when compared to the PER group.
It is trustworthy to note that in the liver, vitamin C levels were obviously worn out in the PER group by − 15% and − 17.42% after 60 and 90 min of incubation, respectively, when compared with group C (Table 6). In the PER+EAF, PER+NBF, and PER+WF groups, an increase of vitamin C levels was observed, while in the PER+ EAF group, a positive effect was similar to that of the control group. The 25-OH vitamin D levels in the liver incubated with PER were considerably lesser than those of the control group (Table 6). In the PER+EAF, PER+NBF, and PER+WF groups, we observed a substantial increase of 25-OH vitamin D levels after 60 and 90 min of incubation was noticed, whereas the PER+EAF group had a powerful effect in comparison with the PER group. ϒ-GT concentrations in the liver incubated with PER were significantly more elevated than those of the control group (Table 6). Thus, ϒ-GT levels were reestablished in the liver incubated with the three flavonoid-rich fractions, while the EAF led to a great change in these levels when compared to group C.
SOD, CAT, and GPx activities were measured as indicators of antioxidant status of tissues (Table 7). A significant increase of hepatic CAT activity (by 71% and 84% at 60 and 90 min, respectively) was observed and associated with lower hepatic SOD and GPx activities (by − 39% and − 50% at 60 min and − 39% and − 36% at 90 min) in the PER group as compared to the control. Flavonoid-rich fractions reduced the effects of permethrin, while EAF reestablished the normal value.
Liver incubated with permethrin alone resulted in an elevation of hepatic GSH level (by 34% at 60 min and 69% at 90 min) as compared to the control group (Table 7). The co-incubation of liver with all fractions caused a momentous restoration of GSH level.
Hepatoprotective activity in vivo
Protective effects of EAF on permethrin-induced hepatic and mitochondrial injury
Food intake and body, absolute, and relative liver weight changes
In the permethrin group, while food consumption and body weight were decreased, absolute and relative liver weights were statistically increased when compared to the control group (Table 8). The co-treatment of the PER group with EAF improved significantly these parameters without reaching the control values.
Biochemical evaluation
The effects of PER and EAF and their combination on some biochemical parameters in rats are exhibited in Table 9. Our findings revealed that permethrin caused a significant decrease in hepatic AST and ALT activities and CRP content with a significant rise in gamma-GT activity when compared to group C. Plasma transaminase activities, CRP, and lactate levels and lactate-to-pyruvate ratio were statistically increased, but pyruvate content was significantly decreased in permethrin-treated rats as compared to the control group (Table 9).
Hepatic and mitochondrial TBARS, PCO, AOPP, and NO levels were significantly increased, while NPSH and vitamin C rates were decreased in the permethrin group as compared to those of the control groups (Table 10). The co-administration of EAF in permethrin-treated rats restored these parameters.
Mitochondrial enzymes and Ca2+ and Mg2+-ATPase activity evaluation
NADH-ubiquinone reductase (complex I), ubiquinol-cytochrome c reductase (complex III), cytochrome c oxidase (complex IV), and Ca2+ and Mg2+-ATPase activities were significantly reduced in permethrin-treated rats (Figs. 3 and 4). The administration of EAF in permethrin-treated rats improved these mitochondrial enzymes and ATPase activities.
Antioxidant enzyme activities in the liver and mitochondria
After permethrin exposure, a significant increase in hepatic and mitochondrial CAT and GPx activities when compared to normal values was found by our results (Table 11). SOD activity was increased in the liver and decreased in mitochondria (Table 11). The administration of EAF in permethrin-treated rats restored these antioxidant enzyme activities.
Non-enzymatic antioxidant levels in the liver and mitochondria
Hepatic and mitochondrial GSH contents were significantly increased in the PER group (by 26.43% and 34.90%, respectively) when compared to those of the control (Table 11). The co-treatment of permethrin-treated rats with EAF improved the level of GSH.
Histopathological changes in the liver
The observed histopathological changes of livers are illustrated in Fig. 5. The control (C) and EAF groups have a normal structure, including normal central vein, hepatocyte cords, and hepatocytes (Fig. 5 (C) and (EAF)). The permethrin (PER) group exhibited an abnormal architecture with a severe vacuole formation, chromatin condensation, degenerations of hepatocytes, and dilatation of sinusoids (Fig. 5 (PER)). The hepatic histopathological damages induced by permethrin were ameliorated by EAF treatment (Fig. 5 (PER+EAF)). Notably, the histopathological observation is consistent with hepatic enzyme evaluation, revealing that the EAF helps in normalizing the tissue marker levels and protecting from the hepato-toxicities induced by permethrin.
Discussion
Increasing evidence has indicated that cellular damage mediated by oxidative stress contributes to the initiation and progression of several human pathologies. Much effort has been made to develop the complementary and alternative medicines in order to limit oxidative stress and restore liver functions (Jiang et al. 2016). Therapeutic agents from natural sources such as flavonoids, as polyphenolic compound, are particularly attractive for treatments with their antioxidant, anti-allergic, anti-inflammatory, anti-microbial, and anti-cancer as well as hepatoprotective activities (Zhi-Qiang et al. 2018; Farhadi et al. 2018). However, from the available literature, insufficient data about pharmacological properties of flavonoids isolated from F. officinalis was found. That is why we investigated its protective effects using ex vivo/in vivo hepatotoxicity model, based on permethrin-induced liver injury. The present in vitro study has demonstrated that all flavonoid-rich fractions have elevated radical scavenging against DPPH, ABTS+, and effective ferric reducing power. Hence, phenolic compounds may play a significant role in its antioxidant properties (Shukla et al. 2009). Similar results were found by Stagos et al. (2018) and Jiang et al. (2016) who have reported that the polyphenol-enriched extract has increased radical scavenging against DPPH, ABTS+, and O2− and powerful reducing power. Numerous research works have proven a direct relationship not only between phenolic compounds and antioxidant activity but also between antioxidant and hepatoprotective activity (Gonçalves et al. 2013; Kalantari et al. 2017). This impact may be explicated by the existence of quercetin that is presented as a very effective agent in hydrogen donors (Terashima et al. 2012). As indicated by Mikropoulou et al. (2018), this feature could be accredited to the absence of hydroxyl groups in its chemical structure that affects its antioxidant property.
Insecticides, like PER, belong to dangerous substances that are highly reactive and toxic. The mechanism of PER might be involved in the liver damage caused by free radicals, which alters membrane structure (Zalba and Hagen 2017; Yang et al. 2018). When weight loss is accompanied with the liver swelling, the cell membrane is damaged and the homeostasis is destroyed, as confirmed in permethrin group. LDH and aminotransferases (AST and ALT) are the first enzymes to be used in the diagnostics of hepatocyte injury and their existence outside the hepatic cells is considered reference in the alterations of cell membrane permeability (Michael et al. 2015; Zentella and Munoz 2016). In fact, our results reported a significant decrease of hepatic AST and ALT activities and an increase of plasma aminotransferases as well as LDH and ALP activities in the liver after permethrin intoxication. However, the co-treatment with EAF extract improved these variations. The improvement in membrane integrity strongly demonstrated the ability of EAF extract to protect the hepatocyte membrane and reduce the leakage of these enzymes into the circulation. This effect could be attributed to the presence of flavonoids and phenolic acids in the EAE extract, especially rosmarinic acid and quercetin, which have a strong antioxidant activity by scavenging free radicals and/or interacting with other antioxidants (Shehab et al. 2015; Parisa and Rosa 2017; Lucarini et al. 2014; Abou Zeid et al. 2017).
The main cellular components susceptible to ROS damage are lipids, proteins, carbohydrates, and nucleic acids. These damages cause structural and functional disturbances of cells (Meo et al. 2016). Our ex vivo and in vivo data revealed an increase in hepatic and mitochondria TBARS, AOPP, PCO, NO, and plasma CRP levels and a decrease in NPSH and CRP rates in the liver of PER groups. Similar results have been reported by Pan et al. (2017), El-Demerdash (2011), and Rosita et al. (2015) in vivo, ex vivo, and in vitro studies. The co-treatment with EAF extract significantly improved PER’s oxidative damages. This could be explained by the richness of this extract in quercetin, ferulic acid, rosmarinic acid, and naringenin (Kalantari et al. 2017; Uzun and Kalender 2013; Marimuthu et al. 2013; Mushtaq et al. 2015; Ramalho et al. 2014). Il is also well known that lipophilic compounds are more active than hydrophilic compounds in in vitro lipid peroxidation assay (Pizzalle et al. 2002; Miura et al. 2002). Yang et al. (2013) have demonstrated that the co-treatment with rosmarinic acid (RA) in HepG2 cells reduced the lipid peroxidation affected by t-BHP. Thus, two hypotheses could explain this property: (i) the molecules of RA in the mass phase could stop the propagation of the free radical before reaching the lipids and/or (ii) the molecules of RA associated with the lipid membrane could block the spread of free radicals within the lipid bilayer (Fadel et al. 2011).
ϒ-GT (gamma-glutamyl transpeptidases) is an enzyme associated with detoxification mediated by GSH and in the cell defense mechanism against oxidative stress (Castellano et al. 2010). In fact, the increase of ϒ-GT activities emanates from the altered levels of GSSG/GSH and overproduction of ROS (Long et al. 2017). In our ex vivo and in vivo studies, the hepatic ϒ-GT levels were significantly increased in the permethrin group when compared with normal values, which is in accordance with the results of El-Shenawy (2010). Actually, the latter have reported that the incubation of hepatocytes with fenitrothion or endosulfan or abamectin significantly increased the gamma-glutamyl transpeptidase levels. The EAF extract seems to protect the structural integrity of hepatic membrane as observed from the significant decrease of ϒ-GT levels in comparison to permethrin values. Indeed, quercetin, which is one of EAF extract compounds, has an important structural role in stabilizing membranes (Oluwatoyin et al. 2018). According to Akinmoladun et al. (2018), the quercetin effect can be attributed to its apparent antioxidant and hepatoprotective properties, which were mainly due to the presence of the 4-Keto group and/or C2-C3 double bonding. These chemical structures have been indicated as the main respect for the bioactivities of flavonoids (Akinmoladun et al. 2018).
The second line of defense consists of the non-enzymes’ scavengers, namely ascorbic acid and 25-OH vitamin D, which scavenge eventual free radicals, generating from decomposition by the antioxidant enzymes. The 25-OH vitamin D and vitamin C levels might play a substantial role in the pathogenesis of liver diseases (Zúñiga et al. 2011). According to Wimalawansa (2019), deficiency in 25-OH vitamin D has been implicated in liver injury, as steatosis, necroinflammation, and fibrosis. Our study demonstrated that these parameters were significantly reduced in the liver and mitochondria after permethrin treatment compared to controls. The significant elevation of 25-OH vitamin D and vitamin C levels was observed in the EAF+PER group. The EAF effect might be explained by the richness of the extract in ferulic acid. Furthermore, Srinivasan et al. (2006) have reported that ferulic acid have electron groups on the benzene ring [3-methoxy or 4-hydroxyl] able to terminate free radical chain reaction.
The cellular antioxidant system comprises integral non-enzymatic antioxidant like GSH. Besides, different enzymatic antioxidants, such as SOD, CAT, and GPx, make up the first line of defense against oxidative damage. In the present ex vivo and in vivo studies, permethrin exposure reduced significantly hepatic and mitochondrial SOD and GPx activities and increased the hepatic and mitochondrial CAT activity and GSH content. In fact, according to Mershiba et al. (2013), the decrease of hepatic and mitochondrial SOD activity may be due to the interaction of permethrin with the metal ions or with the active amino acids and/or due to the accumulation of the mostly reactive free radicals, inducing the deleterious effects as the least of membrane integrity and function. The decrease of GPx activity induced by permethrin may be attributed to a direct inhibitory oxidative effect on the enzyme. It has also been shown that pesticides cause a reduction in GPx activity both in vivo, in vitro, and ex vivo (El-Shenawy 2010; Altuntas et al. 2003; Verma and Srivastava 2003). In addition, the increase of CAT activity could be explained as an adaptive response to equilibrate the oxidative status affected by PER (Pan et al. 2017). The time-dependent increase of the GSH content may be explained by the primary protective response of liver against that of permethrin time-dependent induced oxidative stress. Our analysis showed that co-treatment with EAF extract improved the SOD, CAT, and GPx activities and the GSH content. The most hepatoprotective effect of EAF extract was probably related to its potent antioxidant property that can be explained by the presence of substance bioactive such as naringenin. In fact, according to Sahu et al. 2020, naringenin could restore the antioxidant enzyme activities by scavenging free radical with its hydroxyl group. Taken together, the exposure to polyphenols could induce SOD and CAT biosynthesis at mRNA and protein levels (Oliveira et al. 2012; Ying et al. 2004).
With respect to Mohajeri et al. (2017), they have proven that pesticides are able to induce oxidative stress by (1) generating free radicals as byproducts of detoxifying metabolism, (2) redox cycling properties, (3) or affecting the mitochondrial respiration. The latter could be investigated by the lactate-to-pyruvate ratio as a marker for retarded mitochondria function (Pinieux et al. 1996). Our results showed a significantly higher lactate-to-pyruvate ratio in permethrin-treated rats, which resulted in the reduction of ATP production and eventually caused mitochondrial dysfunction. According to Feldman et al. 2016, the highest lactate and lactate-to-pyruvate ratio is caused by altered fluid balance or hypoxia. As reported by Langeswaran et al. (2012), the free radical overproduction compromises the capacity to meet the energy demands of cells by decreasing the mitochondrial TCA cycle enzyme levels. However, the co-treatment with EAF extract into the permethrin-treated rats improved this TCA cycle by reducing lactate levels. The EAF extract effect could be due to its antioxidant and free radical scavenges’ properties coupled with its richness of kaempferol. The latter proves its hepatoprotective effect through modulating the levels of TCA cycle enzymes, which accelerates the clearance of lactate and returned mitochondria status (Langeswaran et al. 2012).
Based on our findings as mentioned above, permethrin was found to be instrumental in increasing the ROS level, which motivates us to estimate the changes of mitochondrial respiratory chain. It has been revealed that mitochondrial activity interference is associated with effects on complex enzymes such as complex I (NADH-ubiquinone reductase), complex III (ubiquino-cytochrome c reductase), and complex IV (cytochrome c oxidase), which result in the increase of mitochondrial electron leakage. The current study revealed that permethrin caused a significant reduction of Ca2+ and Mg2+-ATPases and complex I, III, and IV activities compared to control values. The diminution of complex I activity observed in our results led to the reduction of oxygen consumption, resulting in the rise of ROS generation, which caused an alteration of the mitochondrial oxidative status (Fato et al. 2009; Thummasorn et al. 2018). The co-administration of EAF extract to the permethrin-treated rats restored these activities. This could be due to its richness in quercetin, which was capable of trapping and neutralizing the effect of the H2O2 and hydroxyl radicals (Chen et al. 2005; Birinci et al. 2020). According to Pozo et al. (2012), quercetin is the most efficient in protecting against mitochondrial dysfunction. In fact, thanks to its lipophilic nature and structural similarity with rotenone, this compound is able to enter in cells and accumulate in mitochondria to bind to the ubiquinone site of complex I, thus ensuring its protection against inhibitors (Pozo et al. 2012).
Permethrin induces a progressive increase of cell death by the apoptosis and imbalance of antioxidant status as demonstrated by the induction of lipid peroxidation, protein oxidation, and oxidative status changes (Gabbianelli et al. 2013; Pan et al. 2017). Our histopathological examination showed that PER caused nuclear morphological and ultrastructure changes, including chromatin condensation and vacuole formation, which agrees well with the findings of Roma et al. (2012) and Latrasse et al. (2016). The authors have reported that permethrin causes severe alterations in hepatic cells, hence reducing the size of nuclei and causing the hydropic degeneration of the hepatocytes. Besides, the co-treatment with EAF extract alleviated the liver cell damage. The hepatoprotective effect of EAF extract might be due to its richness of quercetin, which has anti-lipo-peroxidative, antioxidant, and metal scavenger properties (Renugadevi and Prabu 2011; Birinci et al. 2020).
Conclusion
In conclusion, EAF extract has important antioxidant compounds, which reduce cellular oxidative stress caused by permethrin administration ex vivo and in vivo. Our findings suggest that EAF extract could be protecting the hepatic and mitochondrial cells. These findings suggested that EAF extract might be considered novel treatment in clinical studies.
Data availability
All the data is contained in the article.
Abbreviations
- 25-OH vitamin D:
-
25 hydroxy-vitamin D
- ABTS:
-
2,2′-azinobis (3-ethylbenzothiazoline)-6-sulfonique
- ALP:
-
alkaline phosphatase
- ALT:
-
alanine aminotransferase
- AOPP:
-
advanced oxidation protein products
- AST:
-
aspartate aminotransferase
- ATP:
-
adenosine triphosphate
- CAT:
-
catalase
- CRP:
-
C-reactive protein
- DNA:
-
deoxyribonucleic acid
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyle
- DW:
-
dry weight
- EAF:
-
ethyl acetate fraction
- FRAP:
-
ferric reducing power
- FT-IR:
-
Fourier transform infrared spectroscopy
- GAE:
-
gallic acid
- GPx:
-
glutathione peroxidase
- GSH:
-
glutathione
- HPLC-DAD:
-
high-performance liquid chromatography with diode array detection
- LDH:
-
lactate dehydrogenase
- NADH:
-
nicotinamide adenosine dinucleotide
- NBF:
-
N-butanol fraction
- NO:
-
nitrite oxide
- NPSH:
-
non-protein bound
- PCO:
-
protein carbonyl
- PER:
-
permethrin
- QE:
-
quercetin
- RNS:
-
reactive nitrogen species
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- TBARS:
-
thiobarbituric acid reactive substances
- TCA:
-
tricarboxylic acid
- Vit C:
-
vitamin C
- WF:
-
water fraction
- ϒ-GT:
-
gamma-glutamyl transpeptidase
References
Abou Zeid AH, Farag MA, Hamed MAA, Abdel Aziz KZ, El-Akad RH, El-Rafie HM (2017) Flavonoid chemical composition and antidiabetic potential of Brachychiton acerifolius leaves extract. Asian Pac J Trop Biomed 7:389–396
Adams L, Franco MC, Estevez AG (2015) Reactive nitrogen species in cellular signaling. Exp Biol Med 240:711–717
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Akinmoladun AC, Oladejo CO, Josiah SS, Famusiwa CD, Ojo OB, Olaleye MT (2018) Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: relevance for therapy in pesticide-induced liver toxicity. Pathophysiology 25:365–371
Altuntas I, Delibas N, Doguc DK, Ozmen S, Gultekin F (2003) Role of reactive oxygen species in organophosphate insecticide phosalone toxicity in erythrocytes in vitro. Toxicol in Vitro 17:153–157
Asada K, Takahashi MA, Nagate M (1974) Agricultural and biological chemistry assay and inhibitors of spinach superoxide dismutase. Agric Biol Chem 38:471–473
Asghari MH, Abdollahi M, de Oliveira MR, Nabavi SM (2017) A review of the protective role of melatonin during phosphine-induced cardiotoxicity: focus on mitochondrial dysfunction, oxidative stress and apoptosis. J Pharm Pharmacol 69:236–243
Ay CO, Ozcan AS, Erdogan Y, Ozcan A (2012) Characterization of Punica granatum L. peels and quantitatively determination of its biosorption behavior towards lead (II) ions and Acid Blue 40. Colloids Surf B: Biointerfaces 100:197–204
Barraza-Garza G, Castillo-Michel H, de la Rosa LA, Martinez-Martinez A, Pérez-León JA, Cotte M, Alvarez-Parrilla E (2016) Infrared spectroscopy as a tool to study the antioxidant activity of polyphenolic compounds in isolated rat enterocytes. Oxidative Med Cell Longev 2016:1–10
Birinci Y, Niazi JH, Aktay-çetin O, Basaga H (2020) Quercetin in the form of a nano-antioxidant (QTiO2) provides stabilization of quercetin and maximizes its antioxidant capacity in the mouse fibrobast model. Enzym Microb Technol 138:1–33
Cantalamessa F (1993) Acute toxicity of two pyrethroids, permethrin and cypermethrin in neonatal and adult rats. Arch Toxicol 67:510–513
Castellano I, Merlino A, Rossi M, La Cara F (2010) Biochemical and structural properties of gamma-glutamyl transpeptidase from Geobacillus thermodenitrificans: an enzyme specialized in hydrolase activity. Biochimie 92:464–474
Chen YR, Chen CL, Zhang L, Green-Church KB, Zweier JL (2005) Superoxide generation from mitochondrial NADH dehydrogenase induces self-inactivation with specific protein radical formation. J Biol Chem 280:37339–37348
Cipolla BG, Mandron E, Lefort JM, Coadou Y, Negra ED, Corbel L, Scodan RL, Azzouzi AR, Mottet N (2015) Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila) 8:712–719
Cirri P, Chiarugi P (2012) Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 31:195–208
Costa C, Tsatsakis A, Mamoulakis C, Teodoro M, Briguglio G, Caruso E, Tsoukalas D, Margina D, Dardiotis E, Kouretas D, Fenga C (2017) Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem Toxicol 110:286–299
Ćujić N, Šavikin K, Janković T, Pljevljakušić D, Zdunić IS (2016) Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem 194:135–142
Cullen JM (2005) Mechanistic classification of liver injury. Toxicol Pathol 33:6–8
Docea AO, Gofita E, Goumenou M, Calina D, Rogoveanu O, Varut M, Olaru C, Kerasioti E, Fountoucidou P, Taitzoglou I, Zlatian O, Rakitskii VN, Hernandez AF, Kouretas D, Tsatsakis A (2018) Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem Toxicol 115:470–481
Doshi P, Adsule P, Banerjee K, Oulkar D (2015) Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L) byproducts. J Food Sci Technol 52:181–190
Dreher D, Junod AF (1996) Role of oxygen free radicals in cancer development. Eur J Cancer 32:30–38
Dubey N, Khan AM, Raina R (2013) Sub-acute deltamethirn and fluoride toxicity induced hepatic oxidative stress and biochemical alterations in rats. Bull Environ Contam Toxicol 91:334–338
El Arem A, Ghrairi F, Lahouar L, Thouri A, Saafi EB, Ayed A, Zekri M, Ferjani H, Haouas Z, Zakhama A, Achour L (2014) Hepatoprotective activity of date fruit extracts against dichloroacetic acid-induced liver damage in rats. J Funct Foods 9:119–130
El-Demerdash FM (2011) Oxidative stress and hepatotoxicity induced by synthetic pyrethroids-organophosphate insecticides mixture in rat. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 29:145–158
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
El-Shenawy NS (2010) Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicol in Vitro 24:1148–1157
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Fadel O, El Kirat K, Morandat S (2011) The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochim Biophys Acta Biomembr 12:2973–2980
Farhadi F, Khameneh B, Iranshahi M, Iranshahy M (2018) Antibacterial activity of flavonoids and their structure-activity relationship: an update review. Phytother Res 33:13–40
Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, Ohnishi T, Lenaz G (2009) Differential effects of mitochondrial complex I inhibitors on production of reactive oxygen species. Biochem Biophys Acta 1787:384–392
Feldman AG, Sokol RJ, Hardison RM, Alonso EM, Squires RH, Narkewicz MR (2016) Lactate and lactate: pyruvate ratio in the diagnosis and outcomes of pediatric acute liver failure. J Pediatr 182:217–222
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120
Fountoucidou P, Veskoukis AS, Kerasioti E, Docea AO, Taitzoglou IA, Liesivuori J, Tsatsakis A, Kouretas D (2019) A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: the time and dose issue. Toxicol Lett 317:24–44
Gabbianelli R, Palan M, Flis DJ, Fedeli D, Nasuti C, Skarydova L, Ziolkowski W (2013) Imbalance in redox system of rat liver following permethrin treatment in adolescence and neonatal age. Xenobiotica 43:1103–1110
Georgiadis N, Tsarouhas K, Tsitsimpikou C, Vardavas A, Rezaee R, Germanakis I, Tsatsakis A, Stagos D, Kouretas D (2018) Pesticides and cardiotoxicity. Where do we stand? Toxicol Appl Pharmacol 353:1–14
Gonçalves S, Gomes D, Costa P, Romano A (2013) The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Ind Crop Prod 72:82–87
Goutzourelas N, Stagos D, Spanidis Y, Liosi M, Apotolou A, Priftis A, Hroutoulan S, Spandidos DA, Tsatsakis AM, Kouretas D (2015) Polyphenolic composition of grape stem extravts affects antioxidant activity in endothelial and muscle cells. Mol Med Rep 12:5856–5856
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaun SR (1982) Analysis of nitrate, nitrite and (15 N) nitrate in biological fluids. Anal Biochem 126:131–138
Hjerten S, Pan H (1983) Purification and characterization of two form of low affinity calcium ion ATPase from erythrocyte membrane. Biochim Biophys Acta 728:281–288
Hoppel C, DiMarco JP, Tandler B (1979) Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J Biol Chem 254:4164–4170
Hou WC, Hsu FL, Lee MH (2002) Yam (Dioscorea batatas) tuber mucilage exhibited antioxidant activities in vitro. Planta Med 68:1072–1076
Jagota SK, Dani HM (1982) A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127:178–182
Jiang W, Guo MH, Hai X (2016) Heapatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J Gastroenterol 22:10180–10188
Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzeneoxide as the hepatotoxic intermediate. Pharmacology 11:151–169
Jung WK, Tae BK, Hyun WK, SANG WP, Hong PK, Sang HS (2017) Hepatoprotective flavonoids in Opuntia ficus-indica fruits by reducing oxidative stress in primary rat hepatocytes. Pharmacogn Mag 13:472–476
Kalantari H, Foruozandeh H, Khodayar MJ, Siahpoosh A, Saki N, Kheradmand P (2017) Antioxidant and hepatoprotective effects of Capparis spinosa L. fractions and quercetin on tert-butyl hydroperoxide-induced acute liver damage in mice. J Tradit Complement Med 8:120–127
Koperska M, Łojewski T, Łojewska J (2011) Vibrational spectroscopy to study degradation of natural dyes. Assessment of oxygen-free cassette for safe exposition of artefacts. Anal Bioanal Chem 399 (9):3271–3283
Langeswaran K, Revathy R, Kumar SG, Vijayaprakash S, Balasubramanian MP (2012) Kaempferol ameliorates aflatoxin B1 (AFB1) induced hepatocellular carcinoma through modifying metabolizing enzymes, membrane bound ATPases and mitochondrial TCA cycle enzymes. Asian Pac J Trop Biomed 2:1653–1659
Lata S, Mittal SK (2017) In vitro and in vivo hepatoprotective activity of flavonoids rich extracts on cucumis dipsaceus Ehrenb. Int J Pharmacol 13:563–572
Latrasse D, Benhamed M, Bergounioux C, Raynaud C, Delarue M (2016) Plant programmed cell death from a chromatin point of view. J Exp Bot 67:5887–5900
Loft S, Poulsen HE (1996) Cancer risk and oxidative DNA damagein man. J Mol Med 74:297–312
Long Y, Jia D, Wei L, Yang Y, Tian H, Chen T (2017) Liver-specific overexpression of gamma-glutamyltransferase ameliorates insulin sensitivity of male C57BL/6 mice. J Diabetes Res 2017:1–9
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lucarini R, Bernardes WA, Tozatti MG, Filho AA, da S, Silva MLA, Momo C, Crotti AEM, Martins CHG, Cunha WR (2014) Hepatoprotective effect of Rosmarinus officinalis and rosmarinic acid on acetaminophen-induced liver damage. Emirates J Food Agric 26:878–884
Lushchak VI, Matviishyyn TM, Husak V, Storey JM, Storey KB (2018) Pesticide toxicity: a mechanistic approach. EXCLI J 17:1101–1136
Malgat M, Durrieu G, Mazat JP (1999) Enzymatic and polarographic measurements of the respiratory chain complexes. In: Lestienne P (ed) Mitochondrial diseases. Springer Verlag, Paris, pp 357–377
Mantle D, Eddeb F, Pickering AT (2000) Comparison of relative antioxidant activities of British medicinal plant species in vitro. J Ethnopharmacol 72:47–51
Margaritis I, Angelopoulou K, Lavrentiadou S, Mavrovouniotis IC, Tsantarliotou M, Taitzoglou I, Theodoridis A, Veskoukis A, Kerasioti E, Kouretas D, Zervos I (2020) Effect of crocin on antioxidant gene expression, fibrinolytic parameters, redox status and blood biochemistry in nicotinamide-streptozotocin-induced diabetic rats. J Biol Res (Thessalon) 2:27–40
Margină D, Ilie M, Grădinaru D, Androutsopoulos VP, Kouretas D, Tsatsakis AM (2015) Natural products-friends or foes? Toxicol Lett 236:154–167
Marimuthu S, Adluri RS, Rajagopalan R, Menon VP (2013) Protective role of ferulic acid on carbon tetrachloride-induced hyperlipidemia and histological alterations in experimental rats. J Basic Clin Physiol Pharmacol 24:59–66
Medina-Holguín AL, Omar Holguín F, Micheletto S, Goehle S, Simon JA, O’Connell MA (2008) Chemotypic variation of essential oils in the medicinal plant, Anemopsis californica. Phytochemistry 69:919–927
Meo SD, Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS sources in physilogical and pathological conditions. Oxidative Med Cell Longev 2016:1–44
Mershiba SD, Dassprakash MV, Saraswathy SD (2013) Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep 40:3681–3691
Michael M, Tamara V, Bruno C, Sara CY, Joost W, Vera R, Mathieu V (2015) Measurements of apoptotic and necrotic cell death in primary hepatocyte cultures. Methods Mol Biol 1250:349–361
Mikropoulou EV, Vougogiannopoulou K, Kalpoutzakis E, Sklirou AD, Skaperda Z, Houriet J, Wolfender JL, Trougakos IP, Kouretas D, Halabalaki M, Mitakou S (2018) Phytochemical composition of the decoctions of Greek edible greens (Chórta) and evaluation of antioxidant and cytotoxic properties. Molecules 27:1541
Miura K, Kikuzaki H, Nakatani N (2002) Antioxidant activity of chemical components from sage (SalVia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J Agric Food Chem 50:1845–1851
Mohajeri SK, Ahmadipour A, Rahimi HR, Abdollahi M (2017) Adverse effects of organophosphorus pesticides on the liver: a brief summary of four decades of research. Arch Hig Rada Toksikol 68:261–275
Mushtaq N, Schmatz R, Ahmed M, Pereira LB, Costa PD, Reichert KP, Dalenogare D, Pelinson LP, Juliana MV, Stefanello N, Oliveira LS, Mulinacci N, Bellumori M, Morsch VM, Schetinger MR (2015) Protective effect of rosmarinic acid against oxidative stress biomarkers in liver and kidney of strepotozotocin-induced diabetic rats. J Physiol Biochem 71:743–751
Nikitovic D, Corsisni E, Kouretas D, Tsatsakis A, Tzanakakis G (2013) ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem Toxicol 61:178–186
Nilsson L (1978) New rapid bioassay of gentamicin based on luciferase assay of extracellular ATP in bacterial cultures. Antimicrob Agents Chemother 14:812–816
Ohinishi T, Suzuki T, Suzuki KY, Ozawa K (1982) A comparative study of plasma membrane Mg2+- ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta 684:67–74
Oliveira SAM, Vidal-Novoa A, Batista-Gonzales AE, Pinto JR, Portari Mancini DA, Reina-Urquijo W, Marccini-Filho J (2012) In vivo and in vitro antioxidant activity and hepatoprotective properties of polyphenols from Halimeda opuntia (Linnaeus). Redox Rep 17:47–53
Oluwatoyin OD, Akinwunmi OA, John O, Olufunso OO (2018) Inhibition of liver mitochondrial membrane permeability transition pore opening by quercetin and vitamin E in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun 504:460–469
Oyaizu M (1986) Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine, Japanese. J Nutr Diet 44:307–315
Ozgen M, Reese RN, Tulio AZ, Scheerens JC, Miller AR (2006) Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1- picrylhydrazyl (DPPH) methods. J Agric Food Chem 54:1151–1157
Pan W, Mig-Yuan X, Liang Y-J, Hui-Ping W, Ying-Jian S, Ding-Xin L, Wu Y-J (2017) Subchronic toxicity of low dose propoxur, permethrin, and their combination on the redox status of rat liver. Chem Biol Interact 272:21–27
Parisa H, Rosa S (2017) Beneficial effects od rosmanrinic acid against alcohol-induced hepatotoxicity in rats. Can J Physiol Pharmacol 96:32–37
Pinieux GD, Chariot P, Ammi-Said M, Louarn F, Lejonc JL, Astier A, Jacotot B, Cherardi R (1996) Lipid-lowering drugs and mitochondrial function: effects of HMG-CoA reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br J Clin Pharmacol 42:333–337
Pizzalle L, Bortolomeazzi R, Vichi S, Uberegger E, Conte LS (2002) Antioxidant activity of sage (SalVia officinalis and S. fruticosa) and oregano (Origanum onites and O intercedens) extracts related their phenolic compound content. J Sci Food Agric 82:1645–1651
Pozo CC, Mizgier ML, Speisky H, Gotteland M (2012) Differential protetive effects of quercetin, resveratrol, rutin and espigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem Biol Interact 195:199–205
Ramalho LNZ, Pasta AAC, Terra VA, Augusto MJ, Sanches SC, Neto FPS, Cecchini R, Gulin F, Ramalho FS (2014) Rosmarinic acid attenuates hepatic ischemia and reperfusion injury in rats. Food Chem Toxicol 74:270–278
Renugadevi J, Prabu SM (2011) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol 62:471–481
Resnik AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Rodrigeuz A, Valladares Cisneros M, Gonzalez Rodriguez J (2015) Use of Salvia officinalis as green corrosion inhibitor for carbon steel in acidic media. Int J Electrochemical Sci 10:4053–4067
Roma CG, Oliveira PR, Bechara GH, Mathias MIC (2012) Cytotoxic effects of permethrin on mouse liver and spleen cells. Microsc Res Tech 75:229–238
Rosita G, Manuel C, Franco M, Cinzia N, Donatella F, Emiliano L, Luca M, Roberta G (2015) Permethrin and its metabolites affect Cu/Zn superoxide conformation: fluorescence and in silico evidences. Mol BioSyst 11:208–217
Sahayaraj PA, Gowri J, Dharmalingama V, Shobanaa R, Prema AA (2015) Phytochemical screening by FTIR spectroscopic analysis of leaf and stem Extracts of wedelia biflora. Int J Nano Corr Sci and Engg 2:322–334
Sahreen S, Khan MR, Khan RA (2011) Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. Altern Med 24:11–48
Sahu N, Mishra G, Ghandra HK, Nirala SK, Bhadauria M (2020) Naringenin mitigates antituberculosis drugs induced hepatic and renal injury in rats. J Tradit Complement Med 10:26–35
Schulz H, Ozkan G, Baranska M, Kruger H, Ozcan M (2005) Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib Spectrosc 39:249–256
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Shehab NG, Abu-Gharbieh E, Bayoumi FA (2015) Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complement Altern Med 9:401
Shukla S, Mehta A, Bajpai VK, Shukla S (2009) In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chem 47:2338–2348
Sibanda S, Chigwada G, Poole M, Gwebu ET, Noletto JA, Schmidt JM, Rea AI, Setzer WN (2004) Composition and bioactivity of the leaf essential oil of Heteropyxis dehniae from Zimbabwe. J Ethnopharmacol 92:107–110
Silva JFM, da Souza MC, de Matta SR, Andrade MR (2006) Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities. Food Chemistry 99:431–435
Sinanoglou VJ, Zoumpoulakis P, Fotakis C, Kalogeropoulos N, Sakellari A, Karavoltsosn S, Strati IF (2018) On the characterization and correlation of compositional, antioxidant and colour profile of common and balsamic vinegars. Antioxidants 7:139
Srinivasan M, Ram Sudheer A, Raveendran Pillai K, Raghu Kumar P, Sudhakaran PR, Menon VP (2006) Influence of ferulic acid on -radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology 228:249–258
Stagos D, Balabanos D, Savva S, Skaperda Z, Priftis A, Kerasioti E, Mikropoulou EV, Vougogiannopoulou K, Mitakou S, Halabalaki M, Kouretas D (2018) Extracts from the Mediterranean food plants Carthamus lanatus, Cichorium intybus, and Cichorium spinosum enhanced GSH levels and increased Nrf2 expression in human endothelial cells. Oxidative Med Cell Longev 2018:1–14
Stintzing FC, Herbach KM, Mosshammer MR, Carle R, Yi W, Sellappan S, Akoh CC, Bunch R, Felker P (2005) Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J Agric Food Chem 53:442–451
Tapas A, Sakarkar D, Kakde R (2008) Flavonoids as Nutraceuticals: a review. Trop J Pharm Res 7:1089–1099
Terashima M, Kakuno Y, Kitano N, Matsuoka C (2012) Antioxidant activity of flavonoids evaluated with myoglobin method. Plant Cell Rep 31:291–298
Thummasorn S, Shinlapawittayatorn K, Khamseekaew J, Jaiwongkam T, Chattipakorn SC, Chattipakorn N (2018) Humanin directly protects cardiac mitochondria against dysfunction initiated by oxidative stress by decreasing complex I activity. Mitochondrion 38:31–40
U.S. EPA (United States Environmental Protection Agency) (2009) Reregistration eligibility decision (RED) for permethrin. Prevention, Pesticides and Toxic Substances, Washington
Uday RS, Prakash T, Surendra V, Roopakarki, Rama Rao N, Divakar G (2012) Hepatoprotective activity of Fumaria officinalis against CCL4-induced liver dammage in rats. Pharmacologia 3:9–14
Uzun FG, Kalender Y (2013) Chlorpyrifos induced hepatotoxic and hematologic changes in rats: the role of quercetin and catechin. Food Chem Toxicol 55:549–596
Vardin H, Tay A, Ozen B, Mauer L (2008) Authentication of pomegranate juice concentrate using FTIR spectroscopy and chemometrics. Food Chem 108:742–748
Venkateswarlu S, Kumar BN, Prathima B, SubbaRao Y, Jyothi NVV (2019) A novel green synthesis of Fe3O4 magnetic nanorods using Punica Granatum rind extract and its application for removal of Pb(II) from aqueous environment. Arab J Chem 12:588–596
Verma N (2011) Protective effect of ethyl acetat fraction of Rhododendron arboreum flowers agianst carbon tetrachloride-induced hepatotoxicity in experimental models. Indian J Pharmacol 43:291–295
Verma RS, Srivastava N (2003) Effect of chlorpyrifos on thiobarbituric acid reactive substances, scavenging enzymes and glutathione in rat tissues. Indian J Biochem Biophys 40:423–428
Veskoukis AS, Tsatsakis AM, Kouretas D (2012) Dietary oxidative stress and antioxidant defence with an emphasis on plant extract administration. Cell Stress Chaperones 17:11–21
Veskoukis AS, Vassi E, Poulas K, Kokkinakis M, Asprodini E, Haroutounian S, Kouretas D (2020) Grape stem extracts from three native Greek vine varieties exhibit strong antioxidant and antimutagenic properties. Anticancer Res 40:2025–2032
Wang X, Martinez MA, Dai M, Chen D, Ares I, Romero A, Castellano V, Martinez M, Rodriguez JL, Martinez-Larranaga MR, Anadon A, Yuan Z (2016) Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ Res 149:86–104
Wimalawansa SJ (2019) Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology Basel 8:30
Witko V, Nguyen AT, Descamps-Latscha B (1992) Microtiter plate assay for phagocyte-derived Taurine-chloramines. J Clin Lab Anal 6:47–53
Yan F, Zhang QY, Jiao L, Han T, Zhang H, Qin LP, Khalid R (2009) Synergistic hepatoprotective effect of Schisandrae lignans with Astragalus polysaccharides on chronic liver injury in rats. Phytomedicin 16:805–813
Yang J, Li Y, Wang F, Wu C (2010) Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J Agric Food Chem 58:6525–6531
Yang SY, Hong CO, Lee GP, Kim CT, Lee KW (2013) The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem Toxicol 55:92–99
Yang JS, Symington S, Clark JM, Park Y (2018) Permethrin, a pyrethroid insecticide, regulates ERK1/2 activation through membrane depolarization-mediated pathway in HepG2 hepatocytes. Food Chem Toxicol 121:387–395
Ying CJ, Sun XF, Zhang SL, Zhang XP, Mao LM, Zuo XZ, Yao P (2004) ROS-related enzyme expressions in endothelial cells regulated by tea polyphenols. Biomed Environ Sci 17:33–39
Zalba S, ten Hagen TLM (2017) Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat Rev 52:48–57
Zentella MLC, Munoz RH (2016) Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxidative Med Cell Longev 2016:1–12
Zhi-Qiang H, Pan C, Wei-Wei S, Yong-Gang W, Hao W, Wei P, Pein-BO L (2018) Antioxidant activity and hepatoprotective potential of quercetin 7-rhamnoside in vitro and in vivo. Molecules 23(5):1188
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zúñiga S, Firrincieli D, Housset C, Chignard N (2011) Vitamin D and the vitamin D receptor in liver pathophysiology. Clinics and Research in Hepatology and Gastroenterology 35(4):295–302
Acknowledgments
The authors acknowledge the “Ministry of higher Education, Scientific Research and Technology, Tunisia,” for the support of this work.
Funding
The project was funded by the Department of biology in faculty of sciences of Sfax (Tunisia).
Author information
Authors and Affiliations
Contributions
N. Aoiadni: investigation, methodology, formal analysis, writing-review and editing. H. Ayadi: methodology. H. Jdidi: methodology. M. Naifer: methodology. S. Maalej: resources. F.A. Makni: resources. A. El Feki: resources. H. Fetoui: conceptualization, writing-review and editing. F.G. Koubaa: project administration, resources, conceptualization, supervision, writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aoiadni, N., Ayadi, H., Jdidi, H. et al. Flavonoid-rich fraction attenuates permethrin-induced toxicity by modulating ROS-mediated hepatic oxidative stress and mitochondrial dysfunction ex vivo and in vivo in rat. Environ Sci Pollut Res 28, 9290–9312 (2021). https://doi.org/10.1007/s11356-020-11250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11250-9









 Cytoplasmic vacuolization.
Cytoplasmic vacuolization.  Pyknotic nuclei
Pyknotic nuclei