Abstract
Cis-diamminedichloroplatinum(II) (cisplatin) (CP) is an important chemotherapeutic agent used in the treatment of several cancers. However, it has several side effects including nephrotoxicity gonadotoxicity, hepatotoxicity, and ototoxicity. In in vitro experiments, antioxidants or reactive oxygen species scavengers have a cytoprotective effect on cells exposed to cisplatin (CP). Ellagic acid (EA) is one such bioactive polyphenol that is abundant in some fruits, nuts, and seeds. Various authors have reported that EA has strong antioxidant and antitumor potential. The present study was, therefore, carried out to explore the protective potential of EA on CP-induced gonadotoxicity and nephrotoxicity in colon tumor-bearing mice. Animals were divided into five groups: Group I: normal control, Group II: DMH treated. After 20 weeks of DMH treatment, the animals were divided into four subgroups, viz., Group III: no treatment, Group IV: EA, Group V: CP, and Group VI: CP + EA. Administration of EA significantly ameliorated the toxicity caused by CP as indicated by improved kidney function tests and reproductive function tests. EA treatment to CP-abused mice also led to a marked reduction in the extent of peroxidative damage to tissue as was evident from the improvement in the histopathological changes in kidney and testis. Blood counts were also improved on administration of EA to CP-treated mice. This article provides the evidence that antioxidant efficacy of EA has beneficial effects on CP-induced nephrotoxicity and gonadotoxicity and contributes to understanding the role of oxidative stress, and suggests several points as part of the mechanism of CP toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is among the most commonly observed malignancies and is the third driving reason for disease-related mortality around the world [1]. Several genes have been directly implicated in the etiology of colorectal cancer [2]. Most colon cancers are adenocarcinomas (tumors developing from the glands lining the inner wall of colon). Inactivation of the adenomatous polyposis coli (APC) tumor-suppressor gene initiates colorectal neoplasia and also encodes a protein involved in cell adhesion and transcription [3, 4]. Rectal bleeding and anemia which are sometimes coupled with weight loss and changes in bowel habits are typical symptoms of colorectal cancer [5, 6]. Although surgical resection is the major curative therapy in early stage, chemotherapy still remains an essential treatment modality for patients diagnosed with cancer. Chemotherapy drugs are powerful against cancer cells since they are intended to meddle with fast dividing cells [7]. Platinum-based drugs, for example, cisplatin (CP) is a generally utilized treatment in colorectal tumor [8]. Most of the tumor patients experience relapse and develop resistance in spite of initial reaction to CP. Increased ROS in cancer cells is crucial for its initiation and progression [9], in many cases, excessive ROS generation can cause danger and render cancer cells to be more prone to damage by increased oxidative stress caused by exogenous agents. Oxidative stress has been accounted to increase cell apoptosis by means of downstream pathways, for example, endoplasmic reticulum (ER) stress and mitochondrial cascade [10]. Along these lines, controlling ROS levels in cancerous cells is an approach to specifically execute growth, and has been included in the antitumor impacts of a few therapeutic drugs. Recognizing potential interactions between polyphenols and chemotherpeutics may provide information regarding the efficiency of polyphenol-containing foods in cancer therapy [11].
Numerous phytochemicals are well known for their anticancer properties, due to their ability to quench reactive oxygen species (ROS) and thereby protect critical cellular targets (i.e., DNA, proteins, lipids) from oxidative injury. Ellagic acid (EA) is a bioactive polyphenol present in some fruits, nuts, and seeds, for example, pomegranates, raspberries, strawberries, walnuts, and almonds [12]. It has been utilized for many years for the treatment of different ailments. Recent research has shown that EA has strong antioxidant and antitumor potential, inhibits malignant cell growth and instigates cancer cell apoptosis by prompting intracellular oxidative stress. Nowadays, combination chemotherapy has been observed to be an unrivaled treatment methodology. Hence, looking for a successful chemosensitizer that could expand the proficiency of anticancer medications, and, all the while, overcome multidrug resistance and side effects, is direly required.
Materials and methods
Animal model
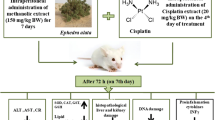
Male laca mice (25–30 g each) procured from Central Animal House, Panjab University, Chandigarh (India) were housed in well-aerated conditions under a constant temperature and humidity in polypropylene cages bedded with sterilized rice husk. Mice in all the groups had free access to standard animal pellet diet (Ashirwad Industries Ltd., Ropar, Panjab, India) and tap water throughout the experiment. All the experimental protocols were approved by the Institutional Ethics Committee (Panjab University, Chandigarh, India) and conducted according to the Indian National Science Academy Guidelines for the use and care of experimental animals. Before starting the various treatments, the animals were allowed to acclimatize to experimental conditions for 1 week, and final day of their acclimatization period was considered as day 0 of the experiment.
Animals were divided into five groups: Group I: normal control, Group II: DMH treated (30 mg/kg body weight once in a week; sub-cutaneous from 0 to 20th week of treatment period), After 20 weeks of DMH treatment, the animals were divided into four subgroups viz. Group IIa: no treatment, Group IIb: EA (10 mg/kg b.wt p.o; orally from the 21st week to 26th week of treatment period), Group IIc: CP (5 mg/kg body weight once in a week; i.p in normal saline from the 23rd to 26th week of treatment period), Group IId: CP + EA (same as in Group IIb and IIc).
Reduced glutathione (GSH)
GSH was estimated according to the method described by Moron et al. [13]. This method is based on the reduction of 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) by SH group of GSH and formation of one mole of 2-nitro-5-mercaptobenzoic acid per mole of –SH. The 2-nitro-5-mercaptobenzoic acid formed has a deep yellow color, which can be used to measure –SH groups at 412 nm. A standard GSH plot was also performed to calculate the content of GSH. The assay was performed within 2–3 h after sacrificing the animal so as to avoid errors due to oxidation of GSH.
Lipid peroxidation
The assay for lipid peroxidation was performed according to the method of Trush [14]. Lipid peroxidation is the oxidative deterioration of lipids by free radicals generated due to various enzymatic and nonenzymatic reactions. Cycloperoxides formed in the deterioration process form Malondialdehyde (MDA) which is a degradation product. MDA forms a pink-colored complex with thiobarbituric acid (TBA) (MDA–TBA chromophore) which can be read at 532 nm.
Total leukocyte count
Total leukocyte counts in blood samples were carried out by method of Dacie and Lewis, 1968 [15]. Blood is diluted with Turk’s solution (distilled water-98 ml, glacial acetic acid-2 ml) and a pinch of crystal violet stain. Turk’s fluid was added to fresh blood to dilute the blood in the ratio 1:20 (v/v). A drop of diluted blood was immediately poured on Neubaur’s chamber and covered with a glass coverslip. WBCs were counted in the four corner square of Neubaur’s chamber.
Differential leukocyte count
The white blood cell differential count determines the number of each type of white blood cell, present in the blood. This was performed using the method described by Dacie and Lewis [16]. Acetone-free methyl alcohol is used as a fixative that preserves the cells in the same chemical and metabolic state (in order to prevent hemolysis). Giemsa stain stains the WBCs differently according to their nucleus (e.g., lymphocytes—nonlobed eccentric nucleus; monocytes—bean-shaped nucleus; and neutrophills—multilobed nucleus).
Hemoglobin
Hemoglobin content in the blood samples was assessed by the oxyhemoglobin method of Dacie and Lewis, 1991 [16]. Twenty microliters of fresh nonclotted blood was made up to 4 ml by adding freshly prepared 0.04% ammonia solution. The OD was measured at 540 nm, and the hemoglobin content was determined from a prestandardized curve.
Reactive oxygen species (ROS) determination
It was determined using the method of Wang and Joseph [17]. In the presence of ROS, H2-DCF is rapidly oxidized to become highly fluorescent dicholorofluorescein (DCF) which can measured. Homogenate was added with DCFH-DA and buffer. This was then incubated at 37 °C for 30 min. After incubation DCF fluorescence intensity was detected at an excitation wavelength of 488 nm and emission wavelength at 525 nm.
Sperm motility
Spermatozoa from the vas deferens were teased out in a watch glass containing 1 ml normal saline (0.9% sodium chloride) at room temperature. Clumps were removed gently by mixing through a Pasteur pipette. 20 µl of this suspension was placed on a slide and was covered with a coverslip. The numbers of motile and nonmotile spermatozoa were counted in four random visual fields. Motility was expressed as percentage of motile sperm according to the following equation:
Sperm concentration
Epididymal spermatozoa were obtained by gentle squeezing and cutting of epididymis, suspended in 1 ml of normal saline. 20 µl of this suspension was placed on hemocytometer, and spermatozoa were counted in all the 64 cells of the hemocytometer and expressed as million per ml.
The sperm concentration was calculated using the following formulas:
Therefore, volume of sperm suspension in 4 WBC squares = 4 mm2 × 0.1 mm = 0.4 mm3, N = total number of sperms in 4 WBC squares. Therefore, number of sperms in 1 mm3 of \({\text{sperm}}\;{\text{suspension}}\,=\,N \times \frac{{{\text{Dilution}}\;{\text{factor}}}}{{0.4}}=N \times 125 \times {10^3}/{\text{ml}}.\)
Histology
Histopathological analysis of tissues was conducted using Hematoxylin and Eosin staining as described by Humanson [18]. Colons were removed and immediately transferred to neutral formalin and allowed to fix for 12 h. Next, the tissue was dehydrated gradually in ascending series of ethanol. For embedding, the dehydrated samples were placed in benzene, then sequentially in 1:1 benzene:paraffin wax and two changes in pure melted wax, before finally embedding in paraffin wax. Thin sections (5 µm) were obtained using a manual hand-driven microtome and transferred to the glass slides. These were dewaxed in xylene, rehydrated in descending series of ethanol, and stained with Hematoxylin and Eosin. Stained sections were mounted in DPX after dehydration and viewed under the light microscope.
Results
GSH
A significant decrease in GSH levels (Table 1) was observed in testis tissue of DMH alone group, DMH + EA, DMH + CP and DMH + CP + EA when compared with control (p ≤ 0.001). CP and EA coadministration showed improvement from DMH alone, DMH + EA and DMH + CP + EA group at a significance level of p ≤ 0.01. There were no significant alterations in GSH levels of kidney tissues when compared with control group (Table 1).
LPO
A significant increase in LPO (Table 2) level was observed in kidney tissue of DMH alone group when compared with control (p ≤ 0.01). DMH + EA-treated group showed a marked increase in the kidney LPO level as compared to control (p ≤ 0.001). A significant decrease was observed in the LPO level of CP and EA coadministered group when compared to DMH + CP group (p ≤ 0.05). DMH + EA group showed a remarkable increase in LPO levels of testis tissue when compared to control (p ≤ 0.001) and decrease when compared with DMH alone group (p ≤ 0.05). DMH + CP group showed an increase when compared to both DMH alone (p ≤ 0.05) and DMH + EA (p ≤ 0.001). CP + EA co administration significantly decreased extent of lipid peroxidation as compared to DMH + CP group (p ≤ 0.01) and increased when compared to control (p ≤ 0.05).
ROS
CP administration to DMH-treated mice significantly increased the ROS levels in kidney tissue when compared with control (Table 3), DMH alone and DMH + EA mice (p ≤ 0.001). Co-treatment group also showed elevated levels of ROS when compared with control, DMH alone and DMH + EA mice (p ≤ 0.001). Similar trends were observed in testis tissue of DMH + CP group when compared with control, DMH alone and DMH + EA mice (p ≤ 0.001). However, coadministered group showed a remarkable decrease in testis ROS levels compared with DMH + CP group (p ≤ 0.001).
Hematological parameters (Table 4)
Hemoglobin
A marked decrease was observed in the Hb levels of DMH alone group, DMH + EA group and DMH + CP group than control (p ≤ 0.001); (p ≤ 0.01); (p ≤ 0.05). DMH + CP group showed decrease in Hb levels than EA administered mice (p ≤ 0.05). Co-treatment group showed improvement in Hb levels than DMH alone as well as DMH + CP group (p ≤ 0.05); (p ≤ 0.01).
Total leukocyte count
A marked decrease was observed in TLC of DMH + CP and DMH + CP + EA when compared to control as well as DMH alone group (p ≤ 0.05); (p ≤ 0.01).
Platelets
DMH + EA group showed decreased platelet count when compared with control and DMH (p ≤ 0.01). DMH + CP animals as well as CP + EA coadministered animals also showed a decreased platelet count as compared to control (p ≤ 0.01) and DMH alone (p ≤ 0.05).
Neutrophils
There were no significant alterations in neutrophil percentage of any group.
Lymphocytes
CP + EA coadministered animals showed increased lymphocyte percentage when compared to DMH + EA group (p ≤ 0.05).
Kidney function tests
Creatinine
A marked increase was observed in serum creatinine levels (Fig. 1) of DMH alone group and DMH + EA group when compared with control (p ≤ 0.05). CP administration to tumor-bearing mice significantly increased the cratinine levels when compared to control (p ≤ 0.01) and DMH alone group (p ≤ 0.01). Co-treatment group also showed elevated levels of creatinine than control (p ≤ 0.05).
Urea and blood urea nitrogen
DMH + EA group showed decreased levels of urea and BUN (Fig. 1) when compared with control (p ≤ 0.001); (p ≤ 0.01) and DMH alone group (p ≤ 0.001); (p ≤ 0.01). CP administration to tumor-bearing mice also showed elevated levels of both when compared with control (p ≤ 0.001); (p ≤ 0.001), DMH alone group and DMH + EA group (p ≤ 0.001); (p ≤ 0.01); (p ≤ 0.001). Co-treatment group also showed increased levels of Urea and BUN when compared with control (p ≤ 0.01); (p ≤ 0.01) and DMH alone group (p ≤ 0.01); (p ≤ 0.01) but a slight decrease was observed from CP-treated animals.
Reproductive potential
Sperm motility
CP administration to tumor-bearing mice significantly decreased the sperm motility (Fig. 2a) when compared to control, DMH alone, DMH + EA group (p ≤ 0.001). Co-treatment group also showed a remarkably decreased sperm motility when compared to control, DMH alone, DMH + EA group (p ≤ 0.001) but showed significant improvement when compared to DMH + CP group (p ≤ 0.01).
Sperm concentration
DMH alone group showed significant decrease in sperm number (Fig. 2b) when compared to control (p ≤ 0.001). DMH + EA group showed improvement in sperm count when compared to DMH alone group (p ≤ 0.001) but a decrease was observed with respect to control (p ≤ 0.01). A marked decrease in sperm count was observed in CP administered group (p ≤ 0.001). Co-treatment group showed improvement in sperm count when compared with DMH + CP group (p ≤ 0.001) but a decrease was observed as compared to other groups.
Histology
Colon from normal animals showed normal histoarchitecture where as DMH administration for 20 weeks showed timorous growth as well as irregular colon epithelium (Fig. 3). Kidneys from control animals show normal morphology with well-preserved glomerulus and no loss of tubular epithelial cells. DMH-treated and DMH + EA groups showed mild tubulointerstitial injury. Cisplatin-treated kidneys show extensive loss of tubular epithelial cells, tubular dilation, intratubular debris, tubular cell atrophy, inflammatory cell infiltration, and increased interstitial areas (Fig. 4). Testis from control animals showed normal spermatogenesis, DMH-treated animals showed marginally low mature sperms than normal, EA-treated animals also showed low mature sperms, DMH + CP-treated group showed atrophy, shrunken tubules as well as low population of germ cells, and sperms all over and reduced leydig cells. CP + EA cotreated animals showed mild atrophy and distorted germinal epithelium but the testicular structure remained intact despite the loss of germ cells, there was no loss of connective tissue when compared to CP + DMH group (Fig. 5).
Statistics
All values are mean ± SD (n = 6). Data are analyzed using one-way ANOVA followed by post-hoc test. Here 3, 2, and 1 denote p < 0.001, p < 0.01, and p < 0.05, respectively. “a” represents the comparison between control and other groups; “b” represents comparison between DMH alone and other groups; “c” represents DMH + EA versus others.
Discussion
The present study was designed to explore the protective potential of EA against toxicity caused by CP in murine colon cancer model (DMH induced) which limits its use as a drug of choice over other available drugs.
DMH is a metabolic precursor of methylazoxymethanol (MAM) which is reported to induce tumors in rodents. Repetitive treatment with this methylating agent was found to induce colon tumors in mice [19]. In the present study, DMH administration for 20 weeks led to development to colon tumors as evidenced by H/E staining.
GSH concentrations were reduced significantly in CP-treated mice. The introduction of an exogenous antioxidant is thought to help the endogenous antioxidant system in scavenging the ROS produced during an imbalance in redox status induced by CP. In the current study, treatment with EA to CP-abused mice seems to maintain the GSH concentrations at a level approaching to those in the control mice. It was reported that when 10 mg/kg EA is combined with CP treatment, the level of GSH is improved [20]. Extent of lipid peroxidation and ROS generation is also a very reliable marker of oxidative damage, in the present study, CP administration to tumor-bearing mice increased lipid peroxidation in both kidney and testis tissue much more than DMH alone group as well as control did. This increase could be attributed to the cytotoxic pathway followed by CP which is known to elevate ROS and LPO in rapidly dividing cells (apart from cancerous cells) [21] and in kidneys due to accumulation. At a dose of 10 mg/kg, EA treatment improved most of the biochemical parameters and histology. The consistency of EA at this dose in improving the kidney and testis status may be due to either the ability to scavenge the ROS as antioxidant [22] and protect the kidney from oxidative stress or EA may affect CP uptake by the kidney cells. It is known that the kidneys accumulate CP more than other organs [23], resulting in necrosis in the terminal portion of the proximal renal tubules and apoptosis in the distal nephron. Biochemical markers such as Cr and BUN elevate significantly following administration of nephrotoxic drugs [24]. However, these markers, although reliable for detection of renal injury, lack the sensitivity and specificity for early kidney damage detection. This increase may be either due to tubular injury, which affects water reabsorption leading to dehydration, or to CP cytotoxic effects on the gastrointestinal tract, which affects the eating behavior, alters gastrointestinal rhythm, and delays gastric emptying, which subsequently causes altered metabolism [25]. Damage is caused by an increase in the glomerular volume and cellular degenerative changes, including cytoplasmic vacuolization of the proximal tubular cells, and tubular dilation [26]. Histopathological examination revealed acute tubular necrosis in most of the examined sections of kidney in mice treated with CP [27]. Testis histology also revealed an altered histoarchitecture in the animals undergoing CP treatment. Many researchers have reported a reduction in germ cell number and deceleration in spermatogenesis [28]. In the present study, decreases in sperm motility and concentration were also observed. Seminiferous tubules contain arrested spermatogenic cells at various stages of division. A reduced mean seminiferous tubule diameter and degenerative changes in germinal cell layer thickness support decreases in sperm motility and concentration [29]. Some improvements were also observed in mice treated with CP combined with EA. Histopathological examination of the kidney is considered to be the golden standard method to detect renal injury. It has been reported that CP can induce epithelial cell atrophy, loss of brush border, tubular dilation, necrosis, and apoptosis [30], leading to severe renal injury through increased expression of intercellular adhesion molecule 1 (ICAM-1) in renal proximal tubules, macrophage infiltration, nuclear factorkappa B (NF-κB) activation, and ROS production [31]. However, Atessahin et al. reported that EA, at a dose of 10 mg/kg, ameliorated the histopathological changes induced by CP in the kidney. They reported that EA at 10 mg/kg protected the kidney from tubular necrosis, degeneration, desquamation, and tubular dilatation. Upon application of chemotherapy, not only the rapidly diving cells get affected but also some of the normal cells get of the body, which are rapidly dividing such as hair, the lining of mouth, the cells lining the intestinal tract, and the blood cells (WBC, RBC, and platelets) [32, 33]. A marked decrease is observed in the blood cells due to chemotherapy which is also known as the “Nadir effect.” Nadir means ‘low point.’ The period of time beginning 7–12 days after each chemotherapy dose—and possibly lasting up to 1 week. This effect renders the patient more prone to infections and fatigue, which further becomes the reason for the plight of patients undergoing chemotherapy [34, 35]. A similar trend was observed in the present study (in the groups administered with CP), which suggests that there must be a supplement given along with the chemotherapeutic drug, which enhances the bone marrow activity so that nadir effect is reduced. Presently, administration of EA reduced the nadir effect significantly in the group undergoing chemotherapy. Many earlier reports have also suggested an increase in blood count on administration of phytochemicals in various diseases. Polyphenols may enhance the total antioxidant-scavenging capacities of blood by binding to the RBCs [36], thereby lowering the reduction in blood counts.
Mice treated with CP showed significant reduction in water intake and urine output post CP treatment. However, it has been reported that CP administration damages the renal tubule and distorts its ability to reabsorb water and cause polyuria [37], which can also lead to polydipsia [38]. Recent studies reported that CP administration leads to increase urine output. Administration of EA at a dose of prior to and post CP treatment increased water intake and urine output (data not shown), which suggests improved kidney function. Additional conventional biochemical markers used in the present study to detect kidney injury were plasma urea, creatinine (Cr), and BUN. A decreased glomerular filtration rate is evidenced by increased plasma creatinine levels, following CP administration [39]. In the present study, plasma urea, Cr, and BUN increased, following either CP treatment or CP combined with EA. However, urea, Cr, and BUN concentrations decreased to some extent when EA was administered prior to and post CP treatment. EA also has antihepatotoxic property that improves the hepatic architecture and functions against toxic and pathological conditions. The molecular mechanisms of EA activates the scavenging of free radicals, regulation of phase I and II enzymes, modulation of proinflammatory and profibrotic cytokines synthesis, the regulation of biochemical pathways involved in synthesis, and degradation of lipids as well as the maintenance of essential trace element levels [40].
It has been reported that EA has been accounted to induce apoptosis particularly in colon adenocarcinoma cells when contrasted with normal colon cells [41]. In outline, our examination showed that EA is an effective agent to be used in combination with CP to reduce the underlying side effects such as nephrotoxicity and gonadotoxicity induced by chemotherapy and may be a potential candidate for CP-based combination treatment in clinical application.
References
Jacobs S, Harmon BE, Ollberding NJ, Wilkens LR, Monroe KR, Kolonel LN, Le Marchand L, Boushey CJ, Maskarinec G (2016) Among 4 diet quality indexes, only the alternate Mediterranean diet score is associated with better colorectal cancer survival and only in African American women in the multiethnic cohort. J Nutr 146:1746–1755
Venook AP, Niedzwiecki D, Lopatin M, Ye X, Lee M, Friedman PN, Frankel W, Clark-Langone K, Millward C, Shak S, Goldberg RM (2013) Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol 31:1775
Feng X, Song Q, Yu A, Tang H, Peng Z, Wang X (2015) Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma 62:592–601
Basu S, Haase G, Ben-Ze’ev A (2016) Wnt signaling in cancer stem cells and colon cancer metastasis. F1000Res
Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D, ESMO Guidelines Working Group (2013) Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi64–vi72
Wong JC, Hasan MR, Rahman M, Angel CY, Chan SK, Schaeffer DF, Kennecke HF, Lim HJ, Owen D, Tai IT (2013) Nucleophosmin 1, upregulated in adenomas and cancers of the colon, inhibits p53-mediated cellular senescence. Int J Cancer 133:1567–1577
Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, La Noce M, Laino L, De Francesco F, Papaccio G (2013) Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J 27:13–24
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14:490–499
Sabharwal SS, Schumacker PT (2014) Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer 14:709–721
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87:1157–1180
Mertens-Talcott SU, Percival SS (2005) Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 218:141–151
Tomás-Barberán FA, García-Villalba R, Gonzalez-Sarrias A, Selma MV, Espín JC (2014) Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem 62:6535–6538
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. BBA-Gen Subj 582:67–78
Trush MA, Mimnaugh EG, Ginsburg E, Gram TE (1981) In vitro stimulation by paraquat of reactive oxygen-mediated lipid peroxidation in rat lung microsomes. Toxicol Appl Pharmacol 15:279–286
Dacie JV, Lewis MS (1968) Practical haematology. Churchill Livingstone Ltd., London
Dacie JV, Lewis SM (1991) Reference ranges and normal values. Practical haematology. Churchill Livingstone, New York
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Humason GL (1962) Animal tissue techniques. W.H. Freeman, San Francisco.
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME (2008) Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 8:299
Ateşşahín A, Çeríbaşi AO, Yuce A, Bulmus Ö, Çikim G (2007) Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol 100:121–126
Borse V, Al Aameri RF, Sheehan K, Sheth S, Kaur T, Mukherjea D, Tupal S, Lowy M, Ghosh S, Dhukhwa A, Bhatta P (2017) Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis 8:2921
Baek B, Lee SH, Kim K, Lim HW, Lim CJ (2016) Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts. Korean J Physiol Pharmacol 20:269–277
Sánchez-González PD, López-Hernández FJ, Dueñas M, Prieto M, Sánchez-López E, Thomale J, Ruiz-Ortega M, López-Novoa JM, Morales AI (2017) Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem Toxicol 107:226–236
Isoda K, Daibo T, Yushina K, Yoshioka Y, Tsutsumi Y, Akimoto Y, Kawakami H, Taira Y, Taira I, Yanoshita R, Nishimura T (2017) Hepatotoxicity, nephrotoxicity, and drug/chemical interaction toxicity of platinum nanoparticles in mice. Pharmazie 72:10–16
Ko JL, Tsai CH, Liu TC, Lin MY, Lin HL, Ou CC (2016) Differential effects of grape juice on gastric emptying and renal function from cisplatin-induced acute adverse toxicity. Hum Exp Toxicol 35:808–817
Aboryag NB, Mohamed DM, Dehe L, Shaqura M, Treskatsch S, Shakibaei M, Schäfer M, Mousa SA (2017) Histopathological changes in the kidney following congestive heart failure by volume overload in rats. Oxid Med Cell Longev. https://doi.org/10.1155/2017/6894040
Ekinci Akdemir FN, Albayrak M, Çalik M, Bayir Y, Gülçin İ (2017) The protective effects of p-coumaric acid on acute liver and kidney damages induced by cisplatin. Biomedicines 5:18
Aminsharifi A, Hekmati P, Noorafshan A, Karbalay-Doost S, Nadimi E, Aryafar A, Hosseinabadi OK, Naseri MM, ZarePoor M (2016) Scrotal cooling to protect against cisplatin-induced spermatogenesis toxicity: preliminary outcome of an experimental controlled trial. Urology 91:90–98
Ateşşahin A, Karahan I, Türk G, Gür S, Yılmaz S, Çeribaşı AO (2006) Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol 21:42–47
Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Perez-Barriocanal F, Morales AI, Lopez-Novoa JM (2011) Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol Dial Transplant 26:3484–3495
Sung MJ, Kim DH, Jung YJ, Kang KP, Lee AS, Lee S, Kim W, Davaatseren M, Hwang JT, Kim HJ, Kim MS (2008) Genistein protects the kidney from cisplatin-induced injury. Kidney Int 74:1538–1547
Dietrich J, Han R, Yang Y, Mayer-Pröschel M, Noble M (2006) CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol 5:22
Elf SE, Chen J (2014) Targeting glucose metabolism in patients with cancer. Cancer 120:774–780
Trump DL, Grem JL, Tutsch KD, Willson JK, Simon KJ, Alberti D, Storer B, Tormey DC (1987) Platinum analogue combination chemotherapy: cisplatin and carboplatin: a phase I trial with pharmacokinetic assessment of the effect of cisplatin administration on carboplatin excretion. J Clin Oncol 5:1281–1289
Seidel C, von Bueren AO, Bojko S, Hoffmann M, Pietsch T, Gielen GH, Warmuth-Metz M, Bison B, Kortmann RD, Kramm CM (2017) Concurrent radiotherapy with temozolomide vs. concurrent radiotherapy with a cisplatinum-based polychemotherapy regimen. Strahlenther Onkol 194:1–10
Koren E, Kohen R, Ginsburg I (2010) Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp Biol Med 235:689–699
Ramadan LA, El-Habit OH, Arafa H, Sayed-Ahmed MM (2001) Effect of cremophor-el on cisplatin-induced organ toxicity in normal rat. J Egypt Nat Cancer Inst 13:139–145
Wong NL, Walker VR, Wong EF, Sutton RA (1993) Mechanism of polyuria after cisplatin therapy. Nephron 65:623–627
Francescato HDC, Costa RS, Camargo SMR, Zanetti MA, Lavrador MA, Bianchi MDLP (2001) Effect of oral selenium administration on cisplatin-induced nephrotoxicity in rats. Pharmacol Res 43:77–82
García-Niño WR, Zazueta C (2015) Ellagic acid: pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res 97:84–103
Larrosa M, Tomás-Barberán FA, Espín JC (2006) The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem 17:611–625
Acknowledgements
The present research was supported by the UGC-BSR fellowship in science, New Delhi, India (Award No: F.25-1/2013-14(BSR)/7-209/2009), DST-PURSE Grant, New Delhi, India (Award No: 49/RPC-29/04/16), and the Departmental Grant from the Panjab University, Sector-25, Chandigarh, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Goyal, Y., Koul, A. & Ranawat, P. Ellagic acid ameliorates cisplatin toxicity in chemically induced colon carcinogenesis. Mol Cell Biochem 453, 205–215 (2019). https://doi.org/10.1007/s11010-018-3446-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3446-1