Abstract
Nectar of honeybee colonies has been used in order to identify heavy metals and establish the benefit of this type of studies as a tool for environmental management. For these goals, samples of nectar were obtained from Apis mellifera hives placed in the city of Córdoba (Spain) and its surroundings. Five stations (each with two hives) were selected and samples were collected from May to July of 2007, 2009 and 2010. Concentrations of Pb, Cr, Ni and Cd in nectar were determined by graphite furnace atomic absorption spectrophotometry. Substantial spatial and temporal differences were detected and compared with the values found in bee bodies in a previously published study based on samples obtained simultaneously with those presented in this work. Upper reference thresholds established for this investigation were surpassed frequently by the measures obtained, being Cr (21.43% of samples), stations S3 (22.22%) and S4 (11.12%) year 2009 (22.22%) and the month of July (23.68%) the metal, the locations and the periods that exceeded more times these references. Regarding the Cd, which was studied only in 2010, 33.33% of the nectar samples exceeded the upper reference thresholds. Comparing the biomonitoring of bee bodies and nectar, some coincidences were found, although they showed different results for highest worrisome values of metal, station and year. This suggests that both methods can give complementary information in the surveillance systems of atmospheric pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal toxicity has proven to be an ecological and global public health concern (Tchounwou et al. 2012); toxicity mechanisms of these metals in humans have been reviewed by Jaishankar et al. (2014). Their dangerous nature represents a threat to the ecological balance that supports life because their non-degradability and their prolonged persistence in the environment (AECOSAN, Spanish Agency of Consumption, Food Security and Nutrition n.d.). In different industrial, combustion or agricultural processes, heavy metals can be released to the air, water and/or soil and transferred from one environmental compartment to another (Järup 2003; Gall et al. 2015). Heavy metals can also be deposited or accumulated in organisms. Plants represent an important point of connection between the biotic and abiotic parts in terrestrial ecosystems (Hamilton 1995). Despite the reduction in emissions of heavy metals that occurred since 2001 in the EU, a part of the surface of ecosystems is at risk due to the atmospheric deposition of Cd, Pb or Hg (EEA, European Environmental Agency 2013).
The monitoring of heavy metals emitted into the air, water or soil must be carried out at the emission sources by those responsible for industrial facilities or activities with a high contamination potential (EC 2010). Other locations are chosen by the governments to monitor the concentrations of heavy metals in the air that generate greater concern for their environmental exposure in order to assess compliance with the value limits established to protect human health (EC 2005; EC 2008).
In any case, the monitoring of heavy metals has been based mainly on physical-chemical methods and is usually carried out in fixed locations.
The Spanish legislation related to the improvement of air quality establishes the criteria to determine the number of sampling points. According to the Royal Decree of 2011(Spain 2011), in agglomerations between 250,000 and 400,000 inhabitants, at least one fixed station is required for the measurement of lead, and in the agglomerations with a population of less than 750,000 inhabitants, a fixed station for the measurement of arsenic, cadmium and nickel is also required. In some places, there are also mobile stations that are used to calibrate fixed stations or to perform point measurements in special circumstances in areas not covered by fixed stations (Boquete et al. 2013). The number of fixed stations in Europe is still relatively small (EEA, European Environment Agency 2013) due to their high costs and the frequent maintenance that these pieces of equipment require (Boquete et al. 2013).
In addition, these fixed stations have two limitations. On the one hand, they need to be complemented by adequate information that allows knowing the degree of collective exposure of the resident population in the zones located between them (Spain 2011). On the other hand, knowing the environmental concentrations of pollutants from physical-chemical methods, being important, means knowing only part of the problem (Ordóñez et al. 2007), since it does not allow direct conclusions to be drawn from environmental damage on habitats and exposed living beings (Klumpp and Klumpp 2004).
It is for this reason that bioindicators are necessary, and among them this study focuses on bees Apis mellifera L., which have exceptional qualities for environmental monitoring (Bromenshenk 1986; Devillers and Pham-Delègue 2002; Celli and Maccagnani 2003; Porrini et al. 2002, 2003). Regarding the biomonitoring of heavy metals, the content of metals in the bee bodies and their concentration in honey or nectar can be used as bioaccumulators (see review in Herrero et al. 2017 or recent works such as Giglio et al. 2017). In the first case, the bees involuntarily can capture these elements during their foraging flights, being retained in the hairs of the surface of their body or, by ingestion or inhalation, to accumulate into them (Porrini et al. 2002).
Honey or nectar (unripe honey, fresh honey) can be used because they can accumulate heavy metals if they are transferred from the soil to flowers or other parts of the plants visited by bees, reaching nectar (Leita et al. 1996; Boyd 2009) and honeydew (Barišić et al. 1999), which are transported to the hives for the formation of honey or honeydew. The type of plant, the mobility of these elements and their availability on the ground influence this transfer (Gall et al. 2015). In fact, it has been proven that the botanical and geographical origin have an important influence on the content of heavy metals in honey (Bogdanov et al. 2007). The hygroscopic nature of honey can also facilitate the absorption of contaminants (Bibi et al. 2008). Honey can also accumulate metals if the metal dust in the air settles or is diluted in these parts by atmospheric deposition, rain or dew (Kalbande et al. 2008; Boyd 2010). The bees themselves can incorporate them into apicultural products if their bodies are previously loaded with heavy metals (Bogdanov et al. 2007).
In biomonitoring studies with colonies of Apis mellifera, two types of samples clearly differentiated by their moisture content have been used for biomonitoring heavy metals: nectar and “mature” honey. While nectar has a higher percentage of water, has been recently collected by bees and is usually found in open cells of the honeycombs, “mature” honey has a lower percentage of water, has been stored in a higher lapse of time and is usually found in operculated cells. There are some works on biomonitoring heavy metal content in mature honey (Roman 2010; Lambert et al. 2012), but there are also some others using nectar (Balestra et al. 1992; Zugravu et al. 2009; Satta et al. 2012; Ruschioni et al. 2013). General studies on chemical characterization of nectar (Nicolson and Thornburg 2007) do not show concrete information about its heavy metal content, only on minerals considered bioelements.
Compared with bee bodies content, honey and nectar have been considered by several researchers matrices with less sensitivity to heavy metal variations in the environment (Conti and Botrè 2001; Lambert et al. 2012; Satta et al. 2012). Multiple factors, such as the species of plant visited, its morphology, the forage range, the date or season of the year and the climatic conditions, could mask the interpretation of the results (Tuzen et al. 2007; Jones 1987). It is also possible that honey or nectar reflects environmental pollution only when the levels of heavy metals in the environment are very high (Conti and Botrè 2001; Ruschioni et al. 2013). In some studies, however, the results have been satisfactory (Zugravu et al. 2009).
Porrini et al. (2002) point out the possibility of generating complete information by integrating the data obtained from the bees and nectar matrices. The bees would provide timely information on the contamination, attributable only to 2 or 3 days prior to their capture and the nectar would be used to obtain contamination information in a greater period of time (between 10 and 15 days) and can include a more extensive area.
During the study of honeybees as bioindicators in the city of Córdoba (southern Spain), samples of bees were studied in order to assess their content of heavy metals (Gutiérrez et al. 2015). Moreover, following Porrini’s suggestions, the potentiality of nectar as bioindicator was also investigated, trying to reach these objectives:
- 1.
Determine the concentration of heavy metals in nectar from several Apis mellifera biomonitoring stations placed in Córdoba (Spain) to estimate the heavy metal pollution in this city and its spatial or temporal differences, focusing on the detection of worrying locations and dates.
- 2.
Compare the results of heavy metal content in nectar with those (previously published) of bee body content obtained in the same stations and dates.
- 3.
Consider if both matrices are complementary and therefore convenient for a regular air pollution monitoring program in cities using A. mellifera hive stations.
Materials and methods
Study area: biomonitoring stations
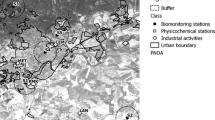
The study was carried out in the surroundings of the city of Córdoba and in its urban area (about 330,000 inhabitants) during the years 2007, 2009 and 2010. Biomonitoring stations were placed in five sites (S1 to S5). Each station consisted of two wood Dadant hives, protected with metal-free paints and placed on a wooden stand 40 cm above the ground with 60 cm from each other. Both hives were painted with different colour to improve bee orientation and minimize drift. The details of the localities are summarized in Table 1 and their particular situation with respect to physicochemical stations and primary industrial activities is indicated in Fig. 1.
Map showing the location of the biomonitoring stations respect to the urban area of Córdoba, physicochemical stations and industrial activities. S1, S2, S3, S4 and S5 are biomonitoring stations, FS the fixed physicochemical station and MS the physicochemical mobile station. Primary industrial activities are nonferrous metals and copper plants (MET), cement plant (CEM), papermaking plant (PMK) and municipal landfill (LAN). The buffer surrounding each biomonitoring station is about 3 km in diameter, which is the foraging area of honeybees. PNOA: = NPAO: National Plan of Aerial Ortophotography. See Table 1 for additional information
Two of these five sites, S2 and S3, were considered to be “comparatively unpolluted” controls due to their geographical location and natural attributes that suggest likely low levels of pollution, even with these contaminants, defined ubiquitous, it is not possible to identify an uncontaminated place with certainty (Porrini et al. 2002).
Sampling
Both hives of each station were visited monthly to collect nectar samples of 10 ml each. These samples were selected using a refractometer from portions of honeycombs in which pollen was absent and the nectar moisture content was higher than 19%. Nectar samples were obtained by mechanical pressing the pieces and were kept at − 28 °C until analysis.
A total of 84 samples were obtained as follows: 24 in May, June and September of 2007; 30 in May, June and July of 2009 and 30 in May, June and July of 2010.
The five stations were tested on the same day whenever possible. The use of smokers was avoided to prevent any chance of contamination.
Analysis of heavy metals
Pb, Cr and Ni were the heavy metals analysed during the 3 years of the study and Cd was incorporated in 2010. These metals were chosen by Gutiérrez et al. (2015) for the study of bee body content because they are usually present in urban areas and are the same metals used for nectar analyses presented in this paper to obtain results with comparative value.
Analyses were conducted by the Centro Studi Ambientali (CSA) in Rimini, Italy, as it was explained in the aforementioned work. No certified reference materials about nectar/honey or the parameters under investigation were found at testing period. This led to perform on nectar repeated tests and some standards were added to test for heavy metals applying the procedure detailed in Bettinelli and Terni (2000) and Gnes et al. (2004).
Before proceeding to instrumental analysis, nectar was diluted in a 1:2 ratio with solution 1% Triton-X; the matrix was digested in the graphite tube during the incineration. Metals were determined by graphite furnace atomic absorption spectrometry with Zeeman background correction (Varian, SpectrAA220Z) in consonance with Method 7010 of the US-EPA (U.S. Environmental Protection Agency 2007). The sample was quantified by standard additions; for cadmium and lead, a 0.5% solution of ammonium dihydrogen phosphate was used as a matrix modifier. The matrix modifier allowed to use higher atomization temperatures, avoiding losing the analyte and at the same time optimally incinerating the matrix and reducing interference. Recoveries between 90 and 105% were obtained. Limits of quantification (LOQ) and limits of detection (LOD) established for the four heavy metals studied are detailed in Table 2.
Data analysis and interpretation
Reference values of the heavy metals studied in nectar, provided by the Department of Agricultural and Food Sciences of the University of Bologna (Italy), have been considered for the analysis and interpretation of the results.
The origin of these reference values was a database (Porrini et al. 2002) updated and calculated on the basis of recent experimental data. The wide variability reflected in the database led to classify the data, so they were organized from least to greatest and divided into quartiles.
The value of Q1 quartile was used as the low reference threshold (LRT) and the value of Q3 quartile was used as the high reference threshold (HRT). When the concentration levels of a sample were equal to or below the LRT, it was considered to correspond to areas with low pollution (and qualified as “acceptable”). Conversely, levels exceeding the HRT were considered “worrisome” and correlated with high-polluted areas. Finally, values between the LRT and the HRT (or equal to this last value) were considered “worthy of attention”, corresponding to districts with an intermediate level of pollution. The reference values are detailed in Table 2 and shown also in Fig. 2.
In the data analysis, the average of the two samples corresponding to both hives of each biomonitoring station was used as one value (one sample).
Qualitative and quantitative ratings were used:
Qualitative ratings were used to represent, by letters, significant differences (Mann-Whitney, p ≤ 0.05 or p ≤ 0.01) between specific locations and periods (years and months); the coincidence of a single letter in two or more stations or dates implies the absence of remarkable differences between locations or periods, whereas different letters express significant differences. Letters closer to the beginning of the alphabet indicate stations or dates with lower amounts of heavy metals. For example, if a value is significantly different from the second one of another station and the first one is lower, it is represented by letters a and b, respectively. But if a third value of a third station does not show remarkable differences neither with the first nor with the second, the pair of letters ab is used to express this situation.
Quantitative ratings were used to represent by numbers the pollution levels: 1 corresponds to “acceptable” results (≤ LRT), 2 indicates “worthy of attention” concentrations (> LRT and ≤ HRT) and 3 is associated to “worrisome” levels (> HRT).
Results
Pb, Cr and Ni
Table 3 shows the concentrations of Pb, Cr and Ni in nectar registered in each station and collection date (absolute data).
Table 4 shows the pollution level (represented by numbers) and the significant differences (represented by letters) between stations, land uses and temporal periods (years and months), taking into account all the investigation period (average data).
Substantial differences between stations and dates are revealed by the Kruskal-Wallis analysis, within a 95% or 99% confidence level. Spatial differences in the amounts of Pb (p ≤ 0.05) and Ni (p ≤ 0.01) were found; temporal variation (monthly and annual) was also observed in Cr levels (p ≤ 0.05 between months and p ≤ 0.01 between years).
A more detailed analysis (Mann-Whitney, p ≤ 0.05 or p ≤ 0.01) showed differences between stations and between dates for each of the heavy metals (Table 4).
Spatial variation
The concentrations of Pb and Ni differed spatially (see Table 4). Pb concentrations in nectar at station S2 were significantly lower (represented by letter a) than at the other stations (letter b in S1, S3, S4 and S5). Samples from stations S3 had significantly higher levels of Ni (indicated with letter c) than those obtained in the remaining stations (indicated with letters a, b or ab); the amount of Ni in S4 was the lowest and the difference with the level of S5 is remarkable (levels of stations S1 and S2 are not significantly different between each other and compared with levels of S4 and S5, so letters ab are used for this situation, as explained in “Material and methods” section).
However, no substantial differences in the level of Cr in nectar were detected among locations (indicated by “a” in all stations).
Regarding land use, industrial zones (stations S1 and S5), agricultural and forest areas (stations S2 and S3) and downtown area (station S4) did not show substantial differences in the levels of Pb and Cr (all of them show letter matching in qualitative rates). Nevertheless, the levels of Ni in nectar from industrial, agricultural and forest areas were significantly higher than those found in the urban area.
Temporal variation
Table 4 also shows that some annual and monthly changes were detected by the continue pollution monitoring, indicating variations in the levels of pollutants.
Annually, significant differences were found only for Cr in nectar. The Cr value was remarkably higher in 2009 than in 2010.
In May, the Pb concentrations in nectar were significantly lower than in June. Moreover, the Cr values in May were appreciably lower than those in the other months. Significant differences were not found for Ni in nectar among months.
Levels of pollution
Table 5 shows the frequencies (%) of concentrations in nectar (for Pb, Cr and Ni) considered acceptable, worthy of attention or worrisome (these ratings are also presented by location and period). Their respective absolute levels of pollution are shown in Table 3.
As Table 5 shows, the highest frequencies of worrisome values occurred for Cr (21.43%); stations S3 (22.22%); year 2009 (22.22%) and the month of July (23.68%).
The highest incidences of worthy of attention values were found in Ni concentrations (52.38%); station S5 (59.26%); year 2007 (58.34%) and the month of June (64.29%).
Finally, the highest frequencies of acceptable values occurred for Pb (59.52%); station S2 (77.78%); year 2010 (60.00%) and the month of May (64.29%).
Figure 3 and Table 3 show the spatial and temporal variability of heavy metal pollution in the city of Córdoba and the severity of some levels in particular dates or locations. The annual averages of Pb, Cr and Ni concentrations in nectar are presented for each station and period in order to be compared with the reference thresholds specified in Fig. 2. Chromium reached worrisome levels in all stations (except in S1) in 2009 and in two stations in 2007 (those close to the urban area but not inside it, and with industrial activities in their buffer surrounding) and nickel reached worrisome levels only in one station in 2009 (curiously, in the northern control station surrounded by forest). Finally, lead never reached worrisome values.
Box and whisker plots showing the variability of concentrations of Pb, Ni and Cr in nectar comparing the values obtained in different years, months and locations. Outlier values are marked with dots. Dashed horizontal lines correspond to the LRT and HRT values established for each metal. The lower and upper limits of each box indicate the limit between the first quartile (Q1) and the second (Q2) and the limit between the third quartile (Q3) and the fourth (Q4), respectively
The monthly level of analysis registered in Table 3 is useful to detect situations of concern that might have been ignored in a global study of more general scale. For example, 2010 resulted to be the year with lower levels of pollution overall (average values represented in Fig. 3 do not show any worrisome value in any heavy metal concentration), but in some specific dates of this year, some worrisome values were found in some particular stations, such as the concentration of Ni in nectar from station S3 in July (Table 3).
Cadmium
An environmental pollution complaint during 2009 suggested the incorporation in 2010 of Cd into the sampling protocol. As shown in Table 6, Cd concentrations exceeded the HRT in 33.33% of the nectar samples and reached worrisome levels at stations S3 and S5 (Fig. 4); July was the month where highest levels were measured (Fig. 5).
Discussion
Interpretation of results in nectar
The biomonitoring of concentrations of heavy metals in nectar of A. mellifera gave important data about the spatial variability and temporal complexity of local environmental circumstances.
In order to assess the results and compare them with those of a previous work on heavy metals in bees (Gutiérrez et al. 2015), two complementary types of analysis have been used. First, for a continuous monitoring of environmental quality, statistical differences among absolute values were recognized to investigate the spatial and temporal variations in pollution. Second, to signal severe and urgent situations, reference values were considered, especially to detect worrisome values that could alert authorities and result in a rapid response to mitigate environmental crises.
As discussed in the aforementioned previous work, “an absence of significant differences among the pollution data does not necessarily indicate that worrisome values have not been reached”. In addition, “when significant differences are found, it cannot be presumed that worrisome values have been reached”. For example, there were no significant differences among locations or months in Cr concentrations in nectar during the 3 years of sampling (Table 4). However, concentrations reached alarming values nine times (Table 3): at station S5 in June and stations S1 and S4 in September 2007; station S4 in June and stations S1, S2, S3, S4 and S5 in July 2009. Moreover, levels of Pb in stations S1 and S2 were significantly different (Table 4), although they never registered worrisome values (Table 3).
Studies carried out by other authors and countries have also found spatial and time-based differences in the levels of heavy metals in floral honey, honeydew or nectar. Some of them do not show apparent differences between natural or contamination-free sites and urban areas (Conti and Botrè 2001; Lambert et al. 2012), as detected in some cases in this study, but some others have found high concentrations in colonies located in the downtown area of the city or in the proximity of industrial areas or roads with heavy traffic (Zugravu et al. 2009; Satta et al. 2012; Ruschioni et al. 2013).
High concentrations of heavy metals found in nectar in some cases were consistent with their dynamics during dry periods characterized by a limitation of the atmospheric purification and the continual deposition and resuspension of the urban dust (Querol 2008).
Unexpected high concentrations were detected in control stations. Stations S3 and S2 were installed a priori in a site that could be considered having less anthropic influences and a more “natural” ecosystem, but the results prove that this “a priori” consideration was wrong. Heavy metal chemodynamics are very complex and a study that relates causes and effects would be necessary. It is possible that wind direction may have occasionally exposed these stations to environmental hazards that may be considered similar or more severe than those found in other stations. For example, station S3 (forest zone, control B) recorded higher annual average concentrations of Ni in nectar than the rest of stations in 2009 and 2010 (Table 3 and Fig. 3). In 2009, station S2 (agricultural zone, control A) and station S3 registered high and worrisome annual average concentration of Cr, analogous to other stations (Fig. 3), and in 2010, station S3 revealed an annual average Cd level that is considered worrisome (Fig. 4).
On the other hand, from the point of view of hygiene and food safety, maximum levels of heavy metals for honey have been established by the 2015/1005 Regulation of EU, which sets the lead content in honey in 0.10 mg/kg (EC 2015). Moreover, Byrne (2000) proposed to the European Union limit values of 0.1 mg/kg for Cd. Tables 2 and 5 show that the values of Pb and Cd in nectar are below those limits.
Comparing heavy metal concentrations in nectar with results in honeybee bodies
The biomonitoring of heavy metals accumulated by bees carried out simultaneously in the same seasons and dates (Gutiérrez et al. 2015) allows comparing and evaluating the results in the nectar. For this comparison, the results obtained in bees in the months of May, June and July have been taken into account (Table 7). In general, there was an interval between 1 and 10 days between the collection of bees and nectar. Only in 2007, the bees were collected in July and the nectar in September. In May 2010, the nectar samples were collected 3 days before those of bees.
Analysing the data as a whole and using the Spearman correlation analysis, the results showed a positive correlation between both matrices (bees and nectar) in the concentration of Pb (p < 0.01; ρ = 0.399) and Ni (p < 0.01; ρ = 0.402); see for example Figs. 6 and 7. However, a negative correlation was found in the concentration of Cr (p < 0.01; ρ = − 0.401), as shown in Fig. 8. All of the correlations calculated can be considered weak. In the case of the Cd, which was analysed only in 2010, no correlation was detected.
Regarding the global levels of pollution reached in the 3 years of study (Tables 5 and 7), both matrices agreed in indicating that the Pb, station S2, the year 2010 and the month of May were respectively the heavy metal, the location and the periods with the highest percentages of acceptable values. They only differed in the metal (Ni in bees and Pb in nectar).
The two matrices differed, however, in the metal, the station and the year where the highest frequencies of worrisome values were detected (Ni in bees and Cr in nectar, S4 in bees and S3 in nectar, 2007 in bees and 2009 in nectar) and they coincided in the month (July).
Moreover, the results obtained with both matrices revealed significant spatial and temporal differences, with bees detecting the greatest number of them. In some cases where significant differences were detected by a matrix, the other matrices confirmed them; in the majority of cases, those differences were not detected but no contradictory results were found, with one exception: the amount of Cr found in bees was significantly higher in 2010 than in 2009 (see Table 3 in Gutiérrez et al. 2015), while that found in nectar was higher in 2009 than in 2010 (Table 3). This fact could be related to the negative correlation of Cr that was detected between the two matrices.
Therefore, coincidences between both matrices are remarkable, but they are not absolute. The dynamics of each heavy metal in the environment, the different routes of exposure to pollution and the nature of the matrix itself have influenced the results. In fact, the amounts of heavy metals in nectar are usually lower than those detected in bees. During foraging, bees can accumulate metals not only by ingestion but also by inhalation through the tracheal system and by deposition on their hairy tegument surface (Porrini et al. 2002), and this could increase the levels of metals in their bodies compared with the levels detected in nectar. Moreover, the morphology of the flower protects the nectaries from increased exposure to contamination and that the contaminants can be partially eliminated or “filtered” in the process of honey formation by the bees (Bogdanov 2006). Some other factors can have influence in heavy metal bioavailability; for example, some studies indicate that some metals in nectar can alter the foraging behavior of Apoidea (Chicas-Mosier et al. 2017; Meindl and Ashman 2013) but nothing can be concluded about how this can modify the concentration levels of nectar samples collected by bees.
Comparing heavy metal concentrations in nectar with the data from physicochemical stations
During the investigation period, a fixed physical-chemical data recording station was installed in the city and, only in 2010, the administration also installed a mobile station (Fig. 1). The physicochemical measurements revealed important spatial differences in the city during 2010 (Mann-Whitney, p ≤ 0.05 for Ni and p ≤ 0.01 for Pb and Cd).
A weak correlation has been found between the high concentration of Ni in both the physicochemical data from the fixed station and the A. mellifera biomonitoring data in 2010 (Fig. 9). In other cases, the physicochemical and biomonitoring records did not show direct correlations; they only coincide in revealing significant spatial differences in the city. An increase in Cd concentration in nectar that occurred in May and July 2010 was not reflected in the physicochemical records (Fig. 10); this increase was detected at station S3 and S5, where this element reached a worrisome level in both months (Table 6). This fact proves that nectar biomonitoring offers complementary information with that obtained using physicochemical technics. Correlations found in 2010 between Ni content in nectar samples vs physicochemical information could be interpreted as contradictory in comparison with those obtained from bee bodies in the same year (correlation between Cd in bees body vs physicochemical system), but it must be considered that the uptake routes of heavy metals are different when using different measurement systems. While physicochemical stations only detect air pollution, honeybee bodies uptake metals directly from different subsystems of the environment (not only air), as explained in the “Introduction” section, and metals in nectar come mainly from soil. When correlations between any pair of measurement tools are detected, they can be associated with similar levels in the different environmental subsystems and/or similar bioavailability of metals, but if correlations are not found, this situation can be associated (but not as a unique reason) with different levels in the subsystems and different characteristics of metals that can have an influence on their different bioavailability routes.
Ni found in nectar and recorded from the fixed physicochemical station (monthly average) in 2010. Averages are calculated from Table 3
It is important to state that the utility of nectar biomonitoring does not represent an alternative method to mechanical devices used for monitoring air pollution, but a complementary system. In this sense, the conclusions of Van der Steen et al. (2015) for relationships between concentrations in bees and in air can be also applied for relationships between concentrations in nectar and in air. Further and more rigorous analysis of risk assessment will be required, considering the sampling in additional years, taking into account more seasonal variables, comparing with similar studies to be performed in other Spanish cities, etc.
Using nectar in integrated biomonitoring strategies
Although the biomonitoring of nectar with colonies of A. mellifera represents a consistent methodology to obtain useful and precise information on environmental quality, further investigations are necessary. First, the methodology used must be standardized, and second, its results should continue to be studied to integrate them into the physicochemical methods of reference. As previously proposed (Ruiz et al. 2013; Gutiérrez et al. 2015), the future approach of such standardization should include:
Complementation and integration with official protocols for the assessment of environmental quality. This integration must take into account that the costs of materials and equipment for honeybee monitoring are lower for governments and institutions than conventional physicochemical methods. It should be noted that biomonitoring with nectar is not possible when spring or blooming has finished.
The analysis of pollen and nectar of bees, combined with a morphological analysis of the contaminating particles, retained by these insects, which would contribute to identify which plants they visit (Porrini et al. 2003) and the possible origin of the contaminants (motorized traffic and industries that could increase the concentration of some metals, particularly those for casting of nonferrous metals or manufacturing cement, etc.).
The use of other bioindicators, such as lichens (Garty 2002), which should be used as complementary tools, mainly in periods of low temperatures when bees are not active out of their hives.
The use of other bee matrices, such as bees (Roman 2010; Perugini et al. 2011; Van der Steen et al. 2012), propolis (Serra-Bonvehí and Orantes 2013) or pollen (Roman 2009; Lambert et al., 2012; Satta et al. 2012) to improve the information provided by nectar, since they could be more exposed to heavy metals.
Finally, not only heavy metals are of concern, as suggested by the increase of mortality of bees at some stations in 2007. In this case, pesticides are suspected cause of death and surely the next objective of biomonitoring.
Conclusions
-
1.
The qualitative information provided by nectar biomonitoring with colonies of A. mellifera allowed the identification of spatial and temporal variations in heavy metal concentrations, detecting significant levels of some of these metals in areas that were disregarded by standard measurement stations.
-
2.
Based on the concentrations found in nectar biomonitoring, the environmental quality of the city of Córdoba and its surroundings shows a high variability depending on the particular type of heavy metal, the location and the period of time (years and months). Overall, the highest frequency of levels of concern was detected in the case of Cr (21.43% of samples), stations S3 (22.22%), year 2009 (22.22%) and in the month of July (23.68%). Although only analysed in 2010, the concentration of Cd also showed a high frequency of problematic values.
-
3.
The simultaneous and compared use of the matrices “bees” and “nectar” has allowed us to observe some coincidence in the assessment of heavy metal contamination in the city of Córdoba. Bees and nectar concentrations match when indicating the metal, the season, the year and the month with the highest frequencies of acceptable values. Both matrices also matched in indicating the month with the highest frequencies of worrisome values, although they varied in metal, season and year. In this case, both matrices offer complementary information.
-
4.
Biomonitoring with honeybees should be incorporated into proposals for tracking and controlling air pollution in cities because they complement and enhance the information derived from physical and chemical methods and its costs are comparatively lower, helping to optimize resources. The use of Apis mellifera as environmental bioindicators is technically viable, integrative and helps to identify the bioavailability of target pollutants and facilitates the analysis of significant differences and reference thresholds as an important tool for the preventive management of environmental crises.
References
AECOSAN Spanish Agency of Consumption, Food Security and Nutrition (n.d.) Heavy metals. http://www.aesan.mspsi.gob.es/AESAN/web/cadena_alimentaria/subdetalle/qui_metales_pesados.shtml. Accessed 7 February 2012 (in Spanish)
Balestra V, Celli G, Porrini C (1992) Bees, honey, larvae and pollen in biomonitoring of atmospheric pollution. Aerobiologia 8:122–126
Barišić D, Vertačnik A, Bromenshenk JJ, Kezić N, Lulić S, Hus M, Kraljević P, Šimpraga M, Seletković Z (1999) Radionuclides and select elements in soil and honey from Gorski Kotar, Croatia. Apidologie 30:277–287
Bettinelli M, Terni C (2000) Determination of trace elements in honey by ICP-MS. In: Minoia C, Bettinelli M, Ronchi A, Spezia S (eds) Application of ICP-MS in chemical and toxicological laboratory. Morgan, Milán, pp 341–349
Bibi S, Husain SZ, Malik RN (2008) Pollen analysis and heavy metal detection in honey samples from seven selected countries. Pak J Bot 40:507–516
Bogdanov S (2006) Contaminants of bee products. Apidologie 37:1–18
Bogdanov S, Haldimann M, Luginbühl W, Gallmann P (2007) Minerals in honey: environmental, geographical and botanical aspects. J Apicult Res 46:269–275
Boquete L, Barea R, Rodríguez JM, Fando R, Cuervo G, Fernández B, Tordera L, de Diego M, Gómez M, Parra I (2013) Environment and sensorization. City 2020: towards a new model of intelligent and sustainable city, IPT 20111006 (in Spanish)
Boyd R (2009) High-nickel insects and nickel hyperaccumulator plants: a review. Insect Sci 16:19–31
Boyd R (2010) Heavy metal pollutants and chemical ecology: exploring new frontiers. J Chem Ecol 36:46–58
Bromenshenk JJ (1986) Public participation in environmental monitoring: a means of attaining networks capability. Environ Monit Assess 6:35–47
Byrne D (2000) Amending Annex II to Council directive 92/118/EEC b (draft)
Celli G, Maccagnani B (2003) Honey bees as bioindicators of environmental pollution. B Insectol 56:137–139
Chicas-Mosier CBA, Melendez AM, Perez M, Abramson CI (2017) The effects of ingested aqueous aluminum on floral fidelity and foraging strategy in honey bees (Apis mellifera). Ecotoxicol Environ Saf 143:80–86
Conti ME, Botrè F (2001) Honeybees and their products as potential bioindicators of heavy metals contamination. Environ Monit Assess 69:267–282
Devillers J, Pham-Delègue MH (2002) Honeybees: estimate the environmental impact of chemicals. Taylor & Francis, London and New York
EC (2005) Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. DO L 23 of 26.1.2005, p. 3–16
EC (2008) Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. DO L 152 of 11.6.2008, p. 1–44
EC (2010) Directive 2010/75/EC of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control). DO L 334 of 17.12.2010, p. 17–19
EC (2015) Commission Regulation (EU) 2015/1005 of 25 June 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs. DO L 161 of 26.6.2015, p. 9–13
EEA European Environment Agency (2013) Air quality in Europe – report 2012. Ministry of Agriculture, Food and Environment, Madrid, p 104 (in Spanish)
Gall J, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187:200–221
Garty J (2002) Biomonitoring heavy metals pollution with lichens. In: Kranner IC, Beckett RP, Varma AK (eds) Protocols in lichenology. Culturing, biochemistry, ecophysiology and use in biomonitoring. Springer, New York, pp 458–482
Giglio A, Ammendola A, Battistella S, Naccarato A, Pallavicini A, Simeon E, Tagarelli A, Giulianini PG (2017) Apis mellifera ligustica, Spinola 1806 as bioindicator for detecting environmental contamination: a preliminary study of heavy metal pollution in Trieste, Italy. Environ Sci Pol R24:659–665
Gnes A, Rondinini T, Pari E, Fonti P, Vincenzi D, Speranza A, Calzoni GL (2004) Mason bees and environment. Insect Social Life 5:111–116
Gutiérrez M, Molero R, Gaju M, Van der Steen J, Porrini C, Ruiz JA (2015) Assessment of heavy metal pollution in Córdoba (Spain) by biomonitoring foraging honeybee. Environ Monit Assess 187:651–665
Hamilton EI (1995) State of the art of trace element determinations in plant matrices: determination of the chemical elements in plant matrices, an overview. Sci Total Environ 176:3–14
Herrero C, Barciela J, García S, Pena RM (2017) The use of honeybees and honey as environmental bioindicators for metals and radionuclides: a review. Environ Rev 25:463–480
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metal. Interdiscip Toxicol 7(2):60–72
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Jones KC (1987) Honey as an indicator of heavy metal contamination. Water Air Soil Pollut 33:179–189
Kalbande DM, Dhadse SN, Chaudhari PR, Wate SR (2008) Biomonitoring of heavy metals by pollen in urban environment. Environ Monit Assess 138:233–238
Klumpp A, Klumpp G (eds) (2004) European network for the assessment of air quality by the use of bioindicator plants (Eurobionet). Final report. University of Hohenheim, Stuttgart
Lambert O, Piroux M, Puyo S, Thorin C, Larhantec M, Delbac F, Pouliquen H (2012) Bees, honey and pollen as sentinels for lead environmental contamination. Environ Pollut 170:254–259
Leita L, Muhlbachova G, Cesco S, Barbattini R, Mondini C (1996) Investigation of the use of honey bees and honey bee products to assess heavy metals contamination. Environ Monit Assess 43:1–9
Meindl GA, Ashman T (2013) The effects of aluminum and nickel in nectar on the foraging behaviour of bumblebees. Environ Pollut 177:78–81
Nicolson SW, Thornburg RW (2007) Nectar chemistry. Nectar chemistry. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, pp 215–264
Ordóñez JM, Iriso A, Aránguez E (2007) Proposal for surveillance of the exposure to environmental chemicals in the Spanish population (biomonitoring). http://www.msssi.gob.es/ciudadanos/saludAmbLaboral/docs/Biomonitorizacion.pdf. Accessed 30 November 2011. (in Spanish)
Perugini M, Manera M, Grotta L, Abete MC, Tarasco R, Amorena M (2011) Heavy metal (Hg, Cr, Cd and Pb) contamination in urban areas and wildlife reserves: honeybees as bioindicators. Biol Trace Elem Res 140:170–176
Porrini C, Ghini S, Girotti S, Sabatini AG, Gattavecchia E, Celli G (2002) Use of honey bees as bioindicators of environmental pollution in Italy. In: Devillers J, Pham-Delègue MH (eds) Honeybees: estimate the environmental impact of chemicals. Taylor & Francis, London and New York, pp 186–247
Porrini C, Sabatini AG, Girotti S, Ghini S, Medrzycki P, Grillenzoni F, Bortolotti L, Gattavecchia E, Celli G (2003) Honey bees and bee products as monitors of the environmental contamination. Apiacta 38:63–70
Querol X (2008) Air quality, suspended particles and metals. Rev Esp Salud Public 82:447–454 (in Spanish)
Roman A (2009) Concentration of chosen trace elements of toxic properties in bee pollen loads. Pol J Environ Stud 18:265–272
Roman A (2010) Levels of copper, selenium, lead, and cadmium in forager bees. Pol J Environ Stud 19:663–669
Ruiz JA, Gutiérrez M, Porrini C (2013) Biomonitoring of bees as bioindicators. Bee World 90:61–63
Ruschioni S, Riolo P, Minuz RL, Stefano M, Cannella M, Porrini C, Isidoro N (2013) Biomonitoring with honeybees of heavy metals and pesticides in nature reserves of the Marche region (Italy). Biol Trace Elem Res 154:226–233
Satta A, Verdinelli M, Ruiu L, Buffa F, Salis S, Sassu A, Floris I (2012) Combination of beehive matrices analysis and ant biodiversity to study heavy metal pollution impact in a post-mining area (Sardinia, Italy). Environ Sci Pollut Res Int 19:3977–3988
Serra-Bonvehí J, Orantes FJ (2013) Element content of propolis collected from different areas of south Spain. Environ Monit Assess 185:6035–6047
Spain (2011) Royal Decree 102/2011, of January 28, on improving air quality. Boletín Oficial del Estado (BOE) n° 25, sec. I pp. 9574–9626 (in Spanish)
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. Exp Suppl 101:133–164
Tuzen M, Silici S, Mendil D, Soyla M (2007) Trace element levels in honeys from different regions of Turkey. Food Chem 103:325–330
US-EPA U.S. Environmental Protection Agency (2007) Graphite furnace atomic absorption spectrophotometry - method 7010 2007
Van der Steen JJM, de Kraker J, Grotenhuis T (2012) Spatial and temporal variation of metal concentrations in adult honeybees (Apis mellifera L.). Environ Monit Assess 184:4119–4126
Van der Steen JJM, de Kraker J, Grotenhuis T (2015) Assessment of the potential of honeybees (Apis mellifera L.) in biomonitoring of air pollution by cadmium, lead and vanadium. J Environ Prot 6:96–102
Zugravu CA, Parvu M, Patrascu D, Stoian A (2009) Correlations between lead and cadmium pollution of honey and environmental heavy metal presence in two Romanian counties. Bulletin UASVM Agriculture 66:230–233
Acknowledgements
We thank José Carlos Casas, from Statistics Department of University of Córdoba, for his advice in the statistical interpretation of results.
Funding
This study has been supported by the Córdoba City Council through the “Evaluation of urban pollution in the municipality of Córdoba using colonies of Apis mellifera as environmental bioindicators” project (Ref: 2006/000228) and by the Spanish Ministry of Environment through the project entitled “Network of biomonitoring stations using colonies of Apis mellifera for real time assessment of urban pollution in the city of Córdoba” (PC 199-003, 2008–2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gutiérrez, M., Molero, R., Gaju, M. et al. Assessing heavy metal pollution by biomonitoring honeybee nectar in Córdoba (Spain). Environ Sci Pollut Res 27, 10436–10448 (2020). https://doi.org/10.1007/s11356-019-07485-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07485-w