Abstract
Cisplatin (CP) is a powerful anticancer agent used in the treatment of a diverse type of cancers. Oxidative stress is one of the most important side effects limiting the use of cisplatin. The protective effects of methanolic extract (ME) and ephedrine (EP), major compound, of Ephedra alata on CP-induced damages were here assessed. Treatment with CP-induced nephrotoxicity and hepatotoxicity characterized by biochemical alterations. In fact, using CP reduced significantly glutathione (GSH) levels, enzymatic activities of superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), and increased malondialdehyde (MDA) content. Nonetheless, CP-treatment induced DNA damage at renal, hepatic, and blood cells and increased interferon gamma (IFNγ) level in serum. Co-treatments of mice with ME normalized relative kidney/body weight, restored biochemical and oxidative stress parameters, reduced DNA damage and IFNγ level. In conclusion, ME exhibited the best protective effect against CP damage compared with ephedrine. This is could be attributed to the presence of polysaccharides, organic acids, flavonoids, and tannins in addition to ephedrine alkaloids. These compounds were reported to play a major role in inhibiting and scavenging free radicals, providing an effective protection against CP- induced oxidative damage.

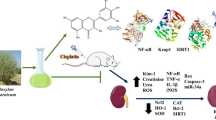
Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cisplatin (CP) is a chemotherapeutic agent widely used in the treatment of cancer since being certified by the Federal Drug Administrationin in 1978. The treatment using high doses of CP revealed an efficient cancer therapy. However, these therapy doses induced in parallel nephrotoxicity and irreversible renal dysfunction side effects limit their effectiveness (Atessahin et al. 2005, Tikoo et al. 2007). In fact, CP induced cytotoxicity in healthy tissues by contributing to the formation of reactive oxygen species (ROS), inducing mitochondrial oxidative damage, and inhibiting antioxidant enzymes (Coskun et al. 2013). Diverse experimental studies were carried out using a combination of CP with radical scavengers or natural foods having antioxidant properties in order to protect healthy tissues against free radicals without reducing its antitumor capacity (Abdelmeguid et al. 2010, Iseri et al. 2007, Mansour et al. 2006). For example, caffeic acid (Choi et al. 2014), apigenin (He et al. 2016), hesperetin (Sahu et al. 2013), and resveratrol (Reddy et al. 2016) were reported to protect against CP toxicity. In this context, medicinal plants have been used for their richness on bioactive compounds. Ephedra alata (Alanda) is a medicinal plant belonging to the Ephedraceae family. This species is widely distributed in several countries of Africa and in Asia (Abourashed et al. 2003). Significant medicinal properties of this genus were reported such as antimicrobial, antioxidant, antidiabetic, hepatoprotective, cardiovascular, and anticancer effects (Hyuga et al. 2016). The preliminary phytochemical analysis of Ephedra alata showed the presence of cardiac glycosides, reducing sugars, flavonoids, phenolic compounds, and alkaloids such as ephedrine, pseudoephedrine, norephedrine, norpseudoephedrine, methylephedrine, and methylpseudoephedrine.
In addition to the above cited alkaloids, Ephedra is rich in antioxidant phenolic compounds such as transcinnamic acid, catechin, syringin, epicatechin, symplocoside, kaempferol 3-O-rhamnoside-7-O-glucoside, and isovitexin-O-rhamnoside which contributed significantly to the antioxidant potential of the plant (Amakura et al. 2013).
Despite the high potential of Ephedra alata up to date, no study has been conducted to evaluate in vivo protective effect on CP toxicity. This research aimed to comparatively evaluate the protective effects of Ephedra alata methaonlic extract and ephedrine against cisplatin-induced nephrotoxicity.
Materials and methods
Collection of plant material
Ephedra alata were collected from the sahara of Tataouine, a region situated in southeast of Tunisia, in February 2017. A voucher specimen has been kept in our laboratory for future reference. The aerial part of plant was shade dried, powdered, and stored in a tightly closed container for further use.
Preparation of methanol extract
The powder (100 g) was macerated into methanol (1 l) for 7 days at room temperature (Ala et al. 2018). The filtrate was concentrated by rotary evaporator under reduced pressure at 40 °C to obtain methanolic extract (ME). The methanol was evaporated to dryness at room temperature to produce the crude extract which was collected and stored at − 4 °C for further test. The yield of extraction was 18.5%.
Total phenolic and flavonoid content analysis
ME was analyzed for its total soluble phenolic content according to the Folin-Ciocalteu colorimetric method (Piccinelli et al. 2004). The result was expressed as mg/g gallic acid equivalent. The colorimetric aluminum chloride assay was used to evaluate and determine the total flavonoid content and expressed as mg/g rutin (Sarker & Oba 2018).
1,1-Diphenyl-2-picryl-hydrazyl assay
The antioxidant activity of the extract was determined against the free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH), as described earlier (Villano et al. 2007) with some modifications. Briefly, 200 μl of each concentration (4–300 μg/ml) were mixed with 3.8 ml DPPH solution and incubated in the dark at room temperature for 1 h. The decrease of optical density (OD) of the samples was measured at 517 nm with methanol as blank. Trolox was used as a positive control. The ability of the sample to scavenge DPPH radical was calculated using the following formula:
Experimental animals
Specific pathogen-free BALB/c mice (6–8 weeks old; male, 25 g) were obtained from the Pasteur Institute (Tunis, Tunisia). All mice were housed under standard conditions of temperature 24 °C, humidity 50%, and light (12 h light/dark) in an accredited pathogen-free facility. All animals were provided ad libitum access to standard rodent chow and filtered water. All experiments were performed in accordance with the guidelines for the care and use of laboratory animals as published by the US National Institute of Health. All experiments received the explicit approval of the Ethics Animal Committee in Tunisia.
Acute toxicity
Mice were randomly divided into groups of 6 animals and treated by intraperitoneal (IP) injection; ME was dissolved in DMSO. The control group was treated by IP saline solution (0.5% DMSO). Groups were treated by different doses of extracts (150, 250, 350, 500, 800, and 1000 mg/kg). The number of dead animals was followed every day for 1 week after treatment in order to determine the 50% lethal dose (LD50).
Experimental design
The mice were fed adaptively for 7 days, divided into six groups of six animals each, and treated as following:
Group 1 (control) were administered IP saline solution (0.5% DMSO; randomly 2 ml/kg) for seven consecutive days, without any drug treatment.
Group 2 (ME) and group 3 ephedrine (EP) were administered IP 150 mg/kg of ME and 15 mg/kg of EP, respectively, for seven consecutive days.
Group 4 (CP) were administered IP saline solution (0.5% DMSO; 2 mL/kg) daily for 7 days. On the 4th day, a single dose of CP (20 mg/kg IP) was given (Nojiri et al. 2016).
Group 5 (ME + CP) and group 6 (EP + CP) were administered IP 150 mg/kg of ME and 15 mg/kg of EP, respectively, daily for 7 days. On the 4th day, a single dose of CP (20 mg/kg IP) was given.
Sample collection and tissue preparation
Trunk blood samples were collected from the sacrificed animals and placed immediately on ice. Heparin was used as an anticoagulant, and plasma samples were obtained by centrifugation at 1500 rpm for 10 min and stored at − 20 °C until measurements. Stored plasma samples were analyzed for biochemical parameters. The liver and kidney of mice were homogenized in the presence of 10 mM Tris–HCl (pH 7.4) and centrifuged at 12.000 rpm for 30 min at 4 °C. The supernatant was collected, aliquoted, and stored at − 80 °C until use. The total protein content was determined according to Bio-Rad Protein Assay (Bradford 1976) using bovine serum albumin as standard.
Determination of liver and kidney function indexes
Aspartate transaminase (AST), alanine transaminase (ALT), and creatinine (CR) levels in plasma were determined by clinical chemistry analyzer COBAS C × 72.
Evaluation of lipid peroxidation status
Lipid peroxidation was determined indirectly by measuring the production of malondialdehyde (MDA) following the method of Aust et al. (1985). Kidney and liver extracts (200 μl) were mixed with trichloroacetic acid (TCA) (250 μl, 20%) and thiobarbituric acid (TBA) (320 μl, 120 mM), then incubated at 95 °C for 2 h. The reaction was stopped by immediate cooling in ice, and then the samples were centrifuged at 2500 rpm for 10 min at 4 °C. The reaction between TBA and the lipids produced by oxidative degradation generates red complexes measured using a microplate reader at 530 nm (ThermoScientific, Vantaa, Finland). The concentration of MDA (micromoles per milligram proteins) was obtained by extrapolating absorbance values to concentrations of standard curve of MDA.
Determination of superoxide dismutase activity
The superoxide dismutase (SOD) activity was evaluated by the inhibition of nitro blue tetrazolium (NBT) reduction assay (Beauchamp & Fridovich 1971, Houmani et al. 2016). Kidney and liver extracts (50 μl) were mixed with NBT (2 mM), methionine (10 mM), riboflavin (2.4 mM), and EDTA (0.1 mM). The reaction was carried out for 15 min illuminated with a UV fluorescent lamp. The absorbance was then measured at 560 nm. The SOD activity was expressed as SOD units (μmol) by milligrams of enzymatic protein.
Determination of catalase activity
Catalase (CAT) activity was performed according to the method of (Brahmi et al. 2012). The reaction mixture consisted of 20 μl of kidney or liver extracts, phosphate buffer, and hydrogen peroxide H2O2 (0.5 M). The reaction was measured at 240 nm using quartz cuvette. One unit of catalase activity was defined as the amount of enzyme required to decompose 1 μmol of H2O2.
Determination of glutathione S-transferase activity
Glutathione S-transferase (GST) activity was analyzed spectrophotometrically at 25 °C following the formation of the conjugate between reduced glutathione (GSH) and 1-chloro-2,4-dinitrobenzene (CDNB) at 340 nm. One milliliter of reagents mixture was prepared (980 μl PBS, 10 μl of 0.02 M CDNB, and 10 μl of 0.1 M GSH). A blank cuvette containing 100 μl of PBS and 900 μl of reagents mixture was used to calibrate the spectrophotometer readings. The test cuvette was filled with 100 μl of sample and 900 μl of reagents mixture. The absorbance was measured every minute during 3 min at 340 nm. The GST activity was expressed as nmol GSH-CDNB/min/mg of protein (Moatamedi Pour et al. 2014).
Determination of glutathione levels
GSH levels were estimated according to Moron et al. 1979 (Moron et al. 1979). A total of 100 μl of kidney and liver extract was mixed with 100 μl of 10% TCA and vortexed. The mixture was centrifuged at 5000 rpm for 20 min. Subsequently, supernatant (100 μl), sodium phosphate dibasic (Na2HPO4) (4 ml, pH 8, 0.3 M), and DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) (5 ml) were added successively. The absorbance was measured within 10 min at 412 nm. Commercially available GSH was used as standard. Level of GSH was expressed as μmol GSH/mg of protein.
Histopathology
For histological studies, the kidney and liver were collected from all the groups and were fixed with 10% phosphate-buffered neutral formalin. Following dehydration in graded (75–100%) alcohol, the tissues were embedded in paraffin. Thin tissue sections, having 5 μM thickness, were cut and stained with routine hematoxylin and eosin stain for photo-microscopic assessment. Kidney tubular damage in stained kidney sections was examined and scored based on the percentage of renal cortical tubular necrosis and hepatic necrosis (Leemans et al. 2005), 0 = normal, 1 = 1–10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = 76–100%. Slides were scored in a blinded manner, and results are means ± SD of 6 representative fields/group.
Genotoxic/antigenotoxic effects
The alkaline comet assay was performed according the method of Singh et al. (1988) with minor modifications. After 3 days of CP administration, animals were sacrificed; the blood, liver, and kidney were isolated. Cellular suspension obtained from each organ were embedded in agarose gel, lysed and subjected to electrophoresis. The total score of DNA damage was determined using the following equation (Collins et al. 1997):
Determination of intracellular cytokines IFN-gamma
Serum interferon gamma levels were assessed using commercially available ELISA kits as per the manufacturer’s instructions.
Statistical analysis
The data were expressed as mean ± SD. Statistical comparisons among groups were analyzed using one-way and two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. All data were analyzed using the GraphPad Prism version 6 (GraphPad SoftwareInc., CA, USA). Statistical significance was considered for p value < 0.05.
Results
Total phenolic and flavonoids contents
The total flavonoids contents (TFC) and total phenolic contents (TPC) of the extracts were reported in Fig. 1. The results showed that ME of Ephedra alata was rich in phenolic and flavonoid compounds. It contains 205 mg/g of phenolic compound expressed as gallic acid equivalents (GAE), and 40.5 mg/g of flavonoid compounds expressed as rutin equivalents (RE) were obtained. Ephedra plant investigated in this study presented higher content of phenolic compounds compared with other Ephedra alata reported by previous studies (TPC, 101.2 GAE (mg/g); TFC, 9.8 RE (mg/g) for ethanolic extract) (Al-Rimawi et al. 2017).
Radical scavenging potential
The radical scavenging potential of ME, measured as decolorizing effect following the trapping of the unpaired electron of DPPH, was shown in Fig. 2. The results revealed that the ME had a potent radical scavenger. In fact, a concentration of 14.7 (μg/ml) recorded was able to quench 50% of the DPPH-free radicals.
Acute toxicity
Intraperitoneal administration of ME did not cause any death and did not show any toxic symptoms or change in general behavior or other physiological activities of mice. The LD50 of this plant was therefore estimated to be above 1000 mg/kg. The dose with minimal cardiotoxicity of EP described by Dunnick et al. (2007) was 15 mg/kg.
Change in body weight and organ coefficient
Before CP administration, the body weights of animals in all the groups were not significantly different. After CP administration, mice showed significant reduction in the average body weight by 10.9% (Fig. 3).
All the groups showed no significant difference of kidney or liver weight. However, an important decrease in organ coefficient of spleen by 41.5% was observed in CP group (Fig. 4). The administration of ME ameliorates these abnormalities. In fact, the differences between body and spleen weights of mice in CP and ME + CP groups were statistically significant (p < 0.01). Whereas, the co-treatment with EP did not ameliorate the weight loss induced by CP treatment.
a Organ coefficient of the kidney, liver, and spleen after treatment. b Effect of cisplatin on morphology of spleen. Results are mean ± SD (n = 6). **p < 0.01 means significant difference between ME, EP, CP groups, and cont group. ##p < 0.01 means significant difference between CP group and co-treated groups
Determination of plasma biochemical parameters
The values of AST, ALT (Fig. 5a), and CR (Fig. 5b) in the serum of CP group were significantly higher compared with other groups which reflect the hepatotoxicity and nephrotoxicity effects of CP treatment. In contrast, the ME + CP group revealed decreased values of biochemical parameters suggesting that ME could effectively reduce the damage of the liver and kidney induced by CP. Moreover, EP did not decrease the level of the above-cited parameters, indicating that it did not promote any nephroprotection or hepatoprotection.
Oxidative stress
Oxidative stress parameters of each group of animals were presented in Table 1. ME and EP treatment alone did not alter the level of MDA and GSH and the activities of SOD, CAT, and GST compared with the control group (p > 0.05). CP treatment revealed a marked decrease in SOD, CAT, and GST activities and GSH level.
Co-treatment with ME and CP decreased the MDA levels and enhanced the levels of antioxidants enzymes. The differences between the CP and ME + CP groups regarding all oxidative stress parameters were statistically significant (p < 0.01). Although, the treatment with both EP and CP increase only the level of liver CAT and liver GST activities and reported significant increase in kidney and liver MDA levels.
Histopathology
The effect on the histology of the kidney of the experimental groups of mice was shown in Fig. 6a–f, and the severity of damage was scored. Histopathological findings of control (Fig. 6a), methanolic extract (Fig. 6b), and ephedrine (Fig. 6c) mice showed normal glomerulus and tubules with regular morphology. Cisplatin-induced renal damage was characterized by glomerular and tubular necrosis, tubular dilatation, and tubular cast formation (Fig. 6d). Methanolic extract of Ephedra alata (Fig. 6e) significantly reversed these changes and showed marked protection against cisplatin-induced kidney damage (Fig. 4e). However, the histological section of mice co-treated by ephedrine (Fig. 6f) still showed damage caused by cisplatin.
Hematoxylin and eosin staining showing kidney histopathology. a Normal control untreated mice with normal glomerular and tubular architecture. b ME-treated mice with intact glomerulus (G) and tubules (T). c EP-treated mice with normal glomerular (G) and tubular (T) architecture. d Cisplatin-intoxicated mice with necrotic tubular cells (NT), glomerular necrosis (GN), tubular dilatation (arrow), and tubular cast formation (stars). e Cisplatin-ME–treated mice with amelioration of undesirable changes produced after cisplatin-intoxication. f Cisplatin-EP–treated mice with less severe tubular dilatation and necrosis. Tubular injury score. Results are mean ± SD (n = 6). ***p < 0.001 means significant difference between ME, EP, CP groups, and cont group. ##p < 0.01 means significant difference between CP group and co-treated groups
Liver sections of control (Fig. 7a), methanolic extract (Fig. 7b), and ephedrine (Fig. 7c) groups showed the normal architecture of the liver tissues. On the other hand, the administration of cisplatin (Fig. 7d) caused a severe liver injury as reflected by hepatic necrosis and inflammatory aggregate. Co-treatment with ME of Ephedra alata decreased the high pathologic changes, suggesting the attenuation of liver damage with the preservation of the liver wall architecture. Liver section of mice co-treated with ephedrine (Fig. 7f) showed more less hepatic necrosis caused by cisplatin which presented by significantly decreased score of hepatic necrosis.
Hematoxylin and eosin staining showing liver histopathology. a Normal control untreated mice with normal hepatic cells. b ME-treated mice with intact hepatic cells. c EP-treated mice with intact hepatic cells. d Cisplatin-intoxicated mice with hepatocyts necrosis (HN) and inflammatory aggregate (IA). e Cisplatin-ME–treated mice with amelioration of the undesirable changes produced after cisplatin-intoxication. f Cisplatin-EP–treated mice with hepatocytes necrosis. Tubular injury score (G). Results are mean ± SD (n = 6). ***p < 0.001 means significant difference between ME, EP, CP groups, and cont group. ##p < 0.01 means significant difference between CP group and co-treated groups
Genotoxic and antigenotoxic properties
The administration of ME and EP to mice did not produce any genotoxic effect in the kidney or in liver and blood cells. Whereas, CP produced a strong genotoxic effect (p < 0.001) in all of the observed types of cells of BALB/c mice (Fig. 8).The co-treatment with ME and CP induced a statistically significant decrease of genotoxic effect of CP alone (p < 0.001). However, EP protect just the liver (p < 0.01) and blood (p < 0.05) against DNA damage induced by CP. These results proved an antigenotoxic effect of ME of Ephedra alata.
Intracellular cytokines
Each of ME or EP alone has no effect on serum cytokine levels (Fig. 9.) The administration of CP to mice caused significant increase in IFNγ levels. Whereas, the co-treatment with ME or EP reduced this level of cytokines significantly (p < 0.001).
Discussion
CP chemotherapy attenuates the normal homeostasis of the body. During the normal clinical treatment, oxidative stress plays a major role in cisplatin-induced toxicity which results in dose escalation limitation and hindrance of the clinical outcome as a consequence (Yousef et al. 2009). Recently, the main goal of scientific research was focused on including new molecules and natural substances in the diet of patients to reduce the toxicity of chemotherapy. In this line, vegetables are rich source of nutraceutical compounds, hence known as protective foods. In fact, their richness on secondary metabolites, presenting important therapeutic potencies, allowed effective health protection. Flavonoids are the major active nutraceutical ingredients in plants. As is typical for phenolic compounds, they can act as potent antioxidants and metal chelators. For a long time, they have been recognized by their anti-inflammatory, antiallergic, hepatoprotective, antithrombotic, antiviral, and anticarcinogenic activities (Komolafe et al. 2014). The present study investigated the hypothesis that Ephedra alata, which is rich in phenolic compounds, may fight oxidative stress and genotoxicity resulting from CP clinical treatment. The first visible change after mice treatment with CP was weight loss. CP group showed a significant reduction of body weight by 10.9% and spleen coefficient by 41.5%. The weight loss of CP group may be ascribed to gastrointestinal tract disorders resulting food intake decrease (Atessahin et al. 2005). Whereas, reduction of spleen weight could be explained by the apoptotic effect on spleen cells, induced by oxidative and inflammatory effect (Banerjee et al. 2018). On the other hand, Treatment of mice groups with ME or EP alone did not induce any significant change in body, kidney, liver, and spleen weight. Compared with CP group, mice group treated with CP and ME exhibited a significant increase (p < 0.01) in both body and spleen weight. We observed that the single administration of a high-dose CP (20 mg/kg) over a 72-h period is responsible of nephrotoxicity and hepatotoxicity. In fact, the obtained results showed an increase in the serum level of ALT, AST, and CR. These effects could be a consequence of the disturbing of several antioxidant system functioning, as CAT, SOD, GST, and GSH (p < 0.001) resulting in lipid peroxide accumulation (p < 0.001) and thus cell damaging (Gong et al. 2015). The observed findings were in correlation with earlier published reports (Farooqui et al. 2016, Niu et al. 2017). Obvious kidney and liver damage including dilation, severe necrosis, and degenerative changes in renal and hepatic cells were also shown by microscopic examination after cisplatin therapy (Omar et al. 2016, Santiago et al. 2016). Co-treatment with Ephedra alata reversed oxidative damage in the kidneys and liver, and prevented acute kidney injury and histopathological liver damage due to cisplatin. The generation of reactive oxygen species and the reduction of the effectiveness of antioxidant defense system in CP group seemed to be responsible of the genotoxicity and apoptosis in non-tumor cells (Attia 2010). Nonetheless, the kidney and liver of CP group exhibited a high comet score (p < 0.001) which indicates high level of DNA damage (class 3 and 4).
Many studies reported that inflammation plays an important role in CP induced nephrotoxicity (Florea & Busselberg 2011). Thereby, the administration of CP increased the IFNγ gene expression (Son 1997). INFγ, one of the inflammatory cytokine, plays an important role in the activation of innate and adaptive immune system signaling pathways in the tumor context (Ortaldo et al. 1983). The results we obtained with CP-treated mice are in accordance with those reporting the role of inflammatory system in CP-induced nephrotoxicity, as far as we observed an increase of IFNγ level in this group. The preliminary chemical study of Ephedra alata revealed the presence of important quantities of polyphenol compounds and flavonoids in ME. These results could be correlated to antioxidant activity towards the free radical DPPH. We believe that ME, thanks to its flavonoids and polyphenol contents, may reduce the oxidative stress induced by CP treatment as far as ME revealed high antioxidant and radical scavenging capacities. These properties of ME should explain the increase of SOD, CAT, GST, and GSH levels in ME + CP–treated animals, as well as the decreasing of ALT, AST, CR, and lipid peroxide levels in the same group of animals. Although, combined treatment of mice by ME and CP reduced significantly the genotoxicity observed in the kidney, blood, and liver induced by CP. Compared with EP, ME revealed a better efficiency in scavenging various free radicals. Otherwise, our experiments revealed that EP restores only liver CAT and GST levels in EP + CP–treated group compared with CP-treated group, whereas SOD and GST remain at the same level in both groups. However, the administration of EP reduced significantly the lipid peroxidation, level of interferon gamma, and genotoxicity in the liver and blood cells, but did not protect the kidney and liver from CP toxicity.
Conclusion
In conclusion, dose-limiting toxicity secondary to antineoplastic chemotherapy constitutes a major obstacle facing the effectiveness and continuity of the treatment. In the present study, it is clear that cisplatin exposure resulted in varying degree of lipid peroxidation, inhibition in the activities of antioxidant enzymes, DNA damage, and alterations of biochemical parameters in the blood plasma. Prior and post exposure to Ephedra alata protect against the liver and kidney injuries by reducing the level of oxidative stress and DNA damage. This study could support that the pharmacological and medicinal properties of Ephedra alata may not linked to one or a few of its constituents, but sometimes to the interaction of plant molecules. Further research is in progress in our laboratory to explore the effect of ME on antitumor activity of cisplatin.
Abbreviations
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GST:
-
Glutathione-S-transferase
- MDA:
-
Malondialdehyde
- IFNγ:
-
Interferon gamma
- ROS:
-
Reactive oxygen species
- DPPH:
-
1.1-diphenyl-2-picryl-hydrazyl
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- CR:
-
Creatinine
- EDTA:
-
Ethylenediaminetetraacetic acid
- CDNB:
-
1-chloro-2,4-dinitrobenzene
- H2O2:
-
Hydrogen peroxide
- DTNB:
-
5,5′-dithiobis-(2-nitrobenzoic acid)
- Na2HPO4:
-
Sodium phosphate dibasic
- TPC:
-
Total phenolic contents
- TFC:
-
Total flavonoids contents
- GAE:
-
Gallic acid equivalents
- RE:
-
Rutin equivalents
- LD50 :
-
Median lethal dose
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- OD:
-
Optical density
- DMSO:
-
Dimethyl sulfoxide
References
Abdelmeguid NE, Chmaisse HN, Abou Zeinab NS (2010) Silymarin ameliorates cisplatin-induced hepatotoxicity in rats: histopathological and ultrastructural studies. Pakistan journal of biological sciences : PJBS 13:463–479
Abourashed EA, El-Alfy AT, Khan IA, Walker L (2003) Ephedra in perspective--a current review. Phytotherapy research : PTR 17:703–712
Ala AA, Olotu BB, Ohia CMD (2018) Assessment of cytotoxicity of leaf extracts of Andrographis paniculata and Aspilia africana on murine cells in vitro. Archives of basic and applied medicine 6:61–65
Al-Rimawi F, Abu-Lafi S, Abbadi J, Alamarneh AAA, Sawahreh RA, Odeh I (2017) Analysis of phenolic and flavonoids of wild Ephedra alata plant extracts by Lc/Pda and Lc/Ms and their antioxidant activity. African journal of traditional, complementary, and alternative medicines : AJTCAM 14:130–141
Amakura Y, Yoshimura M, Yamakami S, Yoshida T, Wakana D, Hyuga M, Hyuga S, Hanawa T, Goda Y (2013) Characterization of phenolic constituents from ephedra herb extract. Molecules 18:5326–5334
Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A (2005) Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 212:116–123
Attia SM (2010) The impact of quercetin on cisplatin-induced clastogenesis and apoptosis in murine marrow cells. Mutagenesis 25:281–288
Aust SD, Morehouse LA, Thomas CE (1985) Role of metals in oxygen radical reactions. Journal of free radicals in biology & medicine 1:3–25
Banerjee S, Sinha K, Chowdhury S, Sil PC (2018) Unfolding the mechanism of cisplatin induced pathophysiology in spleen and its amelioration by carnosine. Chem Biol Interact 279:159–170
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brahmi D, Ayed Y, Hfaiedh M, Bouaziz C, Mansour HB, Zourgui L, Bacha H (2012) Protective effect of cactus cladode extract against cisplatin induced oxidative stress, genotoxicity and apoptosis in balb/c mice: combination with phytochemical composition. BMC Complement Altern Med 12:111
Choi J, Kim SH, Rah YC, Chae SW, Lee JD, Md BD, Park MK (2014) Effects of caffeic acid on cisplatin-induced hair cell damage in HEI-OC1 auditory cells. Int J Pediatr Otorhinolaryngol 78:2198–2204
Collins AR, Dobson VL, Dusinska M, Kennedy G, Stetina R (1997) The comet assay: what can it really tell us? Mutat Res 375:183–193
Coskun N, Hatipoglu MT, Ozogul C, Korkmaz C, Akyol SN, Micili SC, Arik GS, Erdogan D (2013) The protective effects of acetyl L-carnitine on testis gonadotoxicity induced by cisplatin in rats. Balkan medical journal 30:235–241
Dunnick JK, Kissling G, Gerken DK, Vallant MA, Nyska A (2007) Cardiotoxicity of Ma Huang/caffeine or ephedrine/caffeine in a rodent model system. Toxicol Pathol 35:657–664
Farooqui Z, Afsar M, Rizwan S, Khan AA, Khan F (2016) Oral administration of Nigella sativa oil ameliorates the effect of cisplatin on membrane enzymes, carbohydrate metabolism and oxidative damage in rat liver. Toxicol Rep 3:328–335
Florea AM, Busselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 3:1351–1371
Gong C, Qian L, Yang H, Ji LL, Wei H, Zhou WB, Qi C, Wang CH (2015) Hepatotoxicity and pharmacokinetics of cisplatin in combination therapy with a traditional Chinese medicine compound of Zengmian Yiliu granules in ICR mice and SKOV-3-bearing nude mice. BMC Complement Altern Med 15:283
He X, Li C, Wei Z, Wang J, Kou J, Liu W, Shi M, Yang Z, Fu Y (2016) Protective role of apigenin in cisplatin-induced renal injury. Eur J Pharmacol 789:215–221
Houmani H, Rodriguez-Ruiz M, Palma JM, Abdelly C, Corpas FJ (2016) Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma 253:885–894
Hyuga S, Hyuga M, Oshima N, Maruyama T, Kamakura H, Yamashita T, Yoshimura M, Amakura Y, Hakamatsuka T, Odaguchi H (2016) Ephedrine alkaloids-free Ephedra herb extract: a safer alternative to ephedra with comparable analgesic, anticancer, and anti-influenza activities. J Nat Med 70:571–583
Iseri S, Ercan F, Gedik N, Yuksel M, Alican I (2007) Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology 230:256–264
Komolafe K, Olaleye TM, Omotuyi OI, Boligon AA, Athayde ML, Akindahunsi AA, Teixeira da Rocha JB (2014) In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. Journal of acupuncture and meridian studies 7:202–210
Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S (2005) Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115:2894–2903
Mansour HH, Hafez HF, Fahmy NM (2006) Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mol Biol 39:656–661
Moatamedi Pour L, Farahnak A, Molaei Rad M, Golmohamadi T, Eshraghian M (2014) Activity assay of glutathione S-transferase (GSTs) enzyme as a diagnostic biomarker for liver hydatid cyst in vitro. Iran J Public Health 43:994–999
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Niu C, Ma M, Han X, Wang Z, Li H (2017) Hyperin protects against cisplatin-induced liver injury in mice. Acta cirurgica brasileira 32:633–640
Nojiri T, Hosoda H, Kimura T, Tokudome T, Miura K, Takabatake H, Miyazato M, Okumura M, Kangawa K (2016) Protective effects of ghrelin on cisplatin-induced nephrotoxicity in mice. Peptides 82:85–91
Omar HA, Mohamed WR, Arab HH, Arafa E-SA (2016) Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: targeting MAPKs and apoptosis. PLoS One 11:e0151649
Ortaldo JR, Mason A, Rehberg E, Moschera J, Kelder B, Pestka S, Herberman RB (1983) Effects of recombinant and hybrid recombinant human leukocyte interferons on cytotoxic activity of natural killer cells. J Biol Chem 258:15011–15015
Piccinelli AL, De Simone F, Passi S, Rastrelli L (2004) Phenolic constituents and antioxidant activity of Wendita calysina leaves (burrito), a folk Paraguayan tea. J Agric Food Chem 52:5863–5868
Reddy KP, Madhu P, Reddy PS (2016) Protective effects of resveratrol against cisplatin-induced testicular and epididymal toxicity in rats. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 91:65–72
Sahu BD, Kuncha M, Sindhura GJ, Sistla R (2013) Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine : international journal of phytotherapy and phytopharmacology 20:453–460
Santiago MJ, Fernández SN, Lázaro A, González R, Urbano J, López J, Solana MJ, Toledo B, del Castillo J, Tejedor A (2016) Cisplatin-induced non-oliguric acute kidney injury in a pediatric experimental animal model in piglets. PLoS One 11:e0149013
Sarker U, Oba S (2018) Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem 252:72–83
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Son K (1997) Cisplatin-based interferon gamma gene therapy of murine ovarian carcinoma. Cancer Gene Ther 4:391–396
Tikoo K, Bhatt DK, Gaikwad AB, Sharma V, Kabra DG (2007) Differential effects of tannic acid on cisplatin induced nephrotoxicity in rats. FEBS Lett 581:2027–2035
Villano D, Fernandez-Pachon MS, Moya ML, Troncoso AM, Garcia-Parrilla MC (2007) Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 71:230–235
Yousef MI, Saad AA, El-Shennawy LK (2009) Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 47:1176–1183
Acknowledgments
The authors acknowledge the “Ministère Tunisien de l’Enseignement Supérieur et de la Recherche Scientifique” for its support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in accordance with the guidelines for the care and use of laboratory animals as published by the US National Institute of Health. All experiments received the explicit approval of the Ethics Animal Committee in Tunisia.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sioud, F., Ben Toumia, I., Lahmer, A. et al. Methanolic extract of Ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ Sci Pollut Res 27, 12792–12801 (2020). https://doi.org/10.1007/s11356-020-07904-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07904-3