Abstract
A double strategy based on the removal of sulfonamide antibiotics by Pleurotus ostreatus and adsorption on spent mushroom substrate was assessed to reclaim contaminated wastewater. P. ostreatus was firstly tested in a liquid medium fortified with five sulfonamides: sulfamethoxazole, sulfadiazine, sulfathiazole, sulfapyridine and sulfamethazine, to evaluate its capacity to remove them and to test for any adverse effects on fungal growth and for any reduction in residual antibiotic activity. P. ostreatus was effective in removing sulfonamides up to 83 to 91% of the applied doses over 14 days. The antibiotic activity of the sulfonamide residues was reduced by 50%. Sulfamethoxazole transformation products by laccase were identified, and the degradation pathway was proposed. In addition, P. ostreatus growth on a semi-solid medium of spent mushroom substrate and malt extract agar was used to develop a biofilter for the removal of sulfonamides from real wastewater. The biofilter was able to remove more than 90% of the sulfonamide concentrations over 24 h by combining adsorption and biodegradation mechanisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The spread of antibiotics into the environment is a global concern because of their potential ecological risk, including the generation of antibiotic-resistant bacteria and their impact on the bacterial community (Rizzo et al. 2013; Santás-Miguel et al. 2020). Wastewater is an important source of antibiotics in the environment (Rizzo et al. 2013; Ben et al. 2018). The main reason is the inefficiency of wastewater treatment plants (WWTPs) to remove antibiotics or other organic pollutants because they were not designed to remove micro-pollutants (Michael et al. 2013; Berendonk et al. 2015; Tran et al. 2018). Antibiotics occur in wastewater because they are only partially metabolized by humans and animals and are excreted in the urine and faeces as parent compound in a high proportion of the given dose (Van der Ven et al. 1994; Halling-Sørensen et al. 2002).
Sulfonamides (SAs) are wide-spectrum antibiotics highly prescribed for animal breeding and as growth promoters in animal husbandry whose sales for veterinary use in UE were 608.3 tons in 2017 (European Medicines Agency 2019). Sulfonamides adsorb weakly to sediments or sludge and can quickly contaminate groundwater (Reis et al. 2020). They are some of the most commonly detected antibiotics in wastewater and different natural water ecosystems in Europe at a concentration of up to μg L−1 (Carvalho and Santos 2016). As a result of the ubiquity of this family of antibiotics, sulfonamide-resistant genes have been detected in the environment (Sharma et al. 2016). SAs can also reach the food chain by the addition of cattle manure or sewage sludge to croplands as soil organic amendments and fertilizers, as well as by irrigation using polluted wastewater (Conde-Cid et al. 2018; Picó et al. 2019; Zhao et al. 2019). Hence, the removal of sulfonamides from wastewater is an imperative action both for the environment and for human health.
The removal of sulfonamides in WWTP follows abiotic and biotic pathways. Natural photodegradation has been shown to be ineffective or can result in the production of toxic compounds (Reis et al. 2020). Their adsorption in sludge can reach appreciable rates; however, this process is highly dependent on the particular operational conditions of the WWTP and is unfavourable due to their chemical properties such as low Kow values (Tran et al. 2018). Advanced treatments, such as membrane processes, activated carbon adsorption or advanced oxidation processes downstream of conventional biological processes, can significantly improve antibiotic removal despite an increment in capital and operational costs (Michael et al. 2013). Hence, the optimization or new approaches to the biological degradation of sulfonamides could represent a novel improvement in the treatment of wastewater. The biodegradation of SAs by bacteria in WWTPs appears to take place via co-metabolism although some bacteria use SAs as their sole source of energy. The co-existence of both a degradation mechanism and a resistance gene in the same bacteria raises important questions concerning the co-evolution of these traits. Since the link between antibiotic degradation and resistance remains unexplored, the direct application of these degraders may promote the undesirable spread of resistance (Reis et al. 2020). In this respect, the use of white rot fungi and their ligninolytic enzymes has emerged as an interesting alternative to bacterial biodegradation (Čvančarová et al. 2015; Lucas et al. 2016; Navada and Kulal 2019). Several biotechnological approaches have been successfully tested to determine the biodegradation of sulfonamides by white rot fungi (Rodríguez-Rodríguez et al. 2012; de Araujo et al. 2017), using free or immobilized laccase enzymes (Schwarz et al. 2010; Rahmani et al. 2015; García-Delgado et al. 2018; Alharbi et al. 2019) or using spent mushroom substrate (SMS) (Chang et al. 2018b).
Several ligninolytic fungi with the potential to degrade sulfonamides are cultivated for human consumption (Chang et al. 2018b) with the consequent co-generation of large quantities of SMS. For example, China produces about 4 million tons of SMS annually (Mohd Hanafi et al. 2018) and Europe produces more than 3.5 million tons (Gea et al. 2014). The re-use of this agricultural waste is a promising issue due to its wide applications for environmental remediation based on the degradation and adsorption of pollutants. The adsorption of organic and inorganic pollutants on SMS occurs as a result of its high organic carbon content and as functional groups that are able to interact with pollutants (Álvarez-Martín et al. 2016; Frutos et al. 2016; García-Delgado et al. 2020). The degradation of organic pollutants is carried out by the active fungal mycelium, the ligninolytic enzymes and the inherent microbiota of the SMS (García-Delgado et al. 2015b; Di Gregorio et al. 2016; Siracusa et al. 2017; Chang et al. 2018a). Therefore, the design of novel devices combining the removal of sulfonamides by ligninolytic fungi and adsorption on SMS could represent an interesting approach to reclaiming wastewater and a reduction of the exposure of bacteria to these antibiotics, resulting in a reduction in the acquisition of specific sulfonamide-resistant genes and their spread into the environment. In addition, it is a further step more in the re-use of an agricultural waste to promote a circular economy.
The aim of this work was to remove sulfonamides from real wastewater using a novel biofilter based on Pleurotus ostreatus and its spent mushroom substrate as a support. Previously, the ability of P. ostreatus to remove five sulfonamides in liquid media was tested based on fungal growth, ligninolytic activity, antibiotic dissipation and residual antibiotic activity.
Materials and methods
Sulfonamides and fungal material
Sulfonamides and the internal standards were purchased from Sigma-Aldrich (St. Louis, USA) (sulfathiazole (STZ, 99%), sulfapyridine (SP, 95%), sulfamethoxazole (SMX, 99%), sulfathiazole-13C6 and sulfamethoxypyridazine-d3), Acros Organics (Geel, Belgium), (sulfadiazine (SDZ, 99%)) and Alfa Aesar (Haverhill, USA) (sulfamethazine (SMZ, 99%)).
The strain of P. ostreatus was isolated from SMS collected from a commercial mushroom cultivation farm in Quintanar del Rey (Cuenca, Spain) and successfully tested for polycyclic aromatic hydrocarbons degradation in a previous work (García-Delgado et al. 2015a). The fungus was maintained on malt extract agar (MEA) plates at 4 °C and sub-cultured every 28 days.
Assessment of P. ostreatus to remove sulfonamides in liquid cultures
P. ostreatus was inoculated in 500-mL Erlenmeyer flasks with 125 mL of 3% malt extract (ME) in the presence or absence of 0.1 mM of each of the SAs (SDZ, SP, STZ, SMZ and SMX) by adding three 5-mm-diameter fungal plugs from a 7-day-old MEA culture. The cultures were incubated for 14 days at 28 °C, under orbital agitation (120 rpm) in the dark. Abiotic controls without P. ostreatus were prepared and incubated under the same conditions. The assay was performed in triplicate. At the end of the assay, the fungal biomass was separated from the liquid medium using a Büchner funnel and rinsed four times with sterile water to remove all traces of the medium. The fungal mass was dried at 65 °C for 2 days to determine the dry weight. One-millilitre aliquots were removed daily to analyse the kinetics of the antibiotic degradation and ligninolytic activity. The concentration of the remaining SAs was determined by placing 1-mL aliquots of liquid broth in Eppendorf tubes and centrifuged at 5000 rpm for 1 min, and the supernatant was filtered through 0.45-μm nylon syringe filters. Then, 0.5 mL of the filtrate was mixed with 0.5 mL methanol in HPLC vials and stored at − 18 °C until analysis. The concentration of SAs was determined by HPLC coupled to a photodiode array detector according to Summa et al. (2015). A detailed description of the analytical method is included in the Supplementary Information.
The removal of SAs by P. ostreatus was adjusted to a pseudo-first-order kinetic model.
where C0 is the initial concentration of SAs, C is the concentration of the target antibiotic at t time (days) and k is the degradation constant (day−1).
The half-lives of SAs were calculated using Eq. (2)
The residual antibiotic activity after fungal removal of SAs was based on growth inhibition of bacteria present in real wastewater collected from the WWTP at the Autonomous University of Madrid according to García-Delgado et al. (2018). Briefly, 5.0 mL of wastewater was added to 500 mL sterilized tryptic soy broth and incubated at 30 °C in the dark under orbital shaking (120 rpm) for 24 h. The optical density at 600 nm (OD600) of the resulting bacterial culture was adjusted to 0.100 by dilution with sterilized tryptic soy broth. Then, 1 mL obtained from the mycodegradation assay of SAs was mixed with 4 mL bacterial culture media. The absorbance increment at 600 nm was monitored after 4 h of incubation at 30 °C. Controls were prepared without antibiotics, and analysis of samples with 0.1 mM of each SA was performed in parallel. The assay was performed in triplicate. The percentage of bacterial growth inhibition (GI%) was calculated by Eq. (3)
where OD600s and OD600c are the optical densities of the sample and the controls without antibiotics, respectively.
Ligninolytic enzyme analysis
Laccase activity was spectrophotometrically determined by oxidation of 10 mM 2,6-dimethoxy phenol in 50 mM sodium acetate (pH 5.0) at 477 nm (ε = 14,600 M−1 cm−1) (García-Delgado et al. 2018). Mn peroxidase (MnP) activity was assayed by the oxidation of 1 mM MnSO4 in 50 mM sodium malonate buffer (pH 4.5), in the presence of 0.1 mM H2O2. Manganic ions by Mn3+ form a complex with malonate, which absorb at 270 nm (ε = 11,590 M−1 cm−1) (Wariishi et al. 1992). One unit of enzyme activity (IU) is defined as the amount of enzyme that produces 1 μmol of product per min, under the assay conditions.
Degradation of SMX by commercial laccase and identification of the transformation products
Ten millilitres of 0.05 mM SMX was incubated together with 5 U mL−1 of commercial laccase from Aspergillus sp. over 24 h at 28 °C in the dark. The reaction was stopped by adding methanol 50%. The controls without laccase were performed in parallel. Then, 1 mL of each sample was filtered through 0.45-μm nylon syringe filters. The degradation products were separated by UPLC (Acquity Series, Waters). Mass spectra were performed using an ultra-high-resolution QTOF instrument (MAXIS II, Bruker, Bremen, Germany). A detailed description of the analytical method is included in the Supplementary Information.
Removal of sulfonamides from real wastewater by biofilter
Wastewater was obtained from an urban WWTP in Almería (Spain) in April 2017. The wastewater contained SMX (423 ng L−1), SP (72.5 ng L−1), sulfamerazine (SM, 13.5 ng L−1) and sulfamonomethoxine (SX, 21.9 ng L−1). The biofilter was based in a sterilized (121 °C, 20 min) mixture of SMS (60 g) and MEA (250 mL) as a support and as a nutrient source for P. ostreatus. The SMS-MEA mixture was placed in Teflon containers, and after solidification, five plugs of 0.5 mm P. ostreatus were inoculated and grown for 3 days at 25 °C in the dark. Following this, 400 mL of wastewater was introduced into the biofilter using a peristaltic pump at a flow rate of 10 mL min−1. The assay took 24 h, and 30-mL aliquots were taken at 30 min, 1 h, 2 h, 4 h, 6 h and 24 h. The abiotic control consisting of the sterilized SMS-MEA mixture, without fungal inoculation, was carried out under the same operational conditions. All the experiments were performed in triplicate.
Laccase and MnP activities were measured in all the aliquots according to methods described above. The concentration of SAs in wastewater before and after the biofilter treatment was determined by UPLC-MS/MS after a concentration and purification step by solid-phase extraction. A detailed description of the analytical procedure is included in the Supplementary Information.
An environmental risk assessment was determined using the hazard quotient (HQ) of the SAs in the water samples following the equation
where [SAs] is the concentration of the antibiotics (μg L−1) and the predicted no-effect concentration (PNEC) values were 0.027 μg L−1 for SMX and 21.61 μg L−1 for SP according to Verlicchi et al. (2012).
Statistical analysis
All statistical tests were carried out using the IBM SPSS Statistics v25 software package. Comparisons of the differences between treatments were determining using the Duncan post hoc test (according to variance homogeneity) at p < 0.05 was used. The Pearson correlation coefficient was calculated between the increments of laccase activity and removal rates SAs, by P. ostreatus in the biofilter assay.
Results
Degradation of sulfonamides by P. ostreatus in liquid cultures
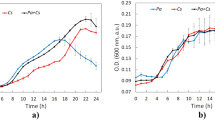
Figure 1 shows the dry fungal biomass and laccase activity profile of the P. ostreatus culture in the presence and absence of SAs. There were no significant differences (p > 0.05) in the dried fungal biomass of the control (0.65 ± 0.05 g) and the culture with SAs (0.85 ± 0.05 g) after 14 days of growth (Fig. 1a). These results indicate that the SAs were neither toxic nor altered the fungal growth under these conditions. Laccase was the extracellular enzyme most expressed in both the presence and absence of SAs. Peak laccase activity (Fig. 1b) was achieved at day 4 and was higher in the control (68 U L−1) than in the presence of SAs (45 U L−1). From day 4, laccase activity decreased progressively until it become undetectable at day 14 in both the control and the SA cultures. MnP was detected during the incubation period with its maximum activity (8 U L−1) at day 6.
The remaining concentrations of SAs in the liquid media during treatment by P. ostreatus over a period of 14 days are shown in Fig. 2. The abiotic control produced a lower removal rate of SAs than the mycodegradation by P. ostreatus. Hence, P. ostreatus played a key role in the degradation of selected SAs. The highest removal rates by P. ostreatus took place during the first week of incubation showing rates of between 60 and 71% of the initial concentration of SAs (Table 1) with a peak occurring between the 4th and 5th days of incubation, coinciding with the maximum activity of laccase. However, degradation of SAs by laccase was not the sole mechanism of degradation as shown by the extensive removal of SAs from day 10 to 14 when laccase activity was very low. Thus, the removal mechanism of SAs is probably a combination of extracellular and intracellular degradations and mycelial adsorption. At the end of the incubation period, P. ostreatus achieved removal rates of SAs between 83 and 91%, denoting the effectivity of P. ostreatus to remove SA antibiotics (Table 1). The pseudo-first-order kinetic model adequately outlined the experimental data of SAs removal by P. ostreatus (Table 1). All the five SAs tested presented a good adjustment to the pseudo-first-order kinetic model. The removal rate of SAs by P. ostreatus was determined by the dissipation constant (k) of between 0.1253 and 0.1506. Four of the five SAs (SDZ, STZ, SP, SMX) showed half-lives of less than 5 days. SMZ showed the lowest removal speed with a half-life of 5.5 days. It is clear that the chemical structure of the SAs has an effect on their dissipation kinetics.
Remaining concentration of sulfonamides incubated in the absence (control) or presence of P. ostreatus for 14 days in malt extract liquid media. a Sulfadiazine (SDZ). b Sulfapyridine (SP). c Sulfathiazole (STZ). d Sulfamethazine (SMZ). e Sulfamethoxazole (SMX). Error bars represent the standard deviation (n = 3)
The residual antibacterial activity of remaining SAs and their transformation products in liquid cultures was determined using a bacterial consortium from the WWTP from the Autonomous University of Madrid. No bacterial growth was observed in the presence of the initial mixture of the 5 SAs at 0.1 mM of each antibiotic. Hence, no resistance towards SAs was detected by the bacterial consortium used. Meanwhile, bacterial growth was partially inhibited by residues of SAs and their transformation products after P. ostreatus treatment compared with the negative controls (without SAs), showing a bacterial growth inhibition rate of 50%. The extensive SA removal rates, which ranged from 83 to 91%, were not proportional to the bacterial activity inhibition rate. Hence, the transformation products of SAs produced by P. ostreatus retained its antimicrobial activity.
Identification of SMX transformation products by laccase
Commercial laccase was tested to degrade SMX in order to determine the role of the main ligninolytic enzyme in the mycodegradation mechanism of SMX. The most important transformation products obtained after SMX oxidation by laccase were identified by QTOF-MS (Fig. S2). The parental compound (SMX) with the molecular ion [M + H]+ of m/z 254.0596 as well as the characteristic SMX transformation product corresponding to 3-amino-5-methylisoxazole (m/z 99.0557) protonated ion were observed. Other transformation products common to the aminobenzenesulfonamide moiety were identified as p-aminobenzenesulfone (m/z 156.0117), aniline (m/z 92.0591) or its typical transformation product, p-aminophenol (m/z 108.0441). Hence, laccase has a clear role in the biochemical mechanisms of SMX mycodegradation. The biodegradation pathway of SMX by laccase is schematized in Fig. 3.
Removal of sulfonamides from wastewater by biofilter
The laccase activity and the percentage of SAs removal from real wastewater by the biofilter are shown in Fig. 4. Laccase was produced at high rates by P. ostreatus growing on a sterilized SMS based on wheat straw, mixed with MEA (SMS-MEA), reaching 510 U L−1. The increment of laccase activity was sustained with growth over time. Meanwhile, MnP exhibited a noticeably lower activity, as had been observed in the previous liquid medium experiments, and an irregular pattern reaching a peak of activity of 2.06 U L−1 in 24 h.
The P. ostreatus biofilter showed high removal rates of SAs from real urban wastewater in a short period (24 h). SM and SX were not detected after wastewater treatment by the P. ostreatus biofilter at any sampling time denoting excellent rates of removal (100%). SMX and SP showed high removal rates from the first sampling time. After 4 h of wastewater treatment, the removal rate of both SAs reached the maximum with the final removal of SMX and SP of 93% and 95%, respectively, over a 24-h period (Fig. 3). SMX and SP fitted well with the pseudo-first-order kinetic model, showing short half-lives of 1.3 h and 0.5 h, respectively (Table 2). SP presented a higher kinetic constant of 15.163 h−1 than SMX with a constant of 0.5350 h−1 after P. ostreatus biofilter performance. In addition, laccase activity and SP and SMX removal rates (Fig. 4) exhibited a statistically significant negative correlation, with the Pearson correlation coefficient value of − 0.71 (p < 0.05). Initially, wastewater showed a HQSMX of 15.7, which was reduced to 0.2 by the P. ostreatus biofilter; meanwhile, HQSP was reduced from 2.7 to 0.5.
The roles of SMS-MEA and P. ostreatus in the biofilter were determined by an abiotic control. The filter without P. ostreatus reached a percentage removal of 48% and 77% for SMX and SP, respectively, and the complete removal of SM and SX after 1 h treatment. At the same time, the P. ostreatus biofilter reached the same removal of SM and SX, but higher removal rates of SMX and SP reaching 70% and 89%, respectively, demonstrating the quick adsorption-mycodegradation ability of the biofilter with P. ostreatus. After 24 h, P. ostreatus removed 95% and 93% of SP and SMX whilst the abiotic control adsorbed 81% and 87%, respectively. Based on a mass balance, the adsorption process on SMS-MEA was responsible for the removal of 91.9% and 85.6% of SP and SMX, respectively, whereas P. ostreatus was responsible for the removal of 8.1% and 14.4% of SP and SMX, respectively.
Discussion
The presence of antibiotics in wastewater is a huge risk to the environment and the population (Berendonk et al. 2015). This concern has led to the search for new ways to remove them from wastewater and has included a wide variety of strategies including chemical, physical and biological methods (Ben et al. 2018). Among the new biological strategies to remove antibiotics from wastewater, ligninolytic fungi are showing promise in biotechnology due to their high degradation and adsorption capacity and the low-cost source of the raw material (Asif et al. 2017a; Dąbrowska et al. 2018; Gao et al. 2018).
Removal of sulfonamides by P. ostreatus and laccase
The growth of P. ostreatus was not affected by the high concentration of SAs in the liquid media. In the same vein, de Araujo et al. (2017) reported an increment in the growing speed of P. ostreatus in the presence of SMX and trimethoprim. However, by contrast, Migliore et al. (2012) described a decrease in the growth rate of P. ostreatus in the presence of tetracyclines, illustrating the different effects on fungal growth as a function of the type of antibiotic.
The remaining concentration of SAs in the controls without P. ostreatus after 14 days of incubation was higher than 90% for SDZ and SP and between 60 and 70% for STZ, SMZ and SMX, indicating the different abiotic degradations of these antibiotics. The decrease of the concentration of SAs in the controls could have occurred as a result of their adsorption on glass and abiotic oxidation. By contrast, P. ostreatus treatment effectively removed all the five SAs in the liquid medium (83% and 91%). Thus, P. ostreatus produced an increment in the removal of SAs with respect to the abiotic control of between 49 and 87%. The most recalcitrant SAs in the abiotic control (SDZ and SP) presented the highest increments of removal by P. ostreatus when compared with these controls. The high effectivity of P. ostreatus to remove SAs was achieved although the maximum activity of the ligninolytic enzymes laccase and MnP (45 U L−1 and 8 U L−1, respectively) was not high. This was probably due to the rich nutrient culture medium (ME). Laccase production is highly influenced by culture conditions. However, a high fungal biomass might not correspond with a high laccase production because the production of ligninolytic enzymes is often triggered by limiting concentrations of carbon or nitrogen. Čvančarová et al. (2015) reported good degradation rates for fluoroquinolones despite a lower laccase and MnP activity for P. ostreatus and other ligninolytic fungi in the liquid media. According to Bhattacharya et al. (2013), CYP450 could be the main enzymatic system expressed by Phanerochaete chrysosporium in a rich liquid medium, whilst lignocellulosic supports might trigger extracellular peroxidases. P. ostreatus might exhibit similar behaviour, and the biochemical mechanisms used to biodegrade antibiotics could be based on a combination of extracellular enzymes mainly laccase (Fig. 1), and the intracellular CYP450 system, which might explain why the removal of SAs in the ME media did not have a significant correlation with laccase activity. de Araujo et al. (2017) reported the same lack of correlation between ligninolytic activity and antibiotic removal. By contrast, they observed a positive correlation between fungal growth and antibiotic degradation.
Ligninolytic fungi have complex extracellular and intracellular enzymatic systems. For example, P. ostreatus has 12 isozymes of laccase (Kempken 2013) and a CYP450 system that consists of 153 genes capable of several chemical reactions such as hydroxylation, epoxidation, decarboxylation and aryl transformation (Golan-Rozen et al. 2011). When organic pollutant degradation is carried out in vivo, as in this work, both systems might be involved depending on the culture conditions. Some researchers have found that the inhibition of CYP450 of P. chrysosporium and Pycnoporus sanguineus growing in potato dextrose broth amended with 1-aminobenzotriazole significantly decreased the removal of SMX, whilst it had no significant effect on the removal of other antibiotics such as ciprofloxacin and norfloxacin (Gao et al. 2018). Rodríguez-Rodríguez et al. (2012) have suggested the implication of CYP450 in STZ degradation, but with regard to SP, the situation was not clear. Therefore, the mycodegradation of pharmaceuticals by ligninolytic fungi might be due to the synergetic performance of extracellular, intracellular and mycelium-bound enzymes (Asif et al. 2017b).
However, the fungal adsorption of antibiotics and specifically SAs could be an additional mechanism for the removal of pollutants from water. For instance, Gao et al. (2018) reported the relatively high biosorption of SMX on P. chrysosporium mycelium (20%) whereas SMX biosorption was not detected on P. sanguineus. Hofmann and Schlosser (2016) did not observe any significant SMX adsorption on Phoma sp. due to the low hydrophobicity of SMX. By contrast, they reported biosorption as an important mechanism in the removal of hydrophobic organic pollutants such as triclosan, 17α-athinylestradiol, nonylphenol or bisphenol A, by fungi.
The antibacterial activity of any remaining SAs and their transformation products was tested on a bacterial consortium from a WWTP. This biological assessment is complementary to the chemical analysis of effluents and is mandatory to perform a complete assessment of the effectiveness of the antibiotic degradation process. Despite the high biodegradation of SAs of between 83 and 91%, the remaining SAs and their transformation products led to a 50% decrease in antibacterial activity. This residual antibacterial activity was probably due to the incomplete degradation of the active site. The partial decrease in antibacterial activity has been described in other works of antibiotic degradation by fungi, ligninolytic enzymes or even enzyme-redox mediator systems (Čvančarová et al. 2015; Rahmani et al. 2015; de Araujo et al. 2017; García-Delgado et al. 2018).
The role of laccase enzyme in the mycodegradation of SAs was supported by the in vitro assay (see section “Degradation of SMX by commercial laccase and identification of the transformation products”) to investigate the transformation of SAs with purified laccase as suggested by Čvančarová et al. (2015). The enzymatic oxidation of SMX by laccase for 24 h yielded 4 transformation products; the typical transformation products of SAs were identified and are shown in Figs. S2 C, D and E, and the characteristic transformation product of SMX is shown in Fig. S2 B, which is in agreement with the results of other researchers (Majewsky et al. 2015; Huynh and Reinhold 2019; Reis et al. 2020). The proposed degradation pathway is in Fig. 3. The transformation product 3-amino-5-methylisoxazole (Fig. 3(B)), characteristic of SMX, was reported as a stable transformation product (Eibes et al. 2011) without antibacterial activity (Reis et al. 2020). Hence, laccase collaborates in the reduction of antibacterial activity of SMX and its transformation products. The degradation of the aminobenzenesulfonamide moiety yielded three degradation products (Fig. 3(C–E)), which are potentially common to all the SAs. The production of p-aminophenol could be performed by substitution of the sulfonamide group by a hydroxyl group. The transformation of aniline into p-aminophenol by laccase is impaired. Although aniline is a putative laccase substrate, aniline is not susceptible to direct oxidation by laccase due to its high ionization energy (7.72 eV) (NIST 2018) with respect to the ionization potential of laccase (≤ 7.45 eV) (Haritash and Kaushik 2009).
The key role of laccase in the removal of SMX and its transformation products is clear. Previous works have reported the importance of laccase and MnP in antibiotic mycodegradation (Prieto et al. 2011; Čvančarová et al. 2015; Alharbi et al. 2019). Hence, in view of present and previous works, mycodegradation processes able to produce abundant and constant ligninolytic activity have a greater possibility of achieving successful antibiotic degradation than those processes with low ligninolytic activity.
Assessment of P. ostreatus biofilter to remove sulfonamides from wastewater
The P. ostreatus biofilter was based on SMS as a way to reuse this abundant and cheap agricultural waste and assure a substrate compatible with the fungus. SMS has valuable microbiota for the biodegradation of organic pollutants, including antibiotics (Chang et al. 2018b). However, in this work, the SMS was previously sterilized in order to eliminate its microbiota and subsequently inoculate it with the same fungal strain used in the degradation assay of SAs in a liquid medium. Therefore, the role of SMS in the biofilter with respect to P. ostreatus was purely that of a support and nutrient source.
Real urban wastewater without the addition of antibiotics was used to test the biofilter to ensure that the biofilter could work in the laboratory conditions, but with a real matrix and SAs concentration. This is important because the chemical and biological composition of real wastewater could interact with the biofilter, reducing its effectivity in comparison with artificial effluent. For example, the fungal growth or enzymatic activity could be negatively affected by real wastewater (Asif et al. 2017b). Laccase activity was appreciable from the first sampling time, reaching a high and constant laccase activity after 6 h (Fig. 3). The presence of lignin in the SMS could induce and increase the production of laccase with respect to the previous assay liquid media (Kunamneni et al. 2007). The activity of P. ostreatus and the adsorption capacity of the biofilter resulted in an effective removal rate of SAs of between 93 and100% in 24 h. The role of laccase in this process was suggested by the significant negative correlation between the removal rates of SMX and SP and laccase activity (R2 = − 0.71 and − 0.64, p < 0.05). Gao et al. (2018) correlated laccase activity with SMX removal, using crude laccase from P. sanguineus, especially when a redox mediator such as ABTS was added which resulted in the complete removal of SMX within 12 h. SAs and other antibiotics or organic pollutants are usually degraded at higher rate by adding redox mediators (Yang et al. 2017; Navada and Kulal 2019). In this work, the biofilter worked in the absence of redox mediators with optimal results. This is a very important fact because it minimized the use of additional chemicals with a subsequent reduction in cost and operational optimization.
The role of SMS-MAE in the removal of SAs was clear because more than 85% SP and SMX adsorbed on it. The adsorption of SAs is not easy due to the anionic nature of SAs in environmental conditions (Reis et al. 2020). Zhou et al. (2016) described SMS as an effective adsorbent for the removal of trace concentrations of SAs with a higher SAs adsorption capacity than recognized adsorbents such as activated carbon. Frutos et al. (2016) reported that more than 80% of the organic carbon from the SMS of P. ostreatus is in the form of O-alkyl groups, such as carbohydrates. This fact is reasonable because the basic component of P. ostreatus SMS is wheat straw. The high carbohydrate content of P. ostreatus SMS could favour the interaction of hydrogen bonds between SAs and SMS and result in the high adsorption ability of this biofilter. García-Delgado et al. (2020) reported the importance of O-alkyl groups in enhancing the hysteresis of organic pollutants.
Adsorption of SAs provided a suitable environment for fungal enzymatic performance. The fungi grown on SMS-MAE secreted laccase to degrade the lignocellulosic compounds from straw, and as a result, laccase also degraded the adsorbed and dissolved SAs. In addition to this process, some lignin degradation products can act as laccase mediators (Zheng et al. 2019), enhancing the oxidation of the adsorbed and dissolved molecules of SAs.
Different kinds of dispositive based on ligninolytic fungi have been tested to evaluate the removal of different pharmaceuticals from the effluents of WWTPs, and some of these have also been based on P. ostreatus. Křesinová et al. (2018) tested the capacity of P. ostreatus to remove endocrine disruptors from spiked wastewater, achieving removal rates of 41% in 3 h on a laboratory scale and 78% in 2 h on a pilot bioreactor scale. Palli et al. (2017) assessed a fluidized bed bioreactor with P. ostreatus to reclaim hospital wastewater containing three pharmaceuticals: atenolol, diclofenac and ketoprofen. They obtained the complete removal of diclofenac in 18 h, 36% of ketoprofen and 8% of atenolol over a period of 42 h.
Conclusions
The growth of the ligninolytic fungus P. ostreatus was not negatively affected by sulfonamides. This fungus was able to remove sulfonamides at high rates by combining different mechanisms. The removal of sulfonamides produced a reduction in antibacterial activity. However, this reduction was lower than the percentage of sulfonamides removed, suggesting the antibacterial activity of the sulfonamide transformation products. The biofilter based on P. ostreatus and its own spent substrate is an effective and sustainable way to reclaim wastewater and reuse this abundant agricultural waste. The device can remove sulfonamides at rates higher than 90% from real wastewater by combining adsorption and mycodegradation processes.
Data availability
Data are available under request to the corresponding author.
Abbreviations
- GI%:
-
Percentage of bacterial growth inhibition
- HQ:
-
Hazard quotient
- IU:
-
Unit of enzyme activity
- ME:
-
Malt extract
- MEA:
-
Malt extract agar
- MnP:
-
Mn peroxidase
- OD600 :
-
Optical density at 600 nm
- PNEC:
-
Predicted no-effect concentration
- SAs:
-
Sulfonamides
- SDZ:
-
Sulfadiazine
- SM:
-
Sulfamerazine
- SMS:
-
Spent mushroom substrate
- SMX:
-
Sulfamethoxazole
- SMZ:
-
Sulfamethazine
- SP:
-
Sulfapyridine
- STZ:
-
Sulfathiazole
- SX:
-
Sulfamonomethoxine
- WWTP:
-
Wastewater treatment plant
References
Alharbi SK, Nghiem LD, van de Merwe JP, Leusch FDL, Asif MB, Hai FI, Price WE (2019) Degradation of diclofenac, trimethoprim, carbamazepine, and sulfamethoxazole by laccase from Trametes versicolor: transformation products and toxicity of treated effluent. Biocatal Biotransformation 37:399–408. https://doi.org/10.1080/10242422.2019.1580268
Álvarez-Martín A, Rodríguez-Cruz MS, Andrades MS, Sánchez-Martín MJ (2016) Application of a biosorbent to soil: a potential method for controlling water pollution by pesticides. Environ Sci Pollut Res 23:9192–9203. https://doi.org/10.1007/s11356-016-6132-4
de Araujo CAV, Maciel GM, Rodrigues EA, Silva LL, Oliveira RF, Brugnari T, Peralta RM, de Souza CGM (2017) Simultaneous removal of the antimicrobial activity and toxicity of sulfamethoxazole and trimethoprim by white rot fungi. Water Air Soil Pollut 228. https://doi.org/10.1007/s11270-017-3525-z
Asif MB, Hai FI, Hou J, Price WE, Nghiem LD (2017a) Impact of wastewater derived dissolved interfering compounds on growth, enzymatic activity and trace organic contaminant removal of white rot fungi – a critical review. J Environ Manag 201:89–109. https://doi.org/10.1016/j.jenvman.2017.06.014
Asif MB, Hai FI, Singh L, Price WE, Nghiem LD (2017b) Degradation of pharmaceuticals and personal care products by white-rot fungi—a critical review. Curr Pollut Reports 3:88–103. https://doi.org/10.1007/s40726-017-0049-5
Ben W, Zhu B, Yuan X, Zhang Y, Yang M, Qiang Z (2018) Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: comparison of wastewater treatment processes. Water Res 130:38–46. https://doi.org/10.1016/j.watres.2017.11.057
Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons MN, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez JL (2015) Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol 13:310–317. https://doi.org/10.1038/nrmicro3439
Bhattacharya SS, Syed K, Shann J, Yadav JS (2013) A novel P450-initiated biphasic process for sustainable biodegradation of benzo[a]pyrene in soil under nutrient-sufficient conditions by the white rot fungus Phanerochaete chrysosporium. J Hazard Mater 261:675–683. https://doi.org/10.1016/j.jhazmat.2013.07.055
Carvalho IT, Santos L (2016) Antibiotics in the aquatic environments: a review of the European scenario. Environ Int 94:736–757. https://doi.org/10.1016/j.envint.2016.06.025
Chang B-V, Fan S-N, Tsai Y-C, Chung YL, Tu PX, Yang CW (2018a) Removal of emerging contaminants using spent mushroom compost. Sci Total Environ 634:922–933. https://doi.org/10.1016/J.SCITOTENV.2018.03.366
Chang BV, Fan SN, Tsai YC, Chung YL, Tu PX, Yang CW (2018b) Removal of emerging contaminants using spent mushroom compost. Sci Total Environ 634:922–933. https://doi.org/10.1016/j.scitotenv.2018.03.366
Conde-Cid M, Álvarez-Esmorís C, Paradelo-Núñez R, Nóvoa-Muñoz JC, Arias-Estévez M, Álvarez-Rodríguez E, Fernández-Sanjurjo MJ, Núñez-Delgado A (2018) Occurrence of tetracyclines and sulfonamides in manures, agricultural soils and crops from different areas in Galicia (NW Spain). J Clean Prod 197:491–500. https://doi.org/10.1016/j.jclepro.2018.06.217
Čvančarová M, Moeder M, Filipová A, Cajthaml T (2015) Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi - metabolites, enzymes and residual antibacterial activity. Chemosphere 136:311–320. https://doi.org/10.1016/j.chemosphere.2014.12.012
Dąbrowska M, Muszyńska B, Starek M, Żmudzki P, Opoka W (2018) Degradation pathway of cephalosporin antibiotics by in vitro cultures of Lentinula edodes and Imleria badia. Int Biodeterior Biodegrad 127:104–112. https://doi.org/10.1016/j.ibiod.2017.11.014
Di Gregorio S, Becarelli S, Siracusa G et al (2016) Pleurotus ostreatus spent mushroom substrate for the degradation of polycyclic aromatic hydrocarbons: the case study of a pilot dynamic biopile for the decontamination of a historically contaminated soil. J Chem Technol Biotechnol 91:1654–1664. https://doi.org/10.1002/jctb.4936
Eibes G, Debernardi G, Feijoo G, Moreira MT, Lema JM (2011) Oxidation of pharmaceutically active compounds by a ligninolytic fungal peroxidase. Biodegradation 22:539–550. https://doi.org/10.1007/s10532-010-9426-0
European Medicines Agency (2019) European Surveillance of Veterinary Antimicrobial Consumption, 2019. ‘Sales of veterinary antimicrobial agents in 31 European countries in 2017’. (EMA/294674/2019)
Frutos I, García-Delgado C, Gárate A, Eymar E (2016) Biosorption of heavy metals by organic carbon from spent mushroom substrates and their raw materials. Int J Environ Sci Technol 13:2713–2720. https://doi.org/10.1007/s13762-016-1100-6
Gao N, Liu CX, Xu QM, Cheng JS, Yuan YJ (2018) Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere 195:146–155. https://doi.org/10.1016/j.chemosphere.2017.12.062
García-Delgado C, Alfaro-Barta I, Eymar E (2015a) Combination of biochar amendment and mycoremediation for polycyclic aromatic hydrocarbons immobilization and biodegradation in creosote-contaminated soil. J Hazard Mater 285:259–266. https://doi.org/10.1016/j.jhazmat.2014.12.002
García-Delgado C, D’Annibale A, Pesciaroli L, Yunta F, Crognale S, Petruccioli M, Eymar E (2015b) Implications of polluted soil biostimulation and bioaugmentation with spent mushroom substrate (Agaricus bisporus) on the microbial community and polycyclic aromatic hydrocarbons biodegradation. Sci Total Environ 508:20–28. https://doi.org/10.1016/j.scitotenv.2014.11.046
García-Delgado C, Eymar E, Camacho-Arévalo R, Petruccioli M, Crognale S, D'Annibale A (2018) Degradation of tetracyclines and sulfonamides by stevensite- and biochar-immobilized laccase systems and impact on residual antibiotic activity. J Chem Technol Biotechnol 93:3394–3409. https://doi.org/10.1002/jctb.5697
García-Delgado C, Marín-Benito JM, Sánchez-Martín MJ, Rodríguez-Cruz MS (2020) Organic carbon nature determines the capacity of organic amendments to adsorb pesticides in soil. J Hazard Mater 390:122162. https://doi.org/10.1016/j.jhazmat.2020.122162
Gea FJ, Carrasco J, Diánez F, Santos M, Navarro MJ (2014) Control of dry bubble disease (Lecanicillium fungicola) in button mushroom (Agaricus bisporus) by spent mushroom substrate tea. Eur J Plant Pathol 138:711–720. https://doi.org/10.1007/s10658-013-0344-y
Golan-Rozen N, Chefetz B, Ben-Ari J, Geva J, Hadar Y (2011) Transformation of the recalcitrant pharmaceutical compound carbamazepine by pleurotus ostreatus: role of cytochrome P450 monooxygenase and manganese peroxidase. Environ Sci Technol 45:6800–6805. https://doi.org/10.1021/es200298t
Halling-Sørensen B, Sengeløv G, Tjørnelund J (2002) Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch Environ Contam Toxicol 42:263–271. https://doi.org/10.1007/s00244-001-0017-2
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15. https://doi.org/10.1016/j.jhazmat.2009.03.137
Hofmann U, Schlosser D (2016) Biochemical and physicochemical processes contributing to the removal of endocrine-disrupting chemicals and pharmaceuticals by the aquatic ascomycete Phoma sp. UHH 5-1-03. Appl Microbiol Biotechnol 100:2381–2399. https://doi.org/10.1007/s00253-015-7113-0
Huynh K, Reinhold D (2019) Metabolism of sulfamethoxazole by the model plant Arabidopsis thaliana. Environ Sci Technol 53:4901–4911. https://doi.org/10.1021/acs.est.8b06657
Kempken F (2013) The Mycota. Springer Nature, Berlin
Křesinová Z, Linhartová L, Filipová A, Ezechiáš M, Mašín P, Cajthaml T (2018) Biodegradation of endocrine disruptors in urban wastewater using Pleurotus ostreatus bioreactor. New Biotechnol 43:53–61. https://doi.org/10.1016/j.nbt.2017.05.004
Kunamneni A, Ballesteros A, Plou FJAM (2007) Fungal laccase—a versatile enzyme for biotechnological applications. Commun Curr Res Educ Top Trends Appl Microbiol 1:233–245
Lucas D, Barceló D, Rodriguez-Mozaz S (2016) Removal of pharmaceuticals from wastewater by fungal treatment and reduction of hazard quotients. Sci Total Environ 571:909–915. https://doi.org/10.1016/j.scitotenv.2016.07.074
Majewsky M, Glauner T, Horn H (2015) Systematic suspect screening and identification of sulfonamide antibiotic transformation products in the aquatic environment. Anal Bioanal Chem 407:5707–5717. https://doi.org/10.1007/s00216-015-8748-5
Michael I, Rizzo L, McArdell CS et al (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47:957–995. https://doi.org/10.1016/j.watres.2012.11.027
Migliore L, Fiori M, Spadoni A, Galli E (2012) Biodegradation of oxytetracycline by Pleurotus ostreatus mycelium: a mycoremediation technique. J Hazard Mater 215–216:227–232. https://doi.org/10.1016/j.jhazmat.2012.02.056
Mohd Hanafi FH, Rezania S, Mat Taib S, Md Din MF, Yamauchi M, Sakamoto M, Hara H, Park J, Ebrahimi SS (2018) Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): an overview. J Mater Cycles Waste Manag 20:1383–1396. https://doi.org/10.1007/s10163-018-0739-0
Navada KK, Kulal A (2019) Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int Biodeterior Biodegrad 138:63–69. https://doi.org/10.1016/j.ibiod.2018.12.012
NIST (2018) WebBook of chemistry. In: Natl. Inst. Stand. Technol. (USA. https://webbook.nist.gov/cgi/cbook.cgi?ID=C62533&Mask=20
Palli L, Castellet-Rovira F, Pérez-Trujillo M, Caniani D, Sarrà-Adroguer M, Gori R (2017) Preliminary evaluation of Pleurotus ostreatus for the removal of selected pharmaceuticals from hospital wastewater. Biotechnol Prog 33:1529–1537. https://doi.org/10.1002/btpr.2520
Picó Y, Alvarez-Ruiz R, Alfarhan AH, el-Sheikh MA, Alobaid SM, Barceló D (2019) Uptake and accumulation of emerging contaminants in soil and plant treated with wastewater under real-world environmental conditions in the Al Hayer area (Saudi Arabia). Sci Total Environ 652:562–572. https://doi.org/10.1016/j.scitotenv.2018.10.224
Prieto A, Möder M, Rodil R, Adrian L, Marco-Urrea E (2011) Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour Technol 102:10987–10995. https://doi.org/10.1016/j.biortech.2011.08.055
Rahmani K, Faramarzi MA, Mahvi AH, Gholami M, Esrafili A, Forootanfar H, Farzadkia M (2015) Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int Biodeterior Biodegrad 97:107–114. https://doi.org/10.1016/j.ibiod.2014.10.018
Reis AC, Kolvenbach BA, Nunes OC, Corvini PFX (2020) Biodegradation of antibiotics: the new resistance determinants – part I. New Biotechnol 54:34–51. https://doi.org/10.1016/j.nbt.2019.08.002
Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360. https://doi.org/10.1016/j.scitotenv.2013.01.032
Rodríguez-Rodríguez CE, Jesús García-Galán M, Blánquez P, Díaz-Cruz MS, Barceló D, Caminal G, Vicent T (2012) Continuous degradation of a mixture of sulfonamides by Trametes versicolor and identification of metabolites from sulfapyridine and sulfathiazole. J Hazard Mater 213–214:347–354. https://doi.org/10.1016/j.jhazmat.2012.02.008
Santás-Miguel V, Arias-Estévez M, Díaz-Raviña M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A, Fernández-Calviño D (2020) Effect of oxytetracycline and chlortetracycline on bacterial community growth in agricultural soils. Agronomy 10:1011. https://doi.org/10.3390/agronomy10071011
Schwarz J, Aust MO, Thiele-Bruhn S (2010) Metabolites from fungal laccase-catalysed transformation of sulfonamides. Chemosphere 81:1469–1476. https://doi.org/10.1016/j.chemosphere.2010.08.053
Sharma VK, Johnson N, Cizmas L, McDonald TJ, Kim H (2016) A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 150:702–714. https://doi.org/10.1016/j.chemosphere.2015.12.084
Siracusa G, Becarelli S, Lorenzi R, Gentini A, di Gregorio S (2017) PCB in the environment: bio-based processes for soil decontamination and management of waste from the industrial production of Pleurotus ostreatus. New Biotechnol 39:232–239. https://doi.org/10.1016/j.nbt.2017.08.011
Summa S, Lo Magro S, Armentano A, Muscarella M (2015) Development and validation of an HPLC/DAD method for the determination of 13 sulphonamides in eggs. Elsevier Ltd
Tran NH, Reinhard M, Gin KYH (2018) Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res 133:182–207. https://doi.org/10.1016/j.watres.2017.12.029
Van der Ven A, Mantel M, Vree T et al (1994) Formation and elimination of sulphamethoxazole hydroxylamine after oral administration of sulphamethoxazole. Br J Clin Pharmacol 38:147–150. https://doi.org/10.1111/j.1365-2125.1994.tb04339.x
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment—a review. Sci Total Environ 429:123–155
Wariishi H, Valli K, Gold MH (1992) Manganese (II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem 267(33):23688–23695
Yang J, Li W, Bun Ng T et al (2017) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.00832
Zhao F, Yang L, Chen L, Li S, Sun L (2019) Bioaccumulation of antibiotics in crops under long-term manure application: occurrence, biomass response and human exposure. Chemosphere 219:882–895. https://doi.org/10.1016/j.chemosphere.2018.12.076
Zheng Y, Guo M, Zhou Q, Liu H (2019) Effect of lignin degradation product sinapyl alcohol on laccase catalysis during lignin degradation. Ind Crop Prod 139:111544. https://doi.org/10.1016/j.indcrop.2019.111544
Zhou A, Zhang Y, Li R, Su X, Zhang L (2016) Adsorptive removal of sulfa antibiotics from water using spent mushroom substrate, an agricultural waste. Desalin Water Treat 57:388–397. https://doi.org/10.1080/19443994.2014.979239
Acknowledgements
This work was supported by the Ministry of Science and Innovation of Spain (Project AGL2016-78490-R). C.G.D. thanks the Ministry of Economy and Competitiveness of Spain by his Juan de la Cierva-Formación (JCFI-2015-23543) contract. The authors thank the Laboratorio Regional de Salud Pública de la Comunidad de Madrid for their analytical support.
Funding
This work was supported by the Ministry of Science and Innovation of Spain (Project AGL2016-78490-R). Carlos García-Delgado was contracted by the Ministry of Economy and Competitiveness of Spain (Grant: JCFI-2015-23543).
Author information
Authors and Affiliations
Contributions
Conceptualization: Enrique Eymar, Carlos García-Delgado, Begoña Mayans and Raquel Camacho-Arévalo. Methodology: Enrique Eymar, Carlos García-Delgado, Begoña Mayans and Raquel Camacho-Arévalo. Formal analysis and investigation: Carlos García-Delgado, Begoña Mayans, Raquel Camacho-Arévalo, Rafael Antón-Herrero and Maria Luz Segura. Writing and original draft preparation: Begoña Mayans, Carlos García-Delgado and Raquel Camacho-Arévalo. Writing and review and editing: Enrique Eymar, Carlos García-Delgado, Begoña Mayans, Raquel Camacho-Arévalo and Consuelo Escolástico. Funding acquisition: Enrique Eymar. Supervision: Enrique Eymar.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
All authors whose names appear on the submission approve the Ethical Responsibilities of Authors of Environmental Science and Pollution Research.
Consent to publish
All authors whose names appear on the submission (1) made substantial contributions to the conception or design of the work or to the acquisition, analysis or interpretation of the data; (2) drafted the work or revised it critically for important intellectual content; (3) approved the version to be published; and (4) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 174 kb)
Rights and permissions
About this article
Cite this article
Mayans, B., Camacho-Arévalo, R., García-Delgado, C. et al. An assessment of Pleurotus ostreatus to remove sulfonamides, and its role as a biofilter based on its own spent mushroom substrate. Environ Sci Pollut Res 28, 7032–7042 (2021). https://doi.org/10.1007/s11356-020-11078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11078-3