Abstract
Dry bubble (caused by Lecanicillium fungicola) is a widespread disease of button mushroom. The objective of the experiments was to determine the efficacy of compost teas made from spent mushroom substrate (SMS) as a biocontrol method against the disease. All SMS teas produced in this study significantly inhibited (100 %) the in vitro mycelial growth of L. fungicola, whereas the fungicide prochloraz at 50 ppm inhibited growth by 91 %. The in vivo effectiveness of two SMS aerated teas, one with mineral soil (MS) and the other with peat (TPT), was evaluated in two mushroom cropping trials inoculated with L. fungicola. The results demonstrated that the most effective treatments were those with TPT applied close to harvest and/or those with the greatest number of applications. The most efficacious treatments were TPT treatments (reducing disease by 34 to 73 % in the two trials, compared to the inoculated control). In contrast, prochloraz reduced disease by 7 % and 4 % in the two trials, compared to the control. These results suggest that dry bubble disease can be controlled by the use of spent mushroom substrate teas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dry bubble, caused by the fungus Lecanicillium fungicola (Preuss) Zare & W. Gams (Zare and Gams 2008), is a serious and common disease of white button mushroom [Agaricus bisporus (Lange) Imbach] and is estimated to cause annual losses of 2–4 % (Berendsen et al. 2012a). Disease symptoms include bubbles (undifferentiated spherical masses), bent and/or split stipes known as blowout, and spotty caps (Fletcher and Gaze 2008; Largeteau and Savoie 2008; Berendsen et al. 2010). The pathogen can be spread by means of dust, flies, mites, debris, containers, watering and pickers. Methods of control include the application of fungicides, mainly prochloraz, and strict hygiene measures. However, some data suggest that the sensitivity of L. fungicola to prochloraz is gradually diminishing (Gea et al. 2005; Grogan 2008), and that its persistence in the casing layer falls considerably at the end of the second flush (Grogan and Jukes 2003). Management strategies for fungal pathogens include reinforcing hygiene practices, the search for new biological control methods and breeding for resistance. In this sense, lemon verbena (Lippia citriodora), oregano (Origanum vulgare) and thyme (Thymus vulgaris) oils and their derivatives may be used as an alternative to synthetic chemicals, but more detailed strategies need to be developed for practical application (Soković and van Griensven 2006; Regnier and Combrinck 2010). On the other hand, potential sources of resistance to dry bubble have been identified in wild types of A. bisporus, but complete resistance has not been found (Dragt et al 1995; Largeteau et al 2005). This partial resistance to L. fungicola in A. bisporus var. burnettii is governed by several QTLs, with the environment also influencing susceptibility (Foulongne-Oriol et al. 2012).
Several studies in recent years have indicated that plant diseases can be suppressed by applying a variety of water-based composts of agricultural wastes. Among such preparations, non-aerated compost teas (NCT) and aerated compost teas (ACT) (Scheuerell and Mahaffee 2002), fermented aqueous extracts of composted materials, have been proposed as potential alternatives to the use of synthetic chemicals for the control of foliar pathogens (Weltzien 1991; McQuilken et al. 1994; Yohalem et al. 1994; Cronin et al. 1996; Segarra et al. 2009; Siddiqui et al. 2009; Koné et al. 2010). Compost teas have also been shown to suppress soil-borne diseases (Scheuerell and Mahaffee 2004; Sang et al. 2010; Alfano et al. 2011; Dionné et al. 2012).
Spent mushroom substrate (SMS), a by-product of the commercial production of button mushrooms, consists of mushroom compost and casing materials, and has the benefit of having disease suppressive characteristics which protect plants from pathogens (Hoitink and Fahy 1986; Hoitink et al. 1997; Yohalem et al. 1994, 1996; Cronin et al. 1996). The mushroom industry in the EU produces more than 3.5 × 106 tonnes of SMS annually, while Spain alone produces more than 5 × 105 tonnes. Most of the SMS collected in Castilla-La Mancha (Spain) is comprised of casing with mineral soils of different origins (from the arable layer as sub-soil), to which peat is added in a low proportion as a bulking agent to improve the water holding capacity (WHC) (Pardo-Giménez and Pardo-González 2008), although another type of SMS, based on casings containing peat alone, is sometimes collected.
To date, teas made from SMS have not been used for the biological control of mushroom diseases, although several preliminary studies have suggested that they might be effective. Favourable results for the control of L. fungicola have been obtained in vitro with grape marc aerated teas (Diánez et al. 2006), and teas made from SMS mixed with amended light peat (Gea et al. 2009). In a recent report, Gea et al. (2012) showed that the SMS tea did not have an inhibitory effect on A. bisporus mycelial growth, and quantitative production parameters were not significantly affected by the SMS treatments. These preliminary studies suggest that SMS teas can be considered a suitable biocontrol agent for the control of dry bubble disease.
The aims of this study were to test the in vitro efficacy of teas made from different SMS teas types against L. fungicola and to test the efficacy of these SMS teas for controlling dry bubble disease in mushroom crops inoculated with L. fungicola.
Materials and methods
Preparation of SMS teas
The two SMSs used in the experiments were obtained from two mushroom growing crops located in Castilla-La Mancha (Spain): one SMS in which mineral soil + Sphagnum peat 4:1 (v/v) was used as the casing layer, named ‘mineral soil’ (MS), and one SMS with a casing based on Topterra®, named ‘peat’ (TPT). These SMSs were given a thermal treatment (70 °C, 12 h) to eliminate any pathogens, and matured for 2 months following the method described by Lohr et al. (1984). The physical and chemical properties of the SMS were characterised after re-composting (Table 1).
The teas were prepared by mixing SMS and water in a 1:4 (w/v) ratio. The mixtures were incubated for 1 day at 25 °C with stirring (aerated compost tea, ACT) and without stirring (non-aerated compost tea, NCT) (Scheuerell and Mahaffee 2002). Each mixture was filtered through two layers of muslin. All SMS teas were used within 24 h of preparation.
Microbial analysis of compost teas
Microbiological analyses of ACT and NCT were performed using the dilution plate technique (Wakelin et al. 1998), according to Koné et al. (2010). The populations of bacteria (total and pseudomonads), fungi, yeast and actinomycetes were counted on different selective culture media. Total bacteria were grown on Tryptic Soy Agar (Sigma–Aldrich) and total pseudomonads on King’s B Medium (King et al. 1954). Fungi were grown on Malt Agar (Becton Dickinson, Sparks, MD, USA) amended with 0.3 g/l streptomycin (Sigma–Aldrich, St. Louis, MO, USA). Yeasts were cultured on Yeast culture medium (YPD) (Cultimed, Panreac, Barcelona, Spain). Actinomycetes were cultured on Actinomycete Isolation Agar (Becton Dickinson) amended with glycerol (10 g/l; Sigma-Aldrich).
Each ACT and NCT was serially diluted (10−2–10−7) and 100 μl of each dilution were plated on the selective media. After an incubation period of 2–6 days at 25 °C, colony forming units (cfu) per plate were counted to estimate the different microbial populations on each selective and non-selective medium. Each test had five replicates.
In vitro effect of SMS teas on mycelium growth of Lecanicillium fungicola
To determine the effect of SMS teas on the mycelial growth of L. fungicola, the NCT or ACT was incorporated into sterile malt extract agar (MEA) medium cooled to 45 °C. The SMS teas were mixed with the cooled MEA medium in two proportions, 10 and 20 % v/v, and immediately poured into the Petri dishes. Two controls (A10 and A20) were also prepared with MEA and sterile water (10 and 20 %, v/v) and a positive control (P50) with the same agar and the fungicide prochloraz 46 % (Sporgon®, Basf Española, Barcelona, Spain), giving a final concentration of 50 ppm of active ingredient (a.i.).
For each tea treatment and control, five Petri dishes were inoculated in the centre with a 5 mm diameter agar plug taken from a culture of L. fungicola, and incubated in the dark at 20 °C for 12 days. Two different isolates (V1, V2) of L. fungicola, collected from two Castilla-La Mancha (Spain) mushroom farms in 2006 and 2007, were independently used per treatment and for the control. Perpendicular colony diameters were measured on each dish after incubation. The results were expressed as percentage inhibition of mycelium growth of the L. fungicola isolates for each of the treatments assayed compared to mycelium growth obtained on the sterile water control.
Efficacy of SMS teas in a mushroom crop inoculated with Lecanicillium fungicola
Two cropping trials (I and II) were placed in experimental mushroom growing rooms, according to the standard practices used in mushroom farms in Spain. A. bisporus was cultivated in experimental trays (16 L in volume, 0.09 m2 in area) filled with 6 kg of commercial mushroom compost spawned at 1 % (Gurelan 45 strain, Gurelan S. Coop., Huarte, Pamplona, Spain). Spawn-run lasted 15 days in the cropping room set at 25 °C and 95 % relative humidity (RH). On day 0 of the cropping cycle, trays of spawn-run compost were cased with a 30 mm layer of a casing soil (2.6 L/tray) made with mineral soil + Sphagnum peat 4:1 (v/v). The environmental conditions were set so that the compost temperature was 26 °C, with a RH of 95 % and a CO2 concentration >2,000 ppm (with no ventilation). Seven days after casing, the surface of the casing soil was ruffled deeply. Two days later, the growing rooms were ventilated with filtered fresh air to stimulate the production of mushroom fruiting bodies, by a gradual reduction of temperature and humidity over a 3-day period to 18 °C and 85–90 % RH. A temperature of 17.5 °C and RH of 85–90 % were maintained throughout cropping. Irrigation of the cultures commenced when sporophores had reached pea size.
One day after casing (day 1), a conidial suspension of L. fungicola isolate V1 was sprayed onto the surface of the casing layer (10 ml/tray, 120 ml/m2) at a rate of 1.15 × 108 conidia/m2. The high concentration of inoculum was designed to cause a high incidence of disease, to create a serious challenge to the crop protection agent. The L. fungicola inoculum was prepared on the day of inoculation and consisted of conidia from a 2-week-old culture on PDA, washed with sterile water before filtering through a polypropylene net filter (25 μm pore size, Millipore®). The concentration of each conidial suspension was determined using a haemocytometer and was adjusted using sterile water to a concentration of 106 conidia/ml. Conidial suspensions were prepared from the same L. fungicola isolate. Control trays were sprayed with 10 ml sterile distilled water.
The two different ACTs (“mineral soil” and “peat”) were applied to the casing mixture at a rate of 100 ml/tray (1.2 L m−2) (Table 2). In trial I, there were four different treatments using each SMS tea: R1 (one drench applied on the same day as the casing material—day 0); R2 (two drenches, applied on days 0 and 3); R3 (three drenches applied on days 0, 3 and 7, before ruffling); and R4 (two drenches applied on days 3 and 7). There were three controls: the first a negative control (C), in which drench applications were 100 ml tap water/tray; the second an inoculated control (CI), each tray receiving a 10 ml conidial spray of L. fungicola, in which irrigation was water alone; and the third, also inoculated, but including the fungicide Sporgon® (prochloraz-Mn 46 % wp) at 0.05 % (w/v) in the third irrigation (P), at a rate of 100 ml/tray. The experiment was a randomised complete block design with six replicates. In trial II, there were three different treatments including each SMS tea: S1 (two drenches applied on days 2 and 4); S2 (three drenches applied on days 2, 4 and 24, between the first and second flushes); and S3 (four drenches applied on days 2, 4, 24 and 31, between the second and third flushes). The same three controls were included in trial II as described for trial I, and as for trial I, the experiment was a randomised complete block design with eight replicates.
The healthy mushrooms and diseased mushrooms (showing symptoms of dry bubbles (spotty cap, stipe blowout and bubbles)) were harvested daily during the three flushes. The numbers and the total weight of the fruiting bodies were recorded for each treatment. Harvested mushrooms were classified as either healthy or infected with L. fungicola. Disease incidence was calculated [(number of diseased sporophores/total number of harvested mushrooms(healthy and diseased)] × 100). SMS tea efficacy was calculated using Abbott’s formula (Abbott 1925): % control(effectiveness) = [(Ic − It)/Ic] × 100 (where Ic = disease incidence in the inoculated control (CI); It = disease incidence in the treated mushrooms) (Gea et al. 2010). The effect of the SMS tea and fungicide treatments was evaluated by comparing the yield with that obtained for the non-treated control. The effect of treatments on the biological efficiency of the crop was calculated as the ratio of the fresh weight of total yield of harvested mushrooms (healthy and diseased) to the weight of dry substrate at spawning and expressing the fraction as kg/100 kg compost. In addition, the earliness (days to first harvest for each treatment) was expressed as the number of days between casing and harvesting of the first flush.
Data analysis
Statistical analyses were done using Statgraphics Plus 5.1 (Statistical Graphics Corp., Princeton, NJ). An analysis of variance (ANOVA) was used to test for the effect of treatment based on relative disease control and the different yield parameters. A Tukey’s means separation test was used to compare means (P = 0.05). Data were analyzed separately for each flush and each trial. Where necessary, log or square-root transformations corrected for heterogeneity of variance, and percentages were arcsine square-root transformed before analysis. Non-normally distributed data were analyzed with a non-parametric Kruskal–Wallis test and a Mann–Whitney U test to confirm the Kruskal–Wallis results. Data are reported as back-transformed means.
Results
Microbial diversity in SMS teas
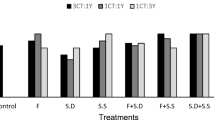
In general, all SMS teas showed large microbial populations—>106 cfu/ml (Table 3). Overall, the populations of total bacteria (F 3, 16 = 0.91, P = 0.4) and pseudomonads (F 3, 8 = 0.74, P = 0.5) did not vary among the four SMS teas, while levels of fungi were significantly higher in the ACT made from mineral soil SMS (F 3, 16 = 10.03, P = 0.0006) compared to the other teas. Teas made from mineral soil SMS provided the highest populations of yeast (F 3, 16 = 23.64, P = 0.0000), while NCT from mineral soil SMS had the lowest population of actinomycetes (F 3, 16 = 6.19, P = 0.005).
In vitro effect of SMS teas on mycelial growth of Lecanicillium fungicola
The diameter of the L. fungicola colonies in the control treatments were: A10 (V1: 35 mm; V2: 37 mm) and A20 (V1: 40 mm; V2: 43 mm). All ACT and NCT treatments gave 100 % inhibition of mycelial growth of both L. fungicola isolates whereas the fungicide prochloraz inhibited growth by 91 % at 50 ppm. There were significant differences between the effect of SMS teas and the addition of prochloraz, both with isolate V1 (F 8, 77 = 3,332.11, P = 0.0000) and V2 (F 8, 79 = 238.21, P = 0.0000).
Efficacy of SMS teas in a mushroom crop inoculated with Lecanicillium fungicola
In the diseased mushrooms harvested in trial I (Table 4), there were significant differences in yield of diseased mushrooms between C and CI at the first flush (F 10, 55 = 4.94, P = 0.0000), second flush (F 10, 55 = 3.87, P = 0.0005), third flush (F 10, 55 = 2.06, P = 0.04) and for total yield (F 10, 55 = 8.13, P = 0.0000). The total yield of diseased mushrooms for the CI treatment was 8.3 kg/m2, which is 47 % of the total production and for the C treatment was 0.1 kg/m2 (less than 1 % of total production). These data show that inoculation with conidia of L. fungicola was appropriate since it led to the appearance of severe dry bubble. The total yield of diseased mushrooms was significantly lower in MS (R4) and all TPT tea treatments compared to the CI treatment. Both TPT (R3) and TPT (R4) had a lower total yield of diseased mushrooms compared to the fungicidal control (P) (F 10, 55 = 8.13, P = 0.0000).
In trial II (Table 4), there were significant differences in yield of diseased mushrooms for C and CI at the first flush (F 8, 63 = 16.14, P = 0.0000), second flush (F 8, 63 = 6.73, P = 0.0000) and for total yield (F 8, 63 = 14.82, P = 0.0000). The total yield of diseased mushrooms for the CI treatment was 7.0 kg/m2, that is 52 % of the total production, and for C treatment was 1.9 kg/m2, 15 % of the total production. The inoculation can be regarded as appropriate, as the CI treatment had a high yield of diseased mushrooms. In the first flush there were significant differences (F 8, 63 = 16.14, P = 0.0000) in the weight of diseased mushrooms between all the treatments using SMS teas and treatment P and treatment CI. These differences in yield of diseased mushrooms were maintained with TPT treatment (S3) in the second (F 8, 63 = 6.73, P = 0.0000) and third (F 8, 63 = 4.12, P = 0.0005) flushes. In the three TPT tea treatments, the total yield of diseased mushrooms was not significantly different from that obtained with the C treatment. All TPT tea treatments provided lower total yields of diseased mushrooms than P (F 8, 63 = 14.82, P = 0.0000).
The biological efficiency (BE) in trial I was higher than in trial II (Table 4). In trial I, there were no significant differences between treatments (F 10, 55 = 1.25, P = 0.3), while in trial II the differences between treatments were significant (F 8, 63 = 4.32, P = 0.0003). Regarding earliness, all treatments in trial I took approximately 23 days (F 10, 49 = 1.79, P = 0.09). However, in trial II, MS (S2) showed a significant delay of 3 days compared to CI (F 8, 61 = 3.06, P = 0.006), but there was no difference among SMS treatments.
The effectiveness of the SMS tea and fungicide treatments assayed against dry bubble (Table 5) was significant for all the treatments in the first flush of trial I (>50 % in all cases) although there were no significant differences among treatments (H = 14.9, P = 0.06). With the second and third flushes, the efficacy of the SMS teas was reduced as was the effect of the fungicide. In terms of total effectiveness, TPT (R3) and TPT (R4) showed significant differences compared with the fungicide treatment (F 8, 45 = 8.57, P = 0.0000).
In trial II, TPT (S1), (S2) and (S3) were the most efficacious treatments, showing significantly better control of dry bubble compared to P at the first flush (F 6, 49 = 5.65, P = 0.0002), second flush (F 6, 49 = 8.48, P = 0.0000), third flush (H = 32.9, P = 0.0000) and in total efficacy (F 6, 49 = 19.64, P = 0.0000). In trial II, the efficacy of the fungicidal treatment at the 1st, 2nd and 3rd flush, and total efficacy, was the same as the MS treatments.
In summary, the most efficacious treatments reducing the incidence of dry bubble disease were those peat-based SMS tea treatments applied closest to the harvest period and/or those which had the highest number of applications.
Discussion
Dry bubble management is based on the application of fungicides and hygiene measures, which have only shown limited control of the disease. Treatment with SMS teas, which are biological control agents, may be an efficacious and economically advantageous way to manage dry bubble disease if used as part of an integrated pest management (IPM) program.
Compost teas are reported to control plant pathogens through single or multiple mechanisms involving microbial antagonism (through antibiosis, parasitism, competition for nutrients and space or induced plant resistance) (Zhang et al. 1998; El-Masry et al. 2002; Al-Mughrabi et al. 2008) or their suppressive physico-chemical properties (Hoitink et al. 1997; Siddiqui et al. 2008). The physical and chemical properties of the nutrients in compost teas may improve the nutritional status of plants, be directly toxic to the pathogen, and/or induce systemic resistance to the pathogen (Koné et al. 2010). In recent work, Marín et al. (2013) studied the physical and chemical properties of several composts, among them spent mushroom compost. ACT produced from SMS had the highest concentrations of sulphates and various cations (K, Ca, Mg, Fe, Cu and Zn), which along with the large microbial population, favours suppressive activity against plant pathogenic microbes.
The microbial populations of compost teas necessary to be considered as suppressive has been discussed by Scheuerell and Mahaffee (2004), who hypothesized that 106 cfu/ml of bacteria represents the threshold value for the transition from non-suppressive to suppressive activity. In general, all SMS teas produced in this study showed large microbial populations (>106 cfu/ml, Table 3) and proved highly suppressive, inhibiting 100 % of mycelial growth in vitro of two isolates of L. fungicola. It should also be borne in mind that the highest populations of actinomycetes shown by the two ACT teas may have favoured the growth of the mushroom mycelium and helped it remain free from fungal contaminants (Fermor et al. 1985).
In the same bioassay, treatment with prochloraz-manganese (50 ppm), achieved 91 % inhibition of the mycelial growth of isolate V1 and V2 of L. fungicola. These results demonstrate that SMS teas are more effective in vitro compared to prochloraz, which agrees with preliminary information on the in vitro suppressive effects of SMS teas used against this pathogen (Gea et al. 2009).
One of the potential parameters that affects the efficacy of compost teas is the target pathosystem (Scheuerell and Mahaffee 2006). In the specific case of mushroom diseases, it must be borne in mind that both the host and the pathogen are fungi, making it necessary to test the effect of the compost tea on the host (Gea et al. 2012). The results demonstrated that the addition of SMS tea to the culture medium did not inhibit the mycelial growth of A. bisporus, and various mushroom production parameters (yield, unitary weight of the mushrooms, biological efficiency, earliness) were not significantly reduced by the SMS tea treatments applied.
The results obtained in the two cropping trials with inoculated treatments of L. fungicola revealed that the TPT treatments were most often more effective than the MS treatments. Specifically, TPT (R3, R4) in trial I and TPT (S1, S2, S3) in trial II led to better control of dry bubble compared to the fungicide prochloraz (P), both regarding the total yield of diseased mushrooms and the relative disease control (Tables 4 and 5, respectively). Therefore, the most effective TPT treatments were those that were applied near harvest and/or those that involved the greatest number of SMS applications. With respect to the fungicide prochloraz, it should be borne in mind that only one application was made (day 7) and that persistence of prochloraz in the casing layer falls considerably 21 days after being applied (Grogan and Jukes 2003). This effect was clearly seen in trial I, in which prochloraz was most effective at the first flush, after which its effectiveness declined. In trial II, prochloraz was the least effective treatment throughout the crop cycle.
In addition, the biological efficiency (BE) values obtained in trial I showed no significant differences between the treatments, reflecting the remarks of Largeteau and Savoie (2008): the total yield (healthy + diseased mushrooms) was not significantly affected by the disease. However, in trial II the BE values were less consistent and the values obtained for treatments MS(S2) and MS(S3) were significantly lower compared to the control inoculated treatment.
Recent findings by Berendsen et al. (2012b) demonstrated that spore germination of L. fungicola is sensitive to the microbial production of antifungal compounds, and Berendsen et al. (2012a) concluded that this pathogen is sensitive to siderophore-mediated competition for iron and to antibiosis by pseudomonads, suggesting that application of microbials that produce effective siderophores and antibiotics could potentially control dry bubble disease. In a previous work, Diánez et al. (2006) also suggested that siderophores played an important role in L. fungicola inhibition. The results obtained in the current study (Table 3) show the ACTs made from SMS (mineral soil and peat) contained a large population of pseudomonads (>106 cfu/ml), which might have contributed to dry bubble suppression.
It can be concluded that SMS teas provide in vitro inhibition of mycelial growth of L. fungicola. In mushroom crops inoculated with L. fungicola, the application of SMS teas decreases the incidence of dry bubble disease. These results and the absence of any fungitoxic effect on A. bisporus (Gea et al. 2012) suggest that dry bubble disease can be controlled by the use of teas made from SMS. However, the major impediment to the use of SMS teas as a biocontrol method might be variation in physical and chemical characteristics, microbial population and efficacy of disease suppression due to SMS types, sources and batches. This problem may be minimized if the SMS tea is prepared by an independent operator (other than the grower) with consistent quality control. Lastly, the ease with which such products can be obtained should also be taken into account.
References
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265–267.
Alfano, G., Lustrato, G., Lima, G., Vitullo, D., & Ranalli, G. (2011). Characterization of composted olive mill wastes to predict potential plant disease suppressiveness. Biological Control, 58, 199–207.
Al-Mughrabi, K. I., Berthélémé, C., Livingston, T., Burgoyne, A., Poirier, R., & Vikram, A. (2008). Aerobic compost tea, compost and a combination of both reduce the severity of common scab (Streptomyces scabiei) on potato tubers. Journal of Plant Sciences, 3, 168–175.
Berendsen, R. L., Baars, J. J. P., Kalkhove, S. I. C., Lugones, L. G., Wösten, H. A. B., & Bakker, P. A. H. M. (2010). Lecanicillium fungicola: causal agent of dry bubble disease in white-button mushroom. Molecular Plant Pathology, 11, 585–595.
Berendsen, R. L., Kalkhove, S. I. C., Lugones, L. G., Baars, J. J. P., Wösten, H. A. B., & Bakker, P. A. H. M. (2012a). Effects of fluorescent Pseudomonas spp. isolated from mushroom cultures on Lecanicillium fungicola. Biological Control, 63, 210–221.
Berendsen, R. L., Kalkhove, S. I. C., Lugones, L. G., Wösten, H. A. B., & Bakker, P. A. H. M. (2012b). Germination of Lecanicillium fungicola in the mycosphere of Agaricus bisporus. Environmental Microbiology Reports, 4, 227–233.
Cronin, M. J., Tohalem, D. S., Harris, R. F., & Andrews, J. H. (1996). Putative mechanism and dynamics of inhibition of the apple scab pathogen Venturia inaequalis by compost extracts. Soil Biology & Biochemistry, 28, 1241–1249.
Diánez, F., Santos, M., Boix, A., De Cara, M., Trillas, I., Avilés, M., & Tello, J. C. (2006). Grape marc compost tea suppressiveness to plant pathogenic fungi: role of siderophores. Compost Science & Utilization, 14, 48–53.
Dionné, R. J., Tweddell, H. A., & Avis, T. J. (2012). Effect of non-aerated compost teas on damping-off pathogens of tomato. Canadian Journal of Plant Pathology, 34, 51–57.
Dragt, J. W., Geels, F. P., Rutjens, A. J., & van Griensven, L. J. (1995). Resistance in wild types of Agaricus bisporus to the mycoparasite Verticillium fungicola var. fungicola. Mushroom Science, 14, 679–683.
El-Masry, M. H., Khalil, A. I., Hassouna, M. S., & Ibrahim, H. A. H. (2002). In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World Journal of Microbiology and Biotechnology, 18, 551–558.
Fermor, T. R., Randle, P. E., & Smith, J. F. (1985). Compost as a substrate and its preparation. In P. B. Flegg, D. M. Spencer, & D. A. Wood (Eds.), The biology and Technology of the cultivated mushroom (pp. 81–109). Chichester: Wiley.
Fletcher, J. T., & Gaze, R. H. (2008). Mushroom pest and disease control. London: Manson Publishing.
Foulongne-Oriol, M., Rodier, A., & Savoie, J.-M. (2012). Relationship between yield components and partial resistance to Lecanicillium fungicola in the button mushroom, Agaricus bisporus, assessed by quantitative trait locus mapping. Applied and Environmental Microbiology, 78, 2435–2442.
Gea, F. J., Navarro, M. J., & Tello, J. C. (2005). Reduced sensitivity of the mushroom pathogen Verticillium fungicola to prochloraz-manganese in vitro. Mycological Research, 109, 741–745.
Gea, F. J., Navarro, M. J., & Tello, J. C. (2009). Potential application of compost teas of agricultural wastes in the control of the mushroom pathogen Verticillium fungicola. Journal of Plant Diseases and Protection, 116, 271–273.
Gea, F. J., Tello, J. C., & Navarro, M. J. (2010). Efficacy and effects on yield of different fungicides for control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop Protection, 29, 1021–1025.
Gea, F. J., Santos, M., Diánez, F., Tello, J. C., & Navarro, M. J. (2012). Effect of spent mushroom compost tea on mycelial growth and yield of button mushroom (Agaricus bisporus). World Journal of Microbiology and Biotechnology, 28, 2765–2769.
Grogan, J. M. (2008). Challenges facing mushroom disease control in the 21st century. In The Proceedings of the sixth international conference on mushroom biology and mushroom products. Bonn: Höntges quickdruck.
Grogan, H. M., & Jukes, A. A. (2003). Persistence of the fungicides thiabendazole, carbendazim and prochloraz-Mn in mushroom casing soil. Pest Management Science, 59, 1225–1231.
Hoitink, H. A. J., & Fahy, P. C. (1986). Basis for the control of soil-borne plant pathogens with composts. Annual Review of Phytopathology, 24, 93–114.
Hoitink, H. A., Stone, A. G., & Han, D. Y. (1997). Suppression of plant diseases by composts. Hortscience, 32, 184–187.
King, E. O., Ward, M. K., & Raney, D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescein. Journal of Laboratory and Clinical Medicine, 44, 301–307.
Koné, S. B., Dionne, A., Tweddell, R. J., Antoun, H., & Avis, T. J. (2010). Suppressive effect of non-aerated compost teas on foliar fungal pathogens of tomato. Biological Control, 52, 167–173.
Largeteau, M. L., Baars, J. J., Juarez del Carmen, S., Regnault-Roger, C., & Savoie, J. M. (2005). Wild strains of Agaricus bisporus : A source of tolerance to dry bubble disease. In A. G. Pisabarro & L. Ramirez (Eds.), Proceedings of the genetics and cellular biology of basidiomycetes VI (pp. 77–87). Pamplona: Universidad Publica de Navarra.
Largeteau, M. L., & Savoie, J. M. (2008). Effect of the fungal pathogen Verticillium fungicola on fruiting initiation of its host, Agaricus bisporus. Mycological Research, 112, 825–828.
Lohr, V. I., Wang, S. H., & Wolt, J. D. (1984). Physical and chemical characteristics of fresh and aged spent mushroom compost. HortScience, 19, 681–683.
Marín, F., Santos, M., Diánez, F., Carretero, F., Gea, F. J., Yau, J. A., et al. (2013). Characters of compost teas from different sources and their suppressive effect on fungal phytopathogens. World Journal of Microbiology and Biotechnology, 29, 1371–1382.
McQuilken, M. P., Whipps, J. M., & Lynch, J. M. (1994). Effects of water extracts of a composted manure-straw mixture on the plant pathogen Botrytis cinerea. World Journal of Microbiology and Biotechnology, 10, 20–26.
Pardo-Giménez, A., & Pardo-González, J. E. (2008). Evaluation of casing materials made from spent mushroom substrate and coconut fibre pith for use in production of Agaricus bisporus (Lange) Imbach. Spanish Journal of Agricultural Research, 6, 683–690.
Regnier, T., & Combrinck, S. (2010). In vitro and in vivo screening of essential oils for the control of wet bubble disease of Agaricus bisporus. South African Journal of Botany, 76, 681–685.
Sang, M. K., Kim, J. G., & Kim, K. D. (2010). Biocontrol activity and induction of systemic resistance in pepper by compost water extracts against Phytophthora capsici. Phytopathology, 100, 774–783.
Scheuerell, S. J., & Mahaffee, W. F. (2002). Compost tea: principles and prospects for plant disease control. Compost Science and Utilization, 10, 313–338.
Scheuerell, S. J., & Mahaffee, W. F. (2004). Compost tea as a container medium drench for suppressing seedling damping-off caused by Pythium ultimum. Phytopathology, 94, 1156–1163.
Scheuerell, S. J., & Mahaffee, W. F. (2006). Variability associated with suppression of gray mold (Botrytis cinerea) on geranium by foliar applications of nonaerated compost teas. Plant Disease, 90, 1201–1208.
Segarra, G., Reis, M., Casanova, E., & Trillas, M. I. (2009). Control of powdery mildew (Erysiphe polygoni) in tomato by foliar applications of compost tea. Journal of Plant Pathology, 91, 683–689.
Siddiqui, Y., Meon, S., Ismail, M. R., & Ali, A. (2008). Trichoderma-fortified compost extracts for the control of choanephora wet rot in okra production. Crop Protection, 27, 385–390.
Siddiqui, Y., Meon, S., Ismail, R., & Rahmani, M. (2009). Bio-potential of compost tea from agro-waste to suppress Choanephora cucurbitarum L. the causal pathogen of wet rot of okra. Biological Control, 49, 38–44.
Soković, M., & van Griensven, L. J. L. D. (2006). Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. European Journal of Plant Pathology, 116, 211–224.
Wakelin, S. A., McCarthy, T., Stewart, A., & Walter, M. (1998). In vitro testing for biological control of Aphanomyces euteiches. Proc. 51st N.Z. Plant Prot. Conf., 90–95.
Weltzien, H. C. (1991). Biocontrol of foliar fungal diseases with compost extracts. In J. H. Andrews & S. S. Hirano (Eds.), Microbial ecology of leaves (pp. 430–450). New York: Springer-Verlag.
Yohalem, D. S., Harris, R. F., & Andrews, J. H. (1994). Aqueous extracts of spent mushroom substrate for foliar disease control. Compost Science and Utilization, 2, 67–74.
Yohalem, D. S., Nordheim, E. V., & Andrews, J. H. (1996). The effect of water extracts of spent mushroom compost on apple scab in the field. Phytopathology, 86, 914–922.
Zare, R., & Gams, W. (2008). A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycological Research, 112, 811–824.
Zhang, W. D., Han, Y., Dick, W. A., Davis, K. R., & Hoitink, H. A. J. (1998). Compost and compost water extract-induced systemic acquired resistance in cucumber and Arabidopsis. Phytopathology, 88, 450–455.
Acknowledgments
Funding for this research was provided by INIA (Ministerio de Economía y Competitividad, Spain) and FEDER (Project RTA2010-00011-C02-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gea, F.J., Carrasco, J., Diánez, F. et al. Control of dry bubble disease (Lecanicillium fungicola) in button mushroom (Agaricus bisporus) by spent mushroom substrate tea. Eur J Plant Pathol 138, 711–720 (2014). https://doi.org/10.1007/s10658-013-0344-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0344-y