Abstract
Apocynum venetum L., belonging to the family Apocynaceae, is a popular medicinal plant, which is commonly used in the treatment of hypertension, neurasthenia, and hepatitis in China. In the present study, the total flavonoids (TFs) were prepared from the leaves of A. venetum, and its protective effects on carbon tetrachloride (CCl4)-induced hepatotoxicity in a cultured HepG2 cell line and in mice were investigated. Cell exposed to 0.4% CCl4 (v/v) for 6 h led to a significant decrease in cell viability, increased LDH leakage, and intracellular reactive oxygen species (ROS). CCl4 also induced cell marked apoptosis, which was accompanied by the loss of mitochondrial membrane potential (MMP). Pretreatment with TFs at concentrations of 25, 50, and 100 μg/mL effectively relieved CCl4-induced cellular damage in a dose-dependent manner. In vivo, TFs (100, 200, and 400 mg/kg BW) were administered via gavage daily for 14 days before CCl4 treatment. The high serum ALT and AST levels induced by CCl4 were dose-dependently suppressed by pretreatment of TFs (200 and 400 mg/kg BW). Histological analysis also supported the results obtained from serum assays. Furthermore, TFs could prevent CCl4-caused oxidative damage by decreasing the MDA formation and increasing antioxidant enzymes (CAT, SOD, GSH-Px) activities in liver tissues. In summary, both in vitro and in vivo data suggest that TFs, prepared from A. venetum, showed a remarkable hepatoprotective and antioxidant activity against CCl4-induced liver damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Being a major organ for detoxification and metabolism of xenobiotic, liver is susceptible to various forms of injury, which may then result in different liver disorders [10]. Up to now, the currently available drugs used for treatment of hepatic injury are limited and some of them have serious side effects [23, 30]. So, new therapeutic approaches with reliable activity and lower toxicity are expected. In the last decade, comprehensive research efforts have demonstrated the natural products from medicinal plants should be the promising resources for treatment of liver injury [12].
Apocynum venetum L., belonging to the family Apocynaceae, is a popular medicinal plant in China and was traditionally used for the treatment of hypertension, neurasthenia, and hepatitis for hundreds of years [33]. Recently, the extract of A. venetum has been studied pharmacologically for its antioxidant [21, 37], antihypertensive [19], antianxiety [15], antidepressant [4], and hepatoprotective actions [34, 36]. Phytochemical investigations have revealed that flavonoids are the major bioactive constituents in A. venetum [1, 33]. Flavonoids are natural polyphenolic compounds found in a wide variety of plants and exhibit various benefits to human health. A broad spectrum of biological effects has been attributed to flavonoids [14]. In particular, the protective efficacy of flavonoids against various drug- or toxin-induced liver injury has been well documented [8, 9, 27].
In the present study, the total flavonoids (TFs) from A. venetum were prepared and its hepatoprotective effects against CCl4-induced liver injury were evaluated in vitro and in vivo. Additionally, the possible mechanisms involved in these effects were also investigated.
Materials and methods
Reagents
Carbon tetrachloride (CCl4) was obtained from Sinopharm Chemical Reagent Co., Ltd., China. The reference standards namely hyperoside, isoquercitrin, and rutin (purity ≥ 98%, HPLC grade) were purchased from Chroma Biotechnology Co., Ltd., China. The standard drug, bifendate drop pills, was purchased from local medical store. Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Chemical (St. Louis, MO). All other chemicals commercially obtained were of analytical grade or HPLC grade and used without further purification.
Preparation of TFs
The leaves of A. venetum were harvested in Shanxi Province, China, in August 2013, and identified according to the identification standard of the Pharmacopeia of the People’s Republic of China. The air-dried leaves of A. venetum (1 kg) were refluxed twice in 70% (v/v) aqueous ethanol (10 L) for 2.0 h at 90 °C. The combined filtration was evaporated under reduced pressure to obtain a crude ethanol extract. The mass was suspended in distilled water and defatted with petroleum ether. The aqueous layer was further concentrated and then chromatographed on HPD100 macroporous resin (Cangzhou Baoen Chemical Co. Ltd., Hebei Province, China). After washing the column with water and 15% ethanol, respectively, to remove polar components, the adsorbed fraction was then eluted with adequate 70% ethanol. The elution was collected, concentrated, and dried to obtain TFs.
Quality control of TFs
The total flavonoid content of the extract was determined using a colorimetric method described by Chen J et al. [5] with slight modification. Briefly, the extract (10 mg) was diluted appropriately and mixed with 1 mL NaNO2 (5%), 1.0 mL of AlCl3 (10%), and 4.0 mL NaOH (1.0 M). The final volume was adjusted to 25 mL with 70% ethanol and allowed to rest for 15 min. The absorbance (A) was measured at 510 nm by UV-vis spectrophotometer (UV-2450, Shimadzu, Japan), with 70% ethanol as a blank control. The total flavonoid contents (%) were estimated using calibration curves with rutin as the reference standard. All determinations were performed in triplicate.
The contents of hyperoside and isoquercitrin in the TFs were determined by HPLC according to the previous report [21]. A Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan) consisting of a solvent delivery pump (LC-10AD), UV-VIS detector (SPD-10A), and column oven (CTO-10A) was used. Analyses were performed with an Agilent SB-C18 column (4.6 mm × 150 mm, 5 μm). Each sample (20 μL) was filtered through a 0.22-μm Minipore filter and injected into the column. The isocratic mobile phase consisted of acetonitrile-0.1% phosphoric acid (13:87, v/v) was used with a flow rate of 0.9 ml/min. The column temperature was maintained at 25 °C, and the detection wavelength was 360 nm. The standards used were hyperoside and isoquercitrin.
In vitro experiments
Cell culture
Human hepatocellular liver carcinoma cell line, HepG2 cell, was obtained from the American Type Culture Collection (ATCC) and cultured at 37 °C under 5% CO2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml of penicillin and 100 μg/ml of streptomycin. When reaching 80–90% confluence, the cells were passaged. HepG2 cells were pretreated with different concentrations (25, 50 and 100 μg/mL) of TFs for 24 h. After the culture medium was discarded, the cells were washed with phosphate buffered saline (PBS) and exposed to 0.4% CCl4 for another 6 h. Then, the treated cells were used for the following assays.
Cell viability assay
Cell viability was assessed by 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, the cells were seeded in 96-well plates at 0.5 × 104 cells/well. After the above treatment, 10 μL of MTT (5 mg/mL) was added to each well and the plate was incubated for 4 h at 37 °C. Finally, the medium was removed and the formazan crystals were dissolved in 100 μL DMSO. The optical density (OD) of each well was measured at 570 nm using a microplate reader (Model 680, Bio-Rad, USA).
Lactate dehydrogenase release assay
Plasma membrane integrity was assessed using the lactate dehydrogenase (LDH) kit from Jiancheng Institute of Biotechnology (Nanjing, China). At the end of treatment, the cell media was collected, and the LDH activity was measured according to the supplied manufacturer’s instructions.
Measurement of intracellular reactive oxygen species
DCFH-DA, a reactive oxygen species (ROS)-sensitive fluorescent probe, was used to determine the intracellular ROS level as described previously [3]. For this assay, the commercially available kit (Applygen Technologies, Beijing, China) was used. In brief, after incubation, cells were harvested, washed twice, and resuspended in PBS. Then, the cells were loaded with 10 μM DCFH-DA for 30 min at 37 °C in the dark, washed with PBS (pH 7.2) three times, and measured by FACS flow cytometry (Becton Dickinson, USA) with 480 nm excitation and 530 nm emission.
Assessment of mitochondrial membrane potential
JC-1 exists as a green fluorescent monomer at low membrane potential and forms red fluorescent aggregates at higher membrane potential. Thus, the ratio of red to green fluorescence can represent changes in mitochondrial membrane potential (MMP) [25]. MMP was measured using the fluorescent probe JC-1 (KeyGen Technology, Nanjing, China) according to the manufacturer’s protocol. After the treatment, cells were harvested, washed, and incubated with 10 μg/mL JC-1 at 37 °C for 30 min in the dark. Then, stained cells were washed, resuspended, and used for flow cytometry analysis.
Cytotoxicity assessment
Cellular apoptosis was analyzed by an Annexin V-FITC/PI double staining kit (KeyGen Technology, Nanjing, China). At the end of the incubation, the cells were harvested and resuspended in 500 μL of binding buffer. Then, the cell suspension was incubated with 5 μL Annexin V conjugated to FITC and 10 μL propidium iodide (PI) in the dark at room temperature for 15 min according to the manufacturer’s instructions. At least 10,000 cells were collected and detected by flow cytometer within 15 min using a FACScan flow cytometer (Becton Dickinson, USA). The Annexin V(−) and PI (−) population was regarded as normal cells, Annexin V(+) and PI (−) cells were defined as early apoptotic cells, Annexin V(+) and PI (+) cells as late apoptotic/secondary necrotic cells, Annexin V(−) and PI (+) cells as primary necrotic (Annexin-V-FITC−/PI+) cells.
In vivo experiments
Animals
Male Kunming mice weighing 18–24 g were purchased from Beijing Vital River Laboratory Animal Co., Ltd. The animals were housed at 23 ± 1 °C, with relative humidity of 55 ± 10%, under a 12-h light/dark cycle and had free access to standard pellet diet and water. All procedures were in strict accordance with the China legislation on the use and care of laboratory animals and were approved by the committee for animal experiments.
Experimental design
After acclimation for 1 week, the mice were randomly divided into six groups with each group containing 10 mice. Mice in group I (control group) and group II (model group) were given 0.5% sodium carboxymethyl cellulose (CMC-Na) solutions orally. Groups III–V (TF-treated groups) were administrated with TFs at the doses of 100, 200, and 400 mg/kg, respectively, while group VI (positive control) was given bifendate (200 mg/kg). Bifendate is a synthetic hepatoprotective compound derived from schisandrin C, a component of Fructus Schizandrae. Bifendate drop pill (BP) is clinically used for the treatment of chronic hepatitis in China, and normally regarded as positive control for exploring other hepatoprotective agents [16, 17]. After the oral administration for 14 consecutive days, the animals were treated as described previously with some modifications [38]. Two hours after the final administration, the mice in groups II–VI were injected intraperitoneally with 0.3% (v/v) CCl4 (10 mL/kg, dissolved in olive oil), while the mice in group I were injected with an equivalent volume of olive oil alone (i.p.). Twenty-four hours after CCl4 challenge followed by fasting, the animals were anesthetized to collect the blood sample and sacrificed to obtain the livers.
Measurement of biochemical parameters in serum
Serum was obtained by centrifugation of blood at 900g for 15 min and stored at − 20 °C until use. Serum aspartate transaminase (AST) and alanine transaminase (ALT) activities were determined spectrophotometrically by commercial kits according to the manufacturer’s instructions (Jiancheng Institute of Biotechnology, Nanjing, China).
Measurement of biochemical parameters in liver tissues
Liver tissue was weighed and homogenized in cold Tris-HCl (5 mmol/L containing 2 mmol/L EDTA, pH 7.4). The homogenate was centrifuged at 10,000g for 20 min at 4 °C. The supernatant was collected and used for the determination of the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPH-x), as well as malondialdehyde (MDA) level in liver by using commercial assay kits (Jiancheng Institute of Biotechnology, Nanjing, China) according to the manufacturer’s protocols. Protein concentrations were determined according to the method of Bradford, using bovine serum albumin as standard.
Histopathological examinations
Liver tissues were fixed in 4% paraformaldehyde, and then embedded in paraffin. The paraffin blocks were sliced into 5-μm sections which were processed for staining with hematoxylin and eosin (HE). Histopathologic changes were observed using an optical microscope (Olympus Optical Co., Tokyo, Japan).
Acute toxicity
The mice were fasted overnight and then orally administered with TFs, with graded doses of up to 1.2 g/kg body weight, while the control group received distilled water. The groups were observed for 14 days and mortality was recorded for each group at the end of the experiment.
Statistical analysis
All experimental values are expressed as means ± standard deviation (SD). A one-way analysis of variance (ANOVA) with the Turkey post hoc test was performed using the Statistical Package for the Social Sciences software (SPSS 17.0 for Windows, 2010, SPSS Inc., Chicago, IL, USA). Values of P < 0.05 were considered to indicate statistical significance. All the experiments were performed in triplicate.
Results
Quality evaluation of TFs
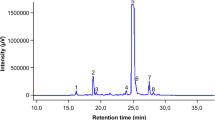
The prepared TFs from A. venetum leaves contained 56.18 ± 2.76% rutin equivalents (n = 3) as determined via UV spectrophotometry. As shown in Fig. 1, two flavonoid compounds, namely hyperoside and isoquercitrin, were identified as main chemicals using HPLC method, and their contents in TFs were 25.19 ± 0.55% (n = 3) and 21.88 ± 0.93% (n = 3), respectively.
In vitro experiments
TFs attenuated CCl4-induced cytotoxicity in HepG2 cells
The toxicity of CCl4 on cell viability was first determined by MTT assay. As shown in Fig. 2b, when cells were exposed to CCl4 for 6 h, there was a concentration-dependent decrease in cell viability. In particular, cell growth was reduced to 52.56 ± 4.31% in the presence of 0.4% CCl4 after 6 h of incubation compared with that of untreated cells. Thus, 0.4% CCl4 and 6 h were selected as the concentration and incubation time, respectively, for the subsequent experiments. On the other hand, TFs did not show apparent cytotoxic effects even up to the concentration of 400 μg/mL for 24 h (Fig. 2a). However, when cells were pretreated with TFs at the different concentrations (25, 50, and 100 μg/mL) for 24 h before CCl4 exposure, cell viability was enhanced to 69.50 ± 2.49, 72.67 ± 4.11, and 76.06 ± 3.74% of the control, respectively (Fig. 2c).
Protective effects of TFs on CCl4-induced cytotoxicity in HepG2 cells. (a) Effect of TF on proliferation of HepG2 cells after treatment for 24 h. (b) HepG2 cells were treated with CCl4 at concentrations of 0.2–0.8% for 6 h. (c) Effect of pretreatment with various concentrations of TFs (25, 50, and 100 μg/mL) for 24 h on the proliferation of HepG2 cells induced by 0.4% CCl4 for 6 h, and cell viability was measured by MTT test. (d) LDH release in culture medium after treatment. Data are presented as means ± SD (n = 3). ##P < 0.01, compared with the control group; **P < 0.01, *P < 0.05, compared with CCl4-treated group
LDH release from cells into the culture medium is considered as an indicator of cellular damage [2]. As shown in Fig. 2d, the LDH release in CCl4-treated cells was observed to be significantly increased when compared with the control group (P < 0.01), pretreatment of HepG2 cells with TFs for 24 h inhibited this leakage in a concentration-dependent manner.
TFs reduced CCl4-induced intracellular ROS generation
To investigate whether ROS is involved in cytoprotective effect of TFs, intracellular ROS levels were measured using the DCFH-DA probe. As illustrated in Fig. 3, treatment of cells with 0.4% CCl4 significantly increased ROS level, and this increment was markedly attenuated by pretreatment with TFs at the concentration of 25, 50, and 100 μg/mL.
Effect of TFs on intracellular ROS generation induced by CCl4 in HepG2 cells. Cells were incubated with or without TFs (25, 50, and 100 μg/mL) for 24 h and then treated with or without 0.4% CCl4 for 6 h. Then, ROS levels were determined by with flow cytometry using DCFH-DA. Data are as means ± SD (n = 3). ##P < 0.01, compared with the control group; **P < 0.01, *P < 0.05, compared with CCl4-treated group
TFs inhibited CCl4-induced MMP disruption
MMP is an important parameter of mitochondrial function and is critical for maintaining the physiological function of the respiratory chain to generate ATP. A collapse of MMP is thought to be an initial and irreversible step of apoptosis [11]. As shown in Fig. 4, when the cells were incubated with 0.4% CCl4 for 6 h, the MMP of cells decreased to 59.36 ± 2.27% compared with that of untreated cells (100%), indicating that CCl4 induced a significant loss of MMP in HepG2 cells. Whereas, pretreatment of cells with 25, 50 and 100 μg/mL of TFs rescued markedly the loss of MMP in a concentration-dependent manner.
Effect of TFs on MMP disruption induced by CCl4 in HepG2 cells. Cells were incubated with or without TFs (25, 50, and 100 μg/mL) for 24 h and then cultured in the presence or absence of 0.4% CCl4 for 6 h. MMP was monitored by flow cytometry using JC-1 probe. Data are as means ± SD (n = 3). ##P < 0.01, compared with the control group; **P < 0.01, *P < 0.05, compared with CCl4-treated group
TF-protected HepG2 cells from CCl4-induced apoptosis
To quantitatively analyze apoptosis, flow cytometry using the FITC-Annexin V/PI double staining method was used to determine whether TFs could protect against CCl4-induced cell apoptosis. As depicted in Fig. 5a, an increase in Annexin V-positive apoptotic cell population was observed posttreatment with 0.4% CCl4, as compared to the untreated control, this elevation was decreased by pretreatment with TF. More intuitively, Fig. 5b indicated that treatment with 0.4% CCl4 for 6 h dramatically increased the frequency of apoptotic cells from 4.07 ± 0.31 to 34.68 ± 1.73% (P < 0.01), while preincubation with TFs (25, 50, and 100 μg/mL) for 24 h decreased this rate to 24.18 ± 1.86, 18.32 ± 1.35, and 17.40 ± 1.29%, respectively. Furthermore, in line with the results from LDH release assay, flow cytometry analysis showed that CCl4 also induced primary necrotic cell death. Compared with the model group, treatment with TFs at all doses could significantly decrease necrotic cells albeit in a rather concentration-independent mode (Fig. 5b). These results suggested that TFs could inhibit both CCl4-induced apoptosis and necrosis in HepG2 cells.
Inhibitory effect of TFs on CCl4-induced HepG2 cell apoptosis. Cells were pretreated with TFs (25, 50, and 100 μg/mL) for 24 h, and then treated with or without 0.4% CCl4 for 6 h. (a) Representative flow cytometric plots after Annexin V-FITC/PI double staining; (b) Frequency of apoptotic and primary necrotic cells shown as histograms after treatment. Data are presented as means ± SD (n = 3). ##P < 0.01, compared with the control group; **P < 0.01, *P < 0.05, compared with CCl4-treated group
In vivo experiments
Effects of TFs on serum ALT and AST levels
The serum ALT and AST enzymatic activities have been commonly considered as indicator of liver toxicity since injury of the hepatocytes could alter their transport functions and membrane permeability, leading to leakage of enzymes from the cells [28]. As shown in Fig. 6, the serum levels of ALT and AST of the CCl4-treated group were markedly elevated at 145.06 ± 13.71 U/L (P < 0.01) and 202.53 ± 26.63 U/L (P < 0.01) compared with those of the normal control group, which were 29.92 ± 3.49 and 75.77 ± 14.52 U/L, respectively. Pretreatment with TFs at doses of 200 and 400 mg/kg/BW once daily for 14 consecutive days significantly prevented these elevations of ALT and AST in comparison with the CCl4 model group (P < 0.05 and P < 0.01). In addition, BP treatment, used as positive control, also markedly reversed the alterations of the AST and ALT levels compared with the model group (P < 0.01).
Effects of TFs on the serum levels of ALT (a) and AST (b) in the CCl4-induced hepatic injury mouse model. The mice were intragastrically administered TFs at 100, 200, or 400 mg/kg/BW or BP at 200 mg/kg/BW once daily for 14 consecutive days prior to a single intraperitoneal administration of CCl4. Values are presented as means ± SD for 10 mice in each group. ##P < 0.01, compared with the control group; **P < 0.01, *P < 0.05, compared with CCl4-treated group
Effect of TFs on histopathological damage in CCl4-induced liver tissue
Histopathological changes were presented in Fig. 7 to support the results obtained from serum assays. The histology of the liver sections of control group showed normal cellular architecture with distinct hepatic cells, sinusoidal spaces, and central veins. In the CCl4-treated group, vacuole formation, inflammatory cells infiltration, centrilobular fatty changes, and widespread hepatocellular necrosis were obviously observed (Fig. 7b). Pretreatment with 100 mg/kg of TFs failed to improve those histological damages in liver. In contrast, the hepatic lesions were markedly prevented by pretreatment with TF (200 and 400 mg/kg) and BP. This result indicated that TFs could protect the liver from CCl4-induced histopathological alterations.
Effects of TFs on CCl4-induced liver histopathological changes in mice. A portion of the liver tissues was stained with hematoxylin and eosin (H&E), and histological assessment was performed under a microscope. Typical photographs of liver sections demonstrate the pathological changes under microscopy (×200). Vacuoles and inflammatory cell clusters are indicated by yellow and black arrows, respectively. (a) Normal control group; (b) CCl4-intoxicated group; (c) positive BP (200 mg/kg BW) + CCl4 group; (d) TFs (100 mg/kg BW) + CCl4 group; (e) TFs (200 mg/kg BW) + CCl4 group; (f) TFs (400 mg/kg BW) + CCl4 group
Effects of TFs on hepatic lipid peroxidation and antioxidant enzymes
MDA, a lipid peroxidation end product, was utilized to evaluate oxidative injury. Figure 8a shows that the CCl4 treatment markedly increased hepatic MDA level by 121% as compared with that of the control (P < 0.01), while administration of TFs (200 and 400 mg/kg) significantly decreased the MDA levels in liver tissue compared with model group (P < 0.05).
Effects of TFs on hepatic lipid peroxidation and antioxidant enzymes activities in the CCl4-treated mice. (a) Level of MDA; (b) SOD activities; (c) CAT activities; (d) GSH-Px activities. The mice were orally administered TFs at 100, 200, or 400 mg/kg/BW or BP at 200 mg/kg/BW once daily for 14 consecutive days prior to a single intraperitoneal administration of CCl4. Values are presented as means ± SD for 10 mice in each group. ##P < 0.01, compared with the control group; **P < 0.01, *P < 0.05, compared with CCl4-treated group
Meanwhile, the activities of antioxidant enzymes (SOD, CAT and GSH-Px) in liver tissue were also determined. As shown in Fig. 8b–d, the hepatic SOD, CAT, and GSH-Px activities in the CCl4-treated group were significantly lower than those in the control group (P < 0.01). However, pretreatment with TFs (200 and 400 mg/kg) afforded a significant and dose-dependent protection against CCl4-induced decrease in activities of all the antioxidant enzymes studied.
Acute toxicity
No any symptom of toxicity or mortality was observed during the 14 consecutive days of oral treatment with TFs at doses of 0.40, 0.80, or 1.20 g/kg body weight. In general, the abnormal changes of body weight have been used as an indicator of adverse effects of testing substances [22]. The body weights of control and TF-treated mice are presented in Fig. 9, there were no significant differences in the body weights of the mice between the groups for each time period. Nevertheless, systemic toxicity should be further investigated hereafter.
Discussion
In China, the leaves of A. venetum are a well-known medicinal herb that have many benefits to human health, especially in prevention and treatment of cardiovascular and liver diseases [33, 34, 36]. These biological activities were largely attributed to flavonoids, which are the main components in A. venetum [1, 21, 33, 37].
Flavonoids, as a group of polyphenolic compounds, possess a wide range of biological properties ranging from antioxidant, antibacterial, anti-inflammatory, anti-allergic, antiviral, and anticancer to hepatoprotective actions [31]. In the last decade, a large body of evidence indicates that flavonoids exhibited significant protective activities against liver damage in vitro and in vivo models [8, 9, 27]. In particular, the hepatoprotective effects of A. venetum and its major flavonoid hyperoside were also confirmed in recent studies [6, 34,35,36].
CCl4-induced hepatic injury is the most common model for investigating various hepatoprotection. In liver cell, CCl4 is metabolized by cytochrome P450 to generate a highly reactive trichloromethyl free radical (CCl3•), which could be further converted to trichloromethylperoxy radical (CCl3OO•). These toxic radicals react with lipids, DNA, or proteins, resulting in lipid peroxidation, cell necrosis, and liver fibrosis radicals [32]. In the current study, the total flavonoids (TFs) were prepared from the leaves of A. venetum, and its protective effects against the CCl4-induced hepatotoxicity were investigated both in vitro and in vivo.
Adsorption resin column chromatography was successfully used for enrichment and purification of TFs, the content of obtained TF was 56.18 ± 2.76% (n = 3). HPLC analysis demonstrated that hyperoside and isoquercitrin are major ingredients in TFs, which constitute 25.19 ± 0.55% (n = 3) and 21.88 ± 0.93% (n = 3), respectively.
HepG2, hepatocellular carcinoma cells, have been shown to express a wide range of liver-specific functions and can be used as a model system for studies of toxicity of xenobiotics, the detection of environmental and dietary cytotoxic and genotoxic agents [24].
As demonstrated in our MTT result, pretreatment with TFs significantly prevented the loss of cell viability induced by CCl4 (Fig. 2c). LDH is an intracellular enzyme present in cell cytoplasm, and its leakage from the cell indicates membrane damage. In this study, treatment with 0.4% CCl4 for 6 h increased the cell LDH release significantly compared to control group, and this increase was attenuated by pretreatment with TFs in a concentration-dependent manner (Fig. 2d). The results of the MTT and LDH leakage assays suggested that TFs could exert a protective effect against CCl4-induced cytotoxicity.
Accumulating evidences have indicated that CCl4-induced liver failure was accompanied by ROS production and peroxidative damage [20, 29]. An excess level of ROS can lead to mitochondrial membrane depolarization, induce MMP loss and cytochrome c release to the cytosol, and eventually trigger cell apoptosis [18]. In this study, we showed that CCl4 markedly increased the intracellular ROS generation and reduced MMP in HepG2 cells (Figs. 3, 4). These CCl4-induced cell events were attenuated significantly when pretreated with TFs. On the other hand, Fig. 3 shows that excessive ROS were not completely eliminated, when pretreated with TFs. ROS, once generated, cause massive oxidation of redox sensitive proteins and lipids leading to mitochondrial damage. Then, the damaged mitochondria decline in function, which in turn generates more ROS [13]. Although TFs cannot reduce the intracellular ROS to its basal level, at least within the range of concentrations tested, its protective effect is reliable. Furthermore, FITC-Annexin V/PI double staining method can provide the direct evidence about cell apoptosis. The result confirmed that TFs decreased CCl4-induced cell apoptosis rate in a concentration-dependent manner. These findings suggest that TFs exhibited protective activity against oxidative stress, and that might contribute to its inhibition of CCl4-induced apoptosis. In view of the fact that a fraction of xenobiotic pathways is active in a potentially genomically instable, transformed cell type, the hepatoprotective effect of TF needs to be further verified in primary hepatocytes.
In vivo experiments, mice administered with CCl4 exhibited serious liver toxicity, as evidenced by significant elevations in serum markers (ALT and AST) for hepatic cell damage (Fig. 6). This observation was in agreement with the previous reports [6, 38]. Whereas, pretreatment with TFs (200 and 400 mg/kg) along with BP (positive control, 200 mg/kg) for 14 days significantly decreased the elevations of serum levels of ALT and AST. Furthermore, the protective effect of TFs on CCl4-induced liver toxicity was also supported by histopathological observations (Fig. 7). The above results suggest that orally administered TFs exerted therapeutic effects on CCl4-induced liver injury in mice, which is in line with previous findings of A. venetum on acetaminophen-induced toxicity [34].
MDA, an end product of lipid peroxidation, is used as an indicator for oxidative damage of tissues [7]. The present study showed that content of MDA in liver homogenate was dramatically increased in the CCl4-treated mice, while TFs (200 and 400 mg/kg) dose-dependently decreased the elevated level of MDA (Fig. 8a). Moreover, organisms possess antioxidant systems including antioxidant enzymes to protect the damage caused by oxidative stress [26]. As shown in Fig. 8b–d, our study showed that the activities of all three antioxidant enzymes, SOD, CAT, and GSH-Px, in CCl4-treated liver tissues decreased significantly (P < 0.01). Compared to the model group, pretreatment with TF (200 and 400 mg/kg) effectively protected the loss of these antioxidant enzymes activities. These observations indicated that TFs can prevent CCl4-induced hepatic impairment in mice via inhibiting excessive radicals and suppressing oxidative stress.
Conclusions
In conclusion, the present study indicated that the obtained TFs from A. venetum exert a protective effect on CCl4-induced hepatocyte damage in vitro and in vivo. The possible mechanisms may be due to its ability to scavenge free radicals, inhibit the apoptosis, and improve the antioxidative defense system. Our finding showed that TFs are a potential candidate to treat oxidative stress-mediated liver disorders.
References
An H, Wang H, Lan Y, Hashi Y, Chen S (2013) Simultaneous qualitative and quantitative analysis of phenolic acids and flavonoids for the quality control of Apocynum venetum L. leaves by HPLC-DAD-ESI-IT-TOF-MS and HPLC-DAD. J Pharm Biomed 85(11):295–304
Bajt ML, Knight TR, Lemasters JJ, Jaeschke H (2004) Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci 80(2):343–349
Behl C, Davis JB, Lesley R, Schubert D (1994) Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77(6):817–827
Butterweck V, Simbrey K, Seo S, Sasaki T, Nishibe S (2003) Long-term effects of an Apocynum venetum extract on brain monoamine levels and beta-AR density in rats. Pharmacol Biochem Be 75(3):557–564
Chen J, Wang JB, Yu CH, Chen LQ, Xu P, Yu WY (2013) Total flavonoids of Mosla scabra leaves attenuates lipopolysaccharide-induced acute lung injury via down-regulation of inflammatory signaling in mice. J Ethnopharmacol 148(3):835–841
Choi JH, Kim DW, Yun N, Choi JS, Islam MN, Kim YS, Lee SM (2011) Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J Nat Prod 74(5):1055–1060
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52(4):601–623
Dhiman RK, Chawla YK (2005) Herbal medicines for liver diseases. Digest Dis Sci 50(10):1807–1812
Dryden GW, Song M, McClain C (2006) Polyphenols and gastrointestinal diseases. Curr Opin Gastroen 22(2):165–170
Duarte S, Baber J, Fujii T, Coito AJ (2015) Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol 46:147–156
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Farghali H, Canova NK, Zakhari S (2015) Hepatoprotective properties of extensively studied medicinal plant active constituents: possible common mechanisms. Pharm Biol 53(6):781–791
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408(6809):239–247
Galleano M, Pechanova O, Fraga CG (2010) Hypertension, nitric oxide, oxidants, and dietary plant polyphenols. Curr Pharm Biotechnol 11(8):837–848
Grundmann O, Nakajima J, Seo S, Butterweck V (2007) Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J Ethnopharmacol 110(3):406–411
Guan LP, Nan JX, Jin XJ, Jin QH, Kwak KC, Chai KY, Quan ZS (2005) Protective effects of chalcone derivatives for acute liver injury in mice. Arch Pharm Res 28(1):81–86
Gui SY, Wei W, Wang H, Wu L, Sun WY, Wu CY (2005) Protective effect of fufanghuangqiduogan against acute liver injury in mice. World J Gastroenterol 11(19):2984–2989
Higuchi M, Honda T, Proske RJ, Yeh ET (1998) Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 17(21):2753–2760
Kwan CY, Zhang WB, Nishibe S, Seo S (2005) A novel in vitro endothelium-dependent vascular relaxant effect of Apocynum venetum leaf extract. Clin Exp Pharmacol Physiol 32(9):789–795
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16(11):26087–26124
Liang T, Yue W, Li Q (2010) Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (Luo-Bu-Ma) and two of its alternative species. Int J Mol Sci 11(11):4452–4464
Liju VB, Jeena K, Kuttan R (2013) Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L). Food Chem Toxicol 53(3):52–61
Malhi H, Gores GJ (2008) Cellular and molecular mechanisms of liver injury. Gastroenterology 134(6):1641–1654
Mersch-Sundermann V, Knasmüller S, Wu XJ, Darroudi F, Kassie F (2004) Use of a human-derived liver cell line for the detection of cytoprotective, antigenotoxic and cogenotoxic agents. Toxicology 198(1–3):329–340
Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques 50(2):98–115
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2(2):219–236
Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F (2013) Novel insights into the pharmacology of flavonoids. Phytother Res 27(11):1588–1596
Schmatz R, Perreira LB, Stefanello N, Mazzanti C, Spanevello R, Gutierres J, Bagatini M, Martins CC, Abdalla FH, Daci da Silva Serres J, Zanini D, Vieira JM, Cardoso AM, Schetinger MR, Morsch VM (2012) Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie 94(2):374–383
Sipes IG, el Sisi AE, Sim WW, Mobley SA, Earnest DL (1991) Reactive oxygen species in the progression of CCl4-induced liver injury. Adv Exp Med Biol 283:489–497
Tacke F, Luedde T, Trautwein C (2009) Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol 36(1):4–12
Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JP (2010) Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients 2(11):1106–1131
Weber LW, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33(2):105–136
Xie W, Zhang X, Wang T, Hu J (2012) Botany, traditional uses, phytochemistry and pharmacology of Apocynum venetum L. (Luobuma): a review. J Ethnopharmacol 141(1):1–8
Xie W, Chen C, Jiang Z, Wang J, Melzig MF, Zhang X (2015) Apocynum venetumattenuates acetaminophen-induced liver injury in mice. Am J Chin Med 43(3):457–476
Xie W, Jiang Z, Wang J, Zhang X, Melzig MF (2016) Protective effect of hyperoside against acetaminophen (APAP) induced liver injury through enhancement of APAP clearance. Chem Biol Interact 246:11–19
Xiong Q, Fan W, Tezuka Y, Adnyana IK, Stampoulis P, Hattori M, Namba T, Kadota S (2000) Hepatoprotective effect of Apocynum venetum and its active constituents. Planta Med 66(2):127–133
Yokozawa T, Kashiwada Y, Hattori M, Chung HY (2002) Study on the components of luobuma with peroxynitrite-scavenging activity. Biol Pharm Bull 25(6):748–752
Zhang S, Lu B, Han X, Xu L, Qi Y, Yin L, Xu Y, Zhao Y, Liu K, Peng J (2013) Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol 55(3):60–69
Funding
Financial supports form the National Natural Science Foundation of China (no. 81302154, 81470865).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, W., Dong, Z., Chang, X. et al. Protective effect of the total flavonoids from Apocynum venetum L. on carbon tetrachloride-induced hepatotoxicity in vitro and in vivo. J Physiol Biochem 74, 301–312 (2018). https://doi.org/10.1007/s13105-018-0618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-018-0618-0