Abstract
Honeybees have become important tools for the ecotoxicological assessment of soil, water and air metal contamination due to their extraordinary capacity to bioaccumulate toxic metals from the environment. The level of heavy metal pollution in the Trieste city was monitored using foraging bees of Apis mellifera ligustica from hives owned by beekeepers in two sites strategically located in the suburban industrial area and urban ones chosen as control. The metal concentration in foraging bees was determined by inductively coupled plasma-mass spectrometry. The chemical analysis has identified and quantified 11 trace elements accumulated in two different rank orders: Zn> Cu > Sr > Bi > Ni > Cr > Pb = Co > V > Cd > As in foraging bees from the suburban site and Zn > Cu > Sr > Cr > Ni > Bi > Co = V > Pb > As > Cd in bees from urban site. Data revealed concentrations of Cr and Cu significantly higher and concentration of Cd significantly lower in bees from urban sites. The spatial difference and magnitude order in heavy metal accumulation along the urban-suburban gradient are mainly related to the different anthropogenic activity within sampled sites and represent a risk for the human health of people living in the city. We discussed and compared results with the range of values reported in literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A number of anthropogenic activities, including farming and urbanization, have a significant impact on the environment and can produce an irreversible damage at different biological levels. Data on the effects of exposure to metal pollution have shown that biological responses of the organism depend on the pollutant concentration and its physical and chemical properties (Dallinger 1994; Holmstrup et al. 2010). Moreover, the tolerance, adaptation or sublethal responses to pollution are closely related to the trophic level of the species (Gall et al. 2015), its physiological properties (metal uptake, elimination and immobilization ability) and its life stages (Morgan et al. 2007; Vijver et al. 2004).
Actually, primary and secondary consumers and decomposers such as nematodes, mites, collembolans, earthworms, isopods, insects and molluscs are well known as potential biological indicators in soil and water pollution (Rosenberg 1986; Cortet et al. 1999; Heikens et al. 2001). However, there is an increasing recognition of pollinators as useful biological indicators of both bioaccumulation and toxicant effects for detecting and monitoring environmental metal pollution (Leita et al. 1996; Kevan 1999; Celli and Maccagnani 2003). Honeybees are exposed through an indirect or direct route to a great variety of potentially toxic chemicals of both natural and synthetic origin due to their foraging behaviour to collect nectar, pollen, water and propolis (Jones 1987; Devillers and Pham-Delegue 2002; Zhelyazkova 2012; Johnson 2015). As a result, the level of metal accumulated in bees and their products can indicate the cumulative level of air, water and soil contamination over bees’ forage area (Rashed et al. 2009; Badiou-Bénéteau et al. 2013; Van der Steen et al. 2012, 2015; Bargańska et al. 2016; Van der Steen 2016). Anthropogenic sources such as industrial plant emissions, non-ferrous metal, iron and steel production, civil heating system, waste disposal and incineration, vehicular traffic, fuel combustion and their additive, coal combustion are the main source of heavy metal exposure and accumulation in living organism. The accumulation is hazardous to both whole-colony health affecting critically on pollination service (Hladun et al. 2016) and human and ecosystem health (Brunekreef and Holgate 2002; Pacyna et al. 2007; Lambert et al. 2012; Ruschioni et al. 2013).
This study focused on the environmental metal contamination of Trieste city. Trieste is a border city located in north-east of Italy, and it is characterized by a major port and an industrial area. Main sources of air pollution in this city are an iron foundry and an incinerator located in the suburban industrial area in addition to domestic heating and emission from moving sources. Generally, working in or living near an industrial area increases one’s risk of exposure to heavy metals by ingestion (drinking or eating) or inhalation (breathing). The bioaccumulation in the human body, via acute and chronic exposition, affects a number of different systems and organs resulting in irritation and respiratory infections, lung cancer, heart disease and oxidative stress (Duruibe et al. 2007; Kampa and Castanas 2008; Jaishankar et al. 2014). Actually, previous epidemiological studies have been shown to have a close relation between the risk of lung cancer in the population and the distance from the industrial site in Trieste as source of environmental pollution (Biggeri et al. 1996; Bidoli et al. 2015). The Regional Agency for Environmental Protection of Friuli Venezia Giulia actively monitors the air of Trieste city (www.arpaweb.fvg.it) to respect pollution limits established by European authorities (Directive 2008/50/EC). However, monitoring stations provide air pollution data for total particulate deposition levels (PM10), sulphur and nitrogen oxide and carbon monoxide and only for fixed points. The heavy metal bioaccumulation has been assessed using bioindicators as lichens (Nimis et al. 1999) and mosses (Miani et al. 2007) in high-risk areas of the Friuli-Venezia Giulia region. The metals recorded in moss, including Al, As, Cd, Cr, Cu, Hg, Fe, Mn, Ni, Pb, V and Zn, indicated a metal fallout gradient of air pollutant through the atmosphere (Miani et al. 2007). Lacking recent information on heavy metal bioaccumulation in living organisms, we have chosen in our current study two sites along the gradient reported in Miani et al. (2007), to determinate bioaccumulation in bees as bioindicators of city metal pollution. The suburban site was located in the area of major metal fallout and the control site in the minor ones in the city.
The aims of this paper was to test the metal accumulation in tissues of foraging bees along an urban-suburban gradient in the city of Trieste to provide qualitative and quantitative information to estimate metal contaminants in relation to the distance from the industrial site.
Material and methods
Animals and study sites
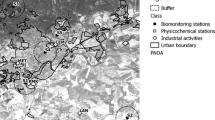
The samples of Apis mellifera ligustica were collected in June 2013 owing to the high foraging activity of bees. Moreover, we choose this sampling period because the low rain and wind season limits atmospheric purification and favours the accumulation of airborne particles. Foraging bees were collected from wooden hives with ten frames owned by beekeepers in two sites (urban and suburban sites, Fig. 1) of Trieste city (Friuli Venezia Giulia, Italy). One site was located close to the industrial area (Domio, locality Lacotišče, suburban site: 45° 36′ 42.6000″ N, 13° 49′ 56.1840″ E, 50 m a.s.l.; Fig. 1). The area has industrial activities with an incinerator and an iron foundry factory, 3.81 km away from the suburban site. Vegetable gardens with fruit trees are the main food source for honeybees in this area. The second group of apiaries, chosen as control, were located 6.07 km south-west of the urban site in a city garden (Parco di San Giovanni, urban site: 45° 39′ 43.9720″ N, 13° 48′ 15.0120″ E, 200 m a.s.l.; Fig. 1), 5.22 km away from the iron foundry factory. A rose garden and different trees, i.e., chestnuts, hackberry, oaks, poplars and Japanese pagoda tree, characterize the area surrounding apiaries. We captured bees coming back from the field whilst alighting boards of hive. They were collected from one hive for each site in sterile, plastic containers. The collection was performed randomly to reduce the difference in metal content owing to the difference in foraging bee age. The samples were transported in a cooler box and frozen at −18 ± 1 °C until chemical analysis.
Metal analyses
Metal concentrations in animals were measured by inductively coupled plasma-mass spectrometry (ICP-MS) using an ELAN DRC-e ICP-MS instrument (PerkinElmer Sciex, Canada). Samples were introduced by means of a PerkinElmer AS-93 plus autosampler and a cross-flow nebulizer with a Scott type spray chamber. For the quantitative analysis, calibration curves were built on seven different concentrations in a calibration range of 0.2–1000 μg/L and having a composition similar to that of sample solutions. Standard solutions were prepared by diluting a multielement solution of As, Bi, Cd, Co, Cr, Cu, Ni, Pb, Sr, V and Zn (100 mg/L, Merck) and a single element solution of Hg (100 mg/L, Merck). Recovery tests were performed using three spike solutions: one near the lower value, one near the centre and one near the upper boundary of the standard curves. Three replicates of each solution were analysed. Satisfactory results were achieved in terms of CV% (<5 %) and accuracy (within +/− 20 %). Moreover, a mid-range calibration standard was measured at the end of each analytical run, for quality control purposes, i.e. to assess instrumental drift throughout the run.
The sample preparation was carried out using the following system for microwave digestion: Anton Paar Multiwave 3000 with programmable power control and rotor XF100 (operating pressure up to 120 bar maximum; operating temperature 260 °C maximum; construction material PTFE-TFM for the vessel). All specimens (n = 20 from each site) were dried at 105 °C (dry weight 0.0531 ± 0.0031 g for bees from urban site and 0.0715 ± 0.0047 g for bees from the suburban site), and each animal was directly weighed in the liner of the microwave system. The digestion was performed by adding 3 mL of HNO3, 3 mL of H2O2 and 1 mL of H2O to each sample and by setting the microwave digestion system at 720 W for 20 min. After digestion, the extracts were quantitatively transferred into a graduated polypropylene test tube, and the volume was adjusted to 50 mL with ultrapure water.
Statistical analyses
Differences in metal concentrations between foraging bees from urban and suburban sites were assessed by non-parametric statistics, i.e. Kruskal–Wallis rank-sum test followed by post hoc Wilcoxon rank-sum test pairwise comparisons with Bonferroni correction, since the null hypothesis of the Bartlett test could not be rejected. The box and whisker plots were drawn with the boxplot command. All values are reported as mean ± SE in the text and metal concentrations expressed as micrograms per gram of dry weight.
Statistical analyses were performed using R version 3.0.1 software (R Development Core Team 2013).
Results
Chemical analysis
The analysis of foraging honeybees from sampling sites has identified and quantified 11 trace element contents: As, Bi, Cd, Co, Cr, Cu, Ni, Pb, Sr, V and Zn (Fig. 2). The ranges of the concentrations of these metals as found in our study were as follows: (a) urban sites As, 0.027 ± 0.053; Bi, 0.416 ± 0.054; Cd, 0.024 ± 0.002; Co, 0.135 ± 0.012; Cr, 0.465 ± 0.041; Cu, 17.93 ± 0.98; Ni, 0.425 ± 0.032; Pb, 0.113 ± 0.014 Sr, 1.90 ± 0.23; V, 0.13 ± 0.05 and Zn, 58.11 ± 4.61; (b) suburban site As, 0.050 ± 0.018; Bi, 0.46 ± 0.062; Cd, 0.052 ± 0.006; Co, 0.125 ± 0.009; Cr, 0.261 ± 0.016; Cu, 12.82 ± 0.92; Ni, 0.358 ± 0.037; Pb, 0.127 ± 0.017; Sr, 1.47 ± 0.175; V, 0.06 ± 0.016 and Zn, 45.92 ± 3.64. Recorded elements were accumulated in two different rank orders Zn > Cu > Sr > Bi > Ni > Cr > Pb = Co > V > Cd > As in foraging bees from the suburban site and Zn > Cu > Sr > Cr > Ni > Bi > Co = V > Pb > As > Cd in bees from urban site. The most abundant elements were Zn, Cu, Sr, Ni, Cr and Bi in all samples from both sites. The highest relative concentration of Zn, Cu, Sr, Ni and Cr was recorded in foraging bees from the urban site. Significantly higher values were recorded in concentrations of Cr and Cu in bees from urban site than in those from the suburban site (Wilcoxon rank-sum test p = 2.104 × 10−4 for Cr and p = 2.58 × 10−4 for Cu), whilst the concentration of Cd (Wilcoxon rank-sum test p = 9.229 × 10−5) was lower in bees from the urban site than in suburban ones. Differences were not significant in concentration of As (Wilcoxon rank-sum test p = 0.9353), Bi (Wilcoxon rank-sum test p = 0.5338), Co (Wilcoxon rank-sum test p = 0.5427), Ni (Wilcoxon rank-sum test p = 0.1441), Pb (Wilcoxon rank-sum test p = 0.5337), Sr (Wilcoxon rank-sum test p = 0.0965) and V (Wilcoxon rank-sum test p = 0.6358). A possible trend towards significance was recorded for Zn (Wilcoxon rank-sum test p = 0.0524) with higher concentration in samples from the urban site.
Heavy metal content in Apis mellifera ligustica foraging bees (μg g−1dry weight, n = 20 from each site, URB urban and SUB suburban sites; for statistics, see the text). The boxplot represents the interquartile range (IQR = Q3 − Q1) and bars represent first (Q1, top) and third quartiles (Q3, bottom) of heavy metal content. The central horizontal black line indicates the median. The ends of dashed lines (ends of the whiskers) represent the lowest datum and the highest datum
Discussion
In this study, we recorded 11 trace metals in the animal’s body that reflect qualitatively pollution levels of monitored sites in the city of Trieste. The spatial difference in heavy metal concentration as well as the magnitude order is mainly related to the different anthropogenic activity within the sampled site. The highest concentration of Cr and Cu in honeybees from the urban site shows that metal pollution of the city centre may be mainly correlated with traffic and domestic heating, whilst the highest concentration of Cd shows that industrial emissions may be the major source of this metal in the suburban area. Furthermore, as the atmospheric deposition is the principal source of accumulated heavy metals in the foraging bees’ body, we did not expect the recorded levels of As, Bi, Co, Ni, Pb, Sr, V and Zn to be qualitatively and quantitatively the same between samples from the urban and suburban sites. It is known that some tree species, planted such as single tree in urban areas or “edge” trees, can have a significant impact on the reduction of PM10 air concentration acting positively on air quality and human health (McDonald et al. 2007; Cavanagh et al. 2009). However, wooded sites may cause higher concentrations of metals than expected given the distance of the pollution source due to local advection and enhanced turbulent exchange, presence of honeydew, particulate concentration in the air, temperature and wind speed (Cavanagh et al. 2009). Thus, we assume that the unexpectedly highest relative concentration of heavy metals recorded in the control urban site was closely related to the relative position of apiaries at the garden edge. Moreover, the city garden is located below a hill that acts as a barrier and favours the accumulation in the bottom of elements transferred by the wind.
Long-term exposure to some elements recorded in this analysis such as As, Cd, Cr and Pb are well known to have an effect on human health (Järup 2003; Jaishankar et al. 2014). The metal uptake and accumulation in honeybees depend mainly in plant flower contamination (Porrini et al. 2002; Rashed et al. 2009; Hladun et al. 2016) and are correlated with the metal presence in the pollen matrix and nectar foraged by bees (Lambert et al. 2012; Bargańska et al. 2016). Thus, high levels of heavy metal bioaccumulated in bees from both sites suggest a high exposure risk for people living in the city. Therefore, epidemiological studies on exposure risk to air pollution should take into account also the heavy metal concentrations in the environment, besides the concentration of particulate deposition (PM10) and nitrogen and sulphur dioxide in the air or the distance from the pollution source (Biggeri et al. 1996; Kampa and Castanas 2008; Pascal et al. 2013; Bidoli et al. 2015).
More difficult is a quantitative interpretation of results. On the one hand, it is because the different levels of metal accumulation depend on a number of factors such as area of bee keeping and its ecological status, method of rearing bee colonies, age of worker bees, physiological and health status of bee specimens and bee colonies. On the other hand, it lacks a general outline of thresholds to define the low and high pollution level for heavy metal monitoring with honeybees and to facilitate the preventive management of environment. The concentration of Cd, Co, Cr, Cu, Ni, Sr, V and Zn measured in our study are all within the range of values reported in literature (Roman 2010; see Van der Steen et al. 2012 for a complete review of published values). The exception is recorded for As, Cd and Pb, which are lower than reported from other previous studies on bees (Perugini et al. 2011; Van der Steen et al. 2012; Ruschioni et al. 2013). The low concentrations of As and Cd are comparable with the bioaccumulation values detected by mosses in the same area (Miani et al. 2007). However, the level of Cr, Cd and Ni in honeybees reported for Italy was lower than that recorded in our samples (Conti and Botré 2001; Satta et al. 2012; Ruschioni et al. 2013). These are geographical differences due to different levels of metal in sampled sites from natural or anthropogenic sources. The comparison of accumulation values in honeybees of metals such as Bi, Co, Sr and V is very difficult because they are rarely reported in previous monitoring studies (Van der Steen et al. 2012, 2015). Actually, the only proposed method for the interpretation of results defines reference thresholds calculated on experimental data, which were derivated into quartiles (Porrini et al. 2002; Gutierrez et al. 2015). Gutierrez et al. (2015) indicate the following reference threshold values for Cd, Cr, Pb and Ni: 0.3 mg/kg ≤ Pb ≥ 0.7 mg/kg, 0.04 mg/kg ≤ Cr ≥ 0.12 mg/kg, 0.1 mg/kg ≤ Ni ≥ 0.3 mg/kg, 0.052 mg/kg ≤ Cd ≥ 0.1 mg/kg. The low value is equivalent to an acceptable low pollution for each metal and the highest value to worrisome high-pollution ones. Based on this method, we can interpret our results only for some metals. Therefore, the level of Cr and Ni recorded in bees from both sites is worrisome, whilst the recorded concentration of Pb and Cd is acceptable.
Conclusions
This study is a preliminary analysis, and more data including additional years, seasonal variable and distance covered by foraging bees (Decourtye et al. 2011) are required for a more rigorous survey of heavy metal risk assessment in Trieste city. Besides, our results confirm that biomonitoring with A. mellifera is a useful approach to assess environmental pollution (Leita et al. 1996; Kevan 1999; Lambert et al. 2012; Bargańska et al. 2016). Moreover, the level of hazardous metals recorded in our investigation may be compared with the value found in other studies to increase the network of information for environmental impact assessment. The metal contamination recorded along the urban-suburban gradient showed that the risk for human health is not related to the distance from the industrial site. Finally, further comparative studies are needed to determinate a range of comparable values to establish thresholds for the interpretation of results that triggers an alarm if its limits are exceeded.
References
Badiou-Bénéteau A, Benneveau A, Géret F, Delatte H, Becker N, Brunet JL, Reynaud B, Belzunces LP (2013) Honeybee biomarkers as promising tools to monitor environmental quality. Environ Int 60:31–41
Bargańska Ż, Ślebioda M, Namieśnik J (2016) Honey bees and their products—bioindicators of environmental contamination. Crit Rev Environ Sci Technol 46(3):235–248
Bidoli E, Barbone F, Collarile P, Valent F, Zanier L, Daris F, Gini A, Birri S, Serraino D (2015) Residence in proximity of an iron foundry and risk of lung cancer in the municipality of Trieste, Italy, 1995–2009. Int. J. Environ. Res. Public Health 12:9025–9035
Biggeri A, Barbone F, Lagazio C, Bovenzi M, Stanta G (1996) Air pollution and lung cancer in Trieste, Italy: spatial analysis of risk as a function of distance from sources. Environ Health Perspect 104(7):750–754
Brunekreef B, Holgate ST (2002) Air pollution and health. Lancet 360(9341):1233–1242
Cavanagh JE, Peyman Zawar-Reza P, Wilson JG (2009) Spatial attenuation of ambient particulate matter air pollution within an urbanised native forest patch. Urban For Urban Green 8(1):21–30
Celli G, Maccagnani B (2003) Honey bees as bioindicators of environmental pollution. Bulletin of Insectology 56(1):137–139
Conti ME, Botrè F (2001) Honeybees and their products as potential bioindicators of heavy metals contamination. Environ Monit Assess 69:267–282
Cortet J, Vauflery AG-D, Poinsot-Balaguer N, Gomot L, Texier C, Cluzeau D (1999) The use of invertebrate soil fauna in monitoring pollutant effects. Eur J Soil Biol 35:115–134
Dallinger R (1994) Invertebrate organisms as biological indicators of heavy metal pollution. Appl Biochem Biotechnol 48:27–31
Decourtye A, Devillers J, Aupinel P, Brun F, Bagnis C, Fourrier J, Gauthier M (2011) Honeybee tracking with microchips: a new methodology to measure the effects of pesticides. Ecotoxicology 20(2):429–437
Devillers J, Pham-Delegue M-H (2002) Honey bees: estimating the environmental impact of chemicals. Taylor & Francis, London 332 pp, ISBN 0 415 27518 0
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences 2(5):112–118
EU (2008) Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off J Eur Union:1–44
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187:1–21
Gutiérrez M, Molero R, Gaju M, Steen J, Porrini C, Ruiz JA (2015) Assessment of heavy metal pollution in Córdoba (Spain) by biomonitoring foraging honeybee. Environ Monit Assess 187:1–15
Heikens A, Peijnenburg WJGM, Hendriks AJ (2001) Bioaccumulation of heavy metals in terrestrial invertebrates. Environ Pollut 113:385–393
Hladun KR, Di N, Liu T-X, Trumble JT (2016) Metal contaminant accumulation in the hive: consequences for whole-colony health and brood production in the honey bee (Apis mellifera L.). Environ Toxicol Chem 35:322–329
Holmstrup M, Bindesbøl A-M, Oostingh GJ, Duschl A, Scheil V, Köhler H-R, Loureiro S, Soares AMVM, Ferreira ALG, Kienle C, Gerhardt A, Laskowski R, Kramarz PE, Bayley M, Svendsen C, Spurgeon DJ (2010) Interactions between effects of environmental chemicals and natural stressors: a review. Science of The Total Environment, Cumulative Stressors—Risk assessment of mixtures of chemicals and combinations of chemicals and natural stressors 408: 3746–3762
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Johnson RM (2015) Honey bee toxicology. Annu Rev Entomol 60:415–434
Jones KC (1987) Honey as an indicator of heavy metal contamination. Water Air Soil Pollut 33:179–189
Kampa M, Castanas E (2008) Human health effects of air pollution. Environ Pollut 151:362–367
Kevan PG (1999) Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agric Ecosyst Environ 74:373–393
Lambert O, Piroux M, Puyo S, Thorin C, Larhantec M, Delbac F, Pouliquen H (2012) Bees, honey and pollen as sentinels for lead environmental contamination. Environ Pollut 170:254–259
Leita L, Muhlbachova G, Cesco S, Barbattini R, Mondini C (1996) Investigation of the use of honey bees and honey bee products to assess heavy metals contamination. Environ Monit Assess 43:1–9
McDonald AG, Bealey WJ, Fowler D, Dragosits U, Skiba U, Smith RI, Donovan RG, Brett HE, Hewitt CN, Nemitz E (2007) Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations. Atmos Environ 41(38):8455–8467
Miani N, Skert N, Grahonja R, Valic I, Abatangelo A, Asquini T (2007) Biomonitoraggio sperimentale delle ricadute al suolo nella provincia di Trieste di metalli in traccia aerodispersi tramite muschi trapiantati come bioaccumulatori. Provincia di Trieste—ARPA FVG Dipartimento di Trieste. Relazione finale 1–28
Morgan AJ, Kille P, Stürzenbaum SR (2007) Microevolution and ecotoxicology of metals in invertebrates. Environmental Science & Technology 41:1085–1096
Nimis PL, Skert N, Castello M (1999) Biomonitoraggio di metalli in traccia tramite licheni in aree a rischio del Friuli-Venezia Giulia. Studía Geobotaníca 18:3–49
Pacyna EG, Pacyna JM, Fudala J, Strzelecka-Jastrzab E, Hlawiczka S, Panasiuk D, Nitter S, Pregger T, Pfeiffer H, Friedrich R (2007) Current and future emissions of selected heavy metals to the atmosphere from anthropogenic sources in Europe. Atmos Environ 41:8557–8566
Pascal M, Pascal L, Bidondo M, Cochet A, Sarter H, Stempfelet M, Wagner V (2013) A review of the epidemiological methods used to investigate the health impacts of air pollution around major industrial areas. J Environ Public Health 1–17
Perugini M, Manera M, Grotta L, Abete MC, Tarasco R, Amorena M (2011) Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: honeybees as bioindicators. Biol Trace Elem Res 140:170–176
Porrini C, Ghini S, Girotti S, Sabatini AG, Gattavecchia E, Celli G (2002) Use of honeybees as bioindicators of environmental pollution in Italy. In: Devillers J, Pham-Delègue MH (eds) Honeybees: estimating the environmental impact of chemicals. Taylor & Francis, London, pp. 186–247
R Development Core Team R (2013) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rashed MN, El-Haty MTA, Mohamed SM (2009) Bee honey as environmental indicator for pollution with heavy metals. Toxicol Environ Chem 91:389–403
Roman A (2010) Levels of copper, selenium, lead, and cadmium in forager bees. Polish J of Environ Stud 19(3):663–669
Rosenberg DM, Danks HV, Lehmkuhl DM (1986) Importance of insects in environmental impact assessment. Environ Manag 10:773–783
Ruschioni S, Riolo P, Minuz RL, Stefano M, Cannella M, Porrini C, Isidoro N (2013) Biomonitoring with honeybees of heavy metals and pesticides in Nature Reserves of the Marche Region (Italy). Biol Trace Elem Res 154:226–233
Satta A, Verdinelli M, Ruiu L, Buffa F, Salis S, Sassu A, Floris I (2012) Combination of beehive matrices analysis and ant biodiversity to study heavy metal pollution impact in a post-mining area (Sardinia, Italy). Environ Sci Pollut Res 19:3977–3988
Van der Steen JJM (2016) Beehold, the colony of the honeybees (Apis mellifera L) as a bio-sampler for pollutants and plant pathogens. Thesis Wageningen UR. ISBN 978-94-6257-751-0
Van der Steen JJM, de Kraker J, Grotenhuis T (2012) Spatial and temporal variation of metal concentrations in adult honeybees (Apis mellifera L.). Environ Monit Assess 184:4119–4126
Van der Steen JJM, de Kraker J, Grotenhuis T (2015) Assessment of the potential of honeybees (Apis mellifera L.) in biomonitoring of air pollution by cadmium, lead and vanadium. J Environ Prot 6:96–102
Vijver MG, van Gestel CAM, Lanno RP, van Straalen NM, Peijnenburg WJGM (2004) Internal metal sequestration and its ecotoxicological relevance: a review. Environ Sci Technol 38:4705–4712
Zhelyazkova I (2012) Honeybees—bioindicators for environmental quality. Bulgarian Journal of Agricultural Science 18:435–442
Acknowledgments
The European program of cross-border collaboration (Slovenia-Italy 2007–2013; Project BioDiNet) supported this work financially.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Headings
• Foraging bees are good bioindicators of air pollution.
• Metal pollution can affect human health.
• Pollution in Trieste city is related to anthropogenic activities
Rights and permissions
About this article
Cite this article
Giglio, A., Ammendola, A., Battistella, S. et al. Apis mellifera ligustica, Spinola 1806 as bioindicator for detecting environmental contamination: a preliminary study of heavy metal pollution in Trieste, Italy. Environ Sci Pollut Res 24, 659–665 (2017). https://doi.org/10.1007/s11356-016-7862-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7862-z