Abstract
Nickel (Ni) is an essential micronutrient but considered toxic for plant growth when present in excess in the soil. Polyamines (PAs) and arbuscular mycorrhiza (AM) play key roles in alleviating metal toxicity in plants. Present study compared the roles of AM and PAs in improving rhizobial symbiosis, ureide, and trehalose (Tre) metabolism under Ni stress in Cajanus cajan (pigeon pea) genotypes (Pusa 2001, AL 201). The results documented significant negative impacts of Ni on plant biomass, especially roots, more in AL 201 than Pusa 2001. Symbiotic efficiency with Rhizobium and AM declined under Ni stress, resulting in reduced AM colonization, N2 fixation, and ureide biosynthesis. This decline was proportionate to increased Ni uptake in roots and nodules. Put-reduced Ni uptake improved plant growth and functional efficiency of nodules and ureides synthesis, with higher positive effects than other PAs. However, AM inoculations were most effective in enhancing nodulation, nitrogen fixing potential, and Tre synthesis under Ni toxicity. Combined applications of AM with respective PAs, especially +Put+AM, were highly beneficial in alleviating Ni-induced nodule senescence by arresting leghemoglobin degradation and improving functional efficiency of nodules by boosting Tre metabolism, especially in Pusa 2001. The study suggested use of Put along with AM as a promising approach in imparting Ni tolerance to pigeon pea plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nickel (Ni) is the 24th most available element in earth and belongs to transition series (group VIII B) in periodic table (Sachan and Lal 2017). The oxidation state, Ni (II), is considered the only accessible form for plants (Seregin and Kozhevnikova 2006; Bhalerao et al. 2015). It plays diverse roles in the metabolism of plants, e.g., ureolysis, methane biogenesis, hydrogen metabolism, and acetogenesis (Collard et al. 1994; Mulrooney and Hausinger 2003; Vatansever et al. 2017). Ni is required more for legumes than other crops because it plays a vital role in nodulation, nitrogen (N) fixation, as well as ureides (allantoin, ALN; allantoic acid, ALA) transport to other plant organs (Zrenner et al. 2006; Sprent and James 2007; González-Guerrero et al. 2014; Freitas et al. 2018). Ni acts as a cofactor for urease and catalyzes the conversion of urea into ammonium ions (NH4+), which is further used by plants as a source of N (Sakamoto and Bryant 2001; Barcelos et al. 2018).
Although Ni is required in numerous metabolic phenomena, its elevated levels in the soil, however, are highly toxic to plants and threaten agricultural productivity (Gajewska and Skłodowska 2008; Shafeeq et al. 2012; Jamil et al. 2014). Ni has been placed at 57th position in the list of hazardous substances by ATSDR (American Agency for Toxic Substances and Disease Registry 2017). Ni-affected areas in the world include Australia, Canada, Cuba, Indonesia, South Africa, the USA, etc. (National Academy of Sciences (NAS) 1975; Duke 1980; Kasprzak 1987; Eisler 1998; Sreekanth et al. 2013). India is largely affected by Ni toxicity because groundwater Ni levels have been reported in the range of 10 to 129 mg kg−1 (Panwar et al. 2010; Sharma et al. 2013). Main sources of Ni pollution include mining, electrical batteries, metallurgical and electroplating industries, and chemical and food industries (Rathor et al. 2014; Bhalerao et al. 2015). In the last decade, Ni toxicity has become a serious issue, since it had reached up to 26,000 ppm in contaminated soils (Yusuf et al. 2011; Alloway 2012; Bhalerao et al. 2015). Excess of Ni in soil reduces seed germination, plant biomass, nutrient absorption by roots, N2 fixation, photosynthesis, respiration, yield, etc. (Pandey and Sharma 2002; Rahman et al. 2005; Gajewska et al. 2006; Vatansever et al. 2017; Shahzad et al. 2018). Moreover, it induces negative impact on legume-rhizobia symbiosis by disturbing nodulation and N2 fixation process (Seregin and Kozhevnikova 2006; Polacco et al. 2013). Soluble Ni (II) is substantially absorbed by cation channels passively and competes with other essential ions of metals (Zn, Cu, and Fe) when absorbed by roots (Gray and Mclaren 2006; Page et al. 2006).
In recent years, trehalose (Tre) (an organic compatible solute) has been reported to act as a carbon (C) source and safeguards the free-living state of rhizobia under HMs and salt stress conditions (Garg and Pandey 2016; Garg and Singla 2016; Garg and Singh 2018). It is synthesized by bacteria such as Rhizobium, yeast, certain species of fungi such as arbuscular mycorrhiza (AM), and plants (Crowe et al. 1992; Zahran 2010) and has been detected in bacteroids as well as root nodules of legumes (Müller et al. 2001). Moreover, synthesis of Tre has been correlated to the efficient N2 fixation and plant tolerance during drought, salinity, and metal stresses in legume species (Farías-Rodríguez et al. 1998; López et al. 2006; Garg and Singh 2018). It is synthesized by trehalose-6-phosphate synthase (T6PS) and trehalose-6-phosphatase (T6PP) enzyme in the nodules. Tre is then converted into two molecules of glucose by trehalase (TRE) (Jorge et al. 1997; Jules et al. 2008). However, reports on the impact of Ni on Tre biosynthesis in legumes are lacking.
Recent studies have indicated that exogenous application of polyamines (PAs), namely, putrescine (Put) [NH2(CH2)4NH2], spermidine (Spd) [NH2(CH2)3NH(CH2)4NH2], and spermine (Spm) [NH2(CH2)3NH(CH2)4NH(CH2)3NH2] has been reported to confer enhanced tolerance to heavy metals (HMs) stress in different crop plants (Bagni and Tassoni 2001; Duan et al. 2008; Gupta et al. 2013; Tiburcio et al. 2014). Nodules of legume crops accumulate 5–10 times higher PAs concentration than other plant organs (Fujihara et al. 1994; Efrose et al. 2008). Alterations in PA concentrations have been reported to modulate the control nodule number as well as biomass (Vassileva and Ignatov 1999; Terakado et al. 2006; Jiménez Bremont et al. 2014). Several researchers have indicated differential role of PAs in different crops under metal stress. Exogenous Spd and Spm have been found to reduce Cu stress in the leaves of Nymphoides peltatum (Wang et al. 2007), while in mung bean, Spm reduced the Cd stress (Nahar et al. 2016). In case of Ni stress, exogenous treatment of Put has been reported to stimulate accumulation of Spd and Spm, especially Put in Brassica napus, thus imparting stress tolerance (Shevyakova et al. 2011). However, reports on differential role of these three PAs (Put, Spd, and Spm) in alleviating Ni stress in case of legumes including pigeon pea are scanty.
In addition, arbuscular mycorrhiza (AM) are an integral part of the rhizosphere and can enhance the phytoremediation of HM-polluted soils through enhanced plant growth, water, nutrient uptake, as well as nitrogen fixation (Gonzalez-Chavez et al. 2002; Augé 2004; Javaid 2010; Shaker-Koohi 2014; Yang et al. 2015). The positive effects of AM inoculation on Ni tolerance in plants have been documented, with an AM Funneliformis mosseae-induced decline in Ni uptake in Festuca arundinacea (Shabani and Sabzalian 2016); Sorghum vulgare with two Glomus etunicatum isolates (Amir et al. 2013); Costularia comosa with Glomus etunicatum (Lagrange et al. 2011). Moreover, AM has also been reported to accumulate Tre in high concentration in the spores and sclerotia which serve as energy and carbohydrate reserves. Synthesis of Tre could help in phosphate release/transport to the plant, thereby improving nodulation potential (Ocón et al. 2007). Moreover, PAs have been reported to improve mycorrhizal colonization and infection in its initial stages of establishment (El Ghachtouli et al. 1995; Wu et al. 2012), since, various PAs especially, Put has been found in the spores of Funneliformis mosseae while Spd and Spm in Gigaspora rosea (El Ghachtouli et al. 1995; Sannazzaro et al. 2004). Additionally, exogenous Put, Spd, and Spm have been found to increase mycorrhizal colonization in Citrus limonia seedlings (Yao et al. 2010). However, relative and interactive effect of AM and PAs in enhancing N2 fixation, ureide synthesis, and Tre metabolism in legume crops under Ni toxicity has not been explored and needs detailed investigations.
Cajanus cajan (L.) Millsp (pigeon pea) is a major legume crop of the tropical and subtropical regions, covering massive areas of developing countries from Africa to Asia to Latin America. It is the third important grain legume worldwide, currently cultivated on 7.02 million hectares with 6.81 million metric tons (MMT) annual production. India is the largest producer with 5.38 million hectares under cultivation and 4.87 MMT annual production (FAOSTAT 2017). It is highly nutritional due to high quantity of proteins and amino acids such as methionine, lysine, and tryptophan. It has been extensively used in sustainable agricultural practices to enhance abiotic stress tolerance.
Therefore, the study was aimed to investigate the relative and interactive impacts of mycorrhization and/or three main PAs (Put, Spd, and Spm) in improving plant biomass, microbial symbiosis, and Tre metabolism, thereby imparting Ni tolerance in pigeon pea genotypes. The objectives of the study were to investigate (i) the impact of Ni toxicity on plant growth, mycorrhizal and rhizobial symbioses, ureide biosynthesis, and Tre metabolism in two differentially tolerant pigeon pea genotypes; (ii) the comparative impact of AM inoculation and PAs (Put, Spd, and Spm) priming in improving biomass accumulation and symbiotic efficiency under Ni stress; and (iii) functional complementarity between PAs and AM improving nitrogen-fixing potential under Ni stress in pigeon pea genotypes.
Materials and methods
Biological materials and experimental set-up
Experimental material taken for study included two differentially Ni-tolerant genotypes of Cajanus cajan L. (pigeon pea); relative tolerant, Pusa 2001; relative sensitive, AL 201, obtained from Indian Agricultural Research Institute (IARI), New Delhi, and Punjab Agricultural University (PAU), Ludhiana, India, respectively. Selection of these genotypes was based on screening of eight genotypes subjected to a range of NiSO4 (50–300 mg/kg) in order to determine upper limit of metal tolerance (TI) for growth and survivability among them.
Pigeon pea-specific rhizobial inoculum (Sinorhizobium fredii AR-4) was procured from Department of Microbiology (IARI), New Delhi. Pure spores of mycorrhizal fungus Rhizoglomus intraradices were sourced from The Energy and Resource Institute (TERI), New Delhi, and the inoculum was prepared through trap cultures using three consecutive host plants species (Sorghum bicolor L., Zea mays L., Coriandrum sativum L). The inoculum consisted of colonized root segments, filamentous hypha, and spores. Experiments were conducted in the Department of Botany, Panjab University, Chandigarh, India, located at 30°45’N, 76°45′E and elevation 305–348 m above sea level, with a relative humidity ranging from 43 to 55% (morning) and 35–48% (afternoon), minimum temperature 21–28 °C, and maximum from 35 to 43 °C. Experimental soil consisted of sand and loam (1:1 v/v) was obtained from agricultural fields, pH 7.4, ECe 0.83.6 dS/m−1, 0.41% total N (Nelson and Sommers 1973), 0.674% organic C (Estefan et al. 2013), 10.2 mg kg−1 P (Olsen and Sommers 1982), 0.79 meq/100 g Ca, 0.16 meq/100 g available K (Mehlich 1953), and 6.02 μg g−1 Ni concentration (Marguí et al. 2007).
Experiment design and nickel treatment
Circular earthenware pots (30 × 25 × 25 cm), sterilized with 70% ethanol, were lined with plastic bags and filled with 8 kg of autoclaved soil. Seeds were surface sterilized with 10% hydrogen peroxide (H2O2v/v) solution for 10 min and then washed four to five times with distilled water. Seeds were priming with 0.5 mM Put, Spd, and Spm (concentration was selected on the basis of preliminary trails) at room temperature for 12 h and coated with the rhizobial inoculum of Sinorhizobium fredii AR-4. Soil-based fungal inoculum (50 g per pot) of R. intraradices was placed underneath (1.5 cm) the seeds before sowing. All non-AM treatments were supplemented with an equal quantity of sterilized inoculum to maintain uniformity. Fifteen-day-old seedlings were treated with 200 mg/kg NiSO4 with/without Put, Spd, and Spm treatments and AM inoculations. Experimental units were organized in a completely randomized block design constituting factorial combination of 2 × 4 × 2 × 2 with two Ni concentrations [0 and 200 mg/kg]; two Put, Spd, and Spm treatments [0 mM (−) and 0.5 mM (+)]; two AM treatments [AM (+) and AM (−)]; and two pigeon pea genotypes (Pusa 2001 and AL 201). The plants were sampled at vegetative stage of 80 DAS (days after sowing), separated into shoots, roots, as well as nodules for analysis and oven dried at 70 °C for 72 h for dry weight measurements.
Mycorrhizal colonization and responsiveness
Mycorrhizal colonization (MC) was determined according to the method of McGonigle et al. (1990) following grid line intersect method. Root samples were autoclaved for 10 min with 10% (w/v) KOH solution, neutralized for 15 min in 20% HCl (v/v) and then stained with 0.05% trypan blue dye. The stained roots (approximately 1-cm length) were examined for root colonization using microscope, and per cent mycorrhizal colonization was recorded. Mycorrhizal responsiveness (MR) was calculated by using the method given by Hetrick et al. (1992).
Leghemoglobin (LHb) concentration and nitrogenase activity (acetylene reduction assay (ARA))
LHb concentration in nodules was analyzed by the method of Hartree (1957), based on the conversion of hematin to pyridine hemochromogen. Nitrogenase (N2ase) activity was measured as acetylene reduction assay according to Herdina and Silsbury (1990). The rate of N2ase activity was calculated as number of ethylene (C2H4) molecules produced per mg dry weight of nodules per hour (nmol C2H4 mg−1 nodule dry wt. h−1).
Nutrients status, metal analysis, and membrane stability index (MSI) in nodules
Dry nodules were digested with sulfuric acid and perchloric acid for the estimation of nitrogen (N) concentration by using colorimetric method of Lindner (1944). Phosphorus (P) was estimated by vanadomolybdophosphoric colorimetric method of Chapman and Pratt (1961). The concentration of Ni (in roots and nodules), Cu, Fe, and Zn (in nodules) was analyzed according to Marguí et al. (2007) through WDXRF spectrometer (TIGER Bruker). MSI was calculated by the method of Sairam et al. (1997) in which the electrical conductivity of nodules (500 mg) in double distilled water was measured in two sets. Set I at 40 °C for 30 min and set II at 100 °C in boiling water bath for 15 min and their respective electrical conductivities (C1 and C2) were measured by using digital conductivity meter. MSI was calculated as follows: MSI = 100 [1–(C1/C2)] × 100.
Ureide metabolism
Total ureide concentrations and allantoinase (ALNase) activity
Total ureide concentrations (TUs) – ALN and ALA – were determined according to Vogels and van der Drift (1970). Nodules were ground in 50-mM potassium phosphate buffer (pH 7), homogenates were centrifuged at 18,000×g for 20 min at 4 °C, and supernatant was collected. About 0.5 mL of 0.5 N NaOH was added to the supernatant, and the mixture was heated for 30 min at 90 °C in water bath. After cooling, 0.5 mL of 0.65 M HCl was added and again incubated for 15 min. Thereafter, 3.5 mL of water, 0.4-M phosphate buffer (pH 7), and 1 mL of phenylhydrazine solution were added. Tubes were then placed in an ice-water bath, and 5 mL of cold concentrated HCl, freshly prepared 1 mL of a potassium ferricyanide [K3Fe(CN)6] solution was added. Absorbance was read at 535 nm. and ALN and ALA were determined.
Fresh nodules were extracted in 50-mM tricine buffer with 2 mM MnSO4 and 35 mM β-mercaptoethanol and then incubated for 30 min at 30 °C. The incubated samples 250 μl were mixed with equal amount of 0.15 N HCl as well as 0.33% phenylhydrazine. Samples were kept in boiling water bath for 2 min and cooled before adding 1 ml cold concentrated HCl and 250 μl of 1.67% K3Fe(CN)6. The absorbance values were read at 520 nm to calculate the ALNase activity.
Urea concentration and urease activity
Modified procedure of Kyllingsbæk (1975) was used to estimate total urea concentration in the leaves. About 0.5 g of fresh material was extracted in 1.0 mL of 10-mM formic acid which was then centrifuged at 13,200×g for 5 min, at 4 °C. In 150 μL of aliquot, 3 mL of color developing reagent was added which was prepared by using a 1:1 proportion of the colorimetric reagent (7%, 0.2 M diacetyl monoxime; 7%, 0.05 M thiosemicarbazide) and the acid reagent (20%, H2SO4; 0.06%, 74 mM FeCl3 hexahydrate; 9%, ortho-phosphoric acid). The samples were incubated for 15 min at 99 °C, then kept in ice-cooled system under dark for 5 min to determine urea concentrations and absorbance was read at 540 nm.
Leaf urease activity was determined by the modified method of Hogan et al. (1983). Homogenate was prepared with phosphate buffer (pH 7.4) and centrifuged at 18,000×g for 20 min at 4 °C and incubated for 1 h at 30 °C. 0.5 mL of aliquot added to 2.5 mL of reagent 1 (0.1 M phenol; 170 μM of sodium nitroprusside) and 2.5 mL of reagent 2 (0.125 M sodium hydroxide; 0.15 M dibasic sodium phosphate; sodium hypochlorite, 3% of Cl2). Samples were then incubated at 37 °C for 35 min and read at 625 nm for determination of urease activity.
Trehalose metabolism
Tre concentration was determined through gas chromatography (Shimadzu GC-14B), according to the method of Streeter and Strimbu (1998). Fresh nodules were extracted in 80% methanol (v/v), incubated at 60 °C for 10 min, and extracts centrifuged at 13,000×g for 10 min. The pellet was re-extracted (thrice), and supernatant was vacuum dried. Solids were dissolved in equal amount of pyridine and STOX reagent. Derivatization of the samples was done by adding hexamethyldisilazane and trifluoroacetic acid (60 min). Trimethylsilyl (TXM)-oxime derivatives were separated using a gas chromatograph (packed column of 3% OV-17 on Chromosorb WHP). T6PS activity was measured by method of Salminen and Streeter (1986) and was read as release of UDP from UDP glucose consuming glucose-6-phosphate. T6PP activity was determined according to Padilla et al. (2004) by observing the liberation of phosphate (Pi) from trehalose-6-phosphate. TRE activity was estimated calorimetrically by quantifying the release of glucose according to method of Müller et al. (1994). Dinitrosulfosalicylic acid method was used to measure the glucose released as described by (Miller 1959).
Statistical analysis
Data was subjected to statistically be analyzed using statistical software SPSS 25.0 for Windows (SPSS, Inc. Chicago, IL, USA) The data presented in figures and tables is expressed as mean values based on six replicates ± standard error (SE) per treatment. Data were analyzed by ANOVA for the main effects (Ni, Spm, Spd, Put, AM, G) and their interactions. Duncan multiple range test (DMRT, p < 0.05) was used to evaluate the differences among the treatments, after performing one-way ANOVA. Regression analysis was applied to compare the individual impacts of six independent variables or factors (Ni, Spm, Spd, Put, AM, G) on a particular parameter and expressed as standardized coefficient (β). Correlational analysis (Pearson’s correlation coefficient-r) was carried out to investigate the correlations between relevant dependent variables for different parameters.
Results
Plant biomass and root/shoot ratio
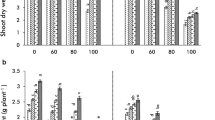
Addition of Ni in the rooting medium had a strong negative effect on both root as well as shoot dry weights (RDW, SDW, Fig. 1), the effects being stronger in roots than shoots as evident by comparing standardized β coefficients through regression analysis (Table 1) [RDW β(Ni) = − 0.624, SDW β(Ni) = − 0.566]. Higher negative effects on roots resulted in disturbed root to shoot ratios (RSR) Table 2. Genotype AL 201 was highly sensitive and displayed greater reductions in shoot and root biomass by 41.30% and 57.04%, respectively. On the other hand, genotype Pusa 2001 had better ability to tolerate Ni stress with reductions of 22.12% and 29.72% observed in SDW and RDW, respectively (Fig. 1), and significant Ni x G interaction (Table 3). Exogenous applications of all three PAs were significantly effective in improving root as well as shoot biomass in the order Put > Spd > Spm [RDW − β(Put) = 0.283, β(Spd) = 0.180, β(Spm) = 0.99; SDW − β(Put) = 0.329, β(Spd) = 0.180, β(Spm) = 0.110]. A complete comparison of data revealed that AM treatment was more effective in improving RDW and SDW as well as able to alleviate maximum Ni stress [RDW − β(AM) = 0.434, SDW − β(AM) = 0.420 (Table 1)] than all PAs treatments with much higher positive effects on roots than shoots which led to stronger RSR in Pusa 2001 even when compared with unstressed controls. All the PAs when combined with AM inoculations prove to be highly beneficial in imparting tolerance to Ni toxicity with treatment +Put+AM providing greatest positive impact in terms of both root and shoot growth in Pusa 2001 with almost complete mitigation of negative effects of Ni toxicity and partial positive effects in AL 201 (significant Ni x Put and Ni x AM interaction (Table 3).
Effect of polyamines (Put, Spd, Spm) and arbuscular mycorrhiza (AM, Rhizoglomus intraradices) on (a) shoot dry weight (SDW, g plant−1) and (b) root dry weight (RDW, g plant−1) in Pusa 2001 and AL 201 pigeon pea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C=PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put+AM = Put and AM added
Mycorrhizal colonization (MC) and mycorrhizal responsiveness (MR)
Microscopic analysis of the root segments revealed a strong percent MC in both genotypes (Pusa 2001–79.21% and AL 201–75.67%) under unstressed control conditions when inoculated with R. intraradices, with no colonization observed in uninoculated pigeon pea plants (Fig. 2). However, with introduction of Ni in the rooting medium, a reduction in MC to 62.34% in Pusa 2001 and 48.68% in AL 201 was recorded and indicated negative impact of Ni on colonization. A negative correlation was observed between MC and Ni toxicity in both the genotypes (Pusa 2001 r(Ni − MC) = − 0.860, p = 0.01; AL 201 r(Ni − MC) = − 0.945, p = 0.01). A combined +Put+AM treatment was able to improve the colonizing ability of both genotypes (90.39% in Pusa 2001 and 84.83% in AL 201, respectively) under unstressed conditions. A complete mitigation of detrimental effects of Ni could be observed with Put treatment in Pusa 2001 where the data was at par with the control series of plants. Spd was relatively more effective and significant in improving MC than Spm under Ni stress in a genotype-dependent manner. On the other hand, MR increased under Ni stress with Pusa 2001 exhibiting greater responsiveness toward R. intraradices than AL 201. PAs (mainly Put) reduced the responsiveness of both the genotypes significantly under stress conditions with almost insignificant effects under unstressed controls.
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM, Rhizoglomus intraradices) inoculation on (a) mycorrhizal colonization (MC, %) and (b) mycorrhizal responsiveness (MR, %) in Pusa 2001 and AL 201 pigeon pea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put+AM = Put and AM added
Rhizobial symbiosis
The symbiotic potential of both genotypes [nodule number, NN; nodule dry weights, NDW (Fig. 3)] declined with addition of Ni in the rooting medium with higher negative impact on AL 201 than Pusa 2001 [NN − β(Ni) = − 0.371, NDW − β(Ni) = − 0.407]. The decline in nodule biomass under Ni had a direct correlation with the functional efficiency of nodules, with a significant decline observed in LHb concentration along with a decline in rate of nitrogenase activity – N2ase (acetylene reduction activity, ARA) [Pusa 2001 r(NDW − LHb) = 0.968, p = 0.01; r(NDW − N2ase) = 0.908, p = 0.01; AL 201 r(NDW − LHb) = 0.982, p = 0.01; r(NDW − N2ase) = 0.929, p = 0.01]. The reduction observed in nodulation potential under stressed conditions could be directly correlated with reduced root biomass of both the genotypes [Pusa 2001 r(RDW − NDW) = 0.946, p = 0.01; AL 201 r(RDW − NDW) = 0.961, p = 0.01]. Exogenous Put application was more effective in improving NN under Ni stress, while AM-inoculated plants led higher nodule biomass as confirmed by comparing their standardized β coefficients [NN − β(Put) = 0.437, β(AM) = 0.402; NDW − β(Put) = 0.411, β(AM) = 0.561]. AM inoculated plants were able to restore LHb degeneration as well as ARA activity much more effectively than all the PAs under Ni stress [LHb − β(AM) = 0.456, β(Put) = 0.346, β(Spd) = 0.231, β(Spm) = 0.131; N2ase − β(AM) = 0.418, β(Put) = 0.321, β(Spd) = 0.199, β(Spm) = 0.103]. Combined exogenous application of both +Put+AM proved to be the best in improving NN, NDW, as well as the N-fixing potential under Ni stress with more positive effects in Pusa 2001 than AL 201.
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM, Rhizoglomus intraradices) inoculation on (a) nodule number (NN) plant−1, (b) dry weights of nodules (NDW, g plant−1), (c) leghemoglobin concentration (LHb, μg g−1 nodule f. wt.) and (d) nitrogenase activity (ARA, nmol ethylene mg NDW h−1) in Pusa 2001 and AL 201 pigeon pea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put+AM = Put and AM added
Nutrient status, Ni concentration, and MSI
The negative effects of Ni on both mycorrhizal and rhizobial symbiotic efficiencies had a direct impact on the nutrient status of nodules, analyzed in terms of N, P, Cu, Fe, and Zn (Table 4). The decline in N and P concentration in nodules was directly related with N2ase and MC, respectively [Pusa 2001 r(N − N2ase) = 0.891, p = 0.01; r(P − MC) = 0.932, p = 0.01; AL 201 r(N − N2ase) = 0.946, p = 0.01; r(P − MC) = 0.983, p = 0.01]. The decline in Cu, Fe, and Zn concentration was proportionate to the increase in Ni concentrations in the nodules, and negative correlation was observed between them [Pusa 2001 r(Ni − Zn) = − 0.532, r(Ni − Cu) = − 0.709, r(Ni − Fe) = − 0.727; AL 201 r(Ni − Zn) = − 0.603, r(Ni − Cu) = − 0.835, r(Ni − Fe) = − 0.914]. Individually, exogenous Put and AM supplementation improved the Ni status of nodules and roots within the nutritional range under unstressed conditions (Table 2). However, there was a significant increase in Ni uptake in both nodule and roots (more in roots, Table 2) when the plants were supplemented with Ni beyond the permissible limits more in AL 201 than Pusa 2001 [nodules β(Ni) = 0.798; roots β(Ni) = 0.873;Table 1]. Put and AM were able to significantly reduce Ni concentration in the nodules as well as roots with a concomitant increase in nutrient concentrations in Ni-stressed plants (significant Put x AM, Put x G, and AM x G interactions, Table 3). AM was comparatively more effective in enhancing nutrient acquisition as compared to PAs application [N concentration − β(AM) = 0.557, β(Put) = 0.377, β(Spd) = 0.237, β(Spm) = 0.121; P concentration − β(AM) = 0.569, β(Put) = 0.420, β(Spd) = 0.261, β(Spm) = 0.122]. The plants gained maximum assistance, when both Put and AM were given in combination leading to significantly lower toxic ion concentrations. In addition, Ni decreases the MSI in nodules of both genotype of pigeon pea plants (Table 2). Thus, in nodules MSI declined from 75.4 to 51.35% in Pusa 2001 and from 71.62 to 32.59 in AL 201 genotype, respectively, under Ni. However, exogenous supplementation of PAs and mycorrhization significantly increased MSI when compared with stressed counterparts. AM inoculation was more effective than PAs in improving the stability of membranes as authenticated through regression analysis (Table 1). The decline in MSI was proportionate to increase Ni concentration in the nodules, and negative correlation was observed between them (Pusa 2001 r(Ni − MSI) = − 0.827; AL 201 r(Ni − MSI) = − 0.944). Thus, +AM plants improved MSI of nodules 68.45% and 44.24% in Pusa 2001 and AL 201, respectively, under Ni exposure. Maximum stability was recorded when +Put+AM were applied simultaneously in a genotype-dependent manner.
Ureides metabolism
Ni stress significantly decreased the total ureides (TUs) concentration in nodules, more in AL201 (37.92%) than Pusa 2001 (19.4%) as compared to unstressed controls (Fig. 4). The reduction observed in TUs under stressed conditions could be directly correlated with N concentration in nodules [Pusa 2001 r(TUs − N) = 0.984, p = 0.01; AL 201 r(TUs − N) = 0.992, p = 0.01]. Both PAs and AM were able to increase ureide concentration under unstressed as well as stressed conditions. AM proved to be much more beneficial than PAs and could nullify the negative effects of Ni stress completely [TUs − β(AM) = 0.508, β(Put) = 0.353, β(Spd) = 0.224, β(Spm) = 0.119]. The combined treatments of AM and PAs were able to increase the ureide concentration in the nodules, even more than unstressed controls in Pusa 2001 (31.04%) (significant Put x AM interaction; Table 3). On the other hand, AL 201 was less responsive to both treatments under Ni stress and could display only a partial improvement.
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM, Rhizoglomus intraradices) inoculation on (a) total ureides concentration (μg g−1 f.wt.), (b) allantoin concentration (μg g−1 f.wt.), (c) allantoinase (μ moles allantoic acid formed mg−1 min−1), and (d) allantoic acid (μg g−1 f.wt.) in nodules of Pusa 2001 and AL 201 pigeon pea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put+AM = Put and AM added
The major ureides (ALN and ALA) were analyzed in the nodules under Ni stress with and without PAs and AM (data is presented in Fig. 4). A moderately positive correlation was observed between the Ni concentration in the nodules and ALN, ALA under Ni stress [Pusa 2001 r(Ni − ALN) = 0.591, p = 0.01; r(Ni − ALA) = 0.529, p = 0.01]. On the other hand, a strong positive correlation was observed between ALN and ALA in the nodules under Ni stress in both the genotypes [Pusa 2001 r(ALN − ALA) = 0.978, p = 0.01; r(ALN − ALA) = 0.968, p = 0.01]. An increase in synthesis of ALA could be directly correlated to an increase in the activity of enzyme ALNase (EC 3.5.2.5) [Pusa 2001 r(ALA − ALNase) = 0.982, p = 0.01; AL 201 r(ALA − ALAase) = 0.973, p = 0.01]. A further increase in the concentrations of ALN and ALA along with a significant increase in ALNase was observed when pigeon pea plants were treated with the three PAs, Put being more effective than Spd and Spm [ALN − β(Put) = 0.364, β(Spd) = 0.241; β(Spm) = 0.112; ALA − β(Put) = 0.418; β(Spd) = 0.281, β(Spm) = 0.128; ALNase − β(Put) = 0.310, β(Spd) = 0.186; β(Spm) = 0.091]. Introduction of R. intraradices was the most efficient in increasing the concentration of ureides through increased enzyme activity [TUs − β(AM) = 0.508; ALN − β(AM) = 0.466; ALNase − β(AM) = 0.488; ALA − β(AM) = 0.579]. The combined treatments of +Put+AM further boosted the synthesis of ALA with an increase of 57.2% in Pusa 2001 and 43.02% in AL 201 over their stressed counterparts in the nodules.
Upon transport of ALA to the leaves, its conversion to urea was analyzed, and the data is presented in Fig. 5. The presence of Ni in the rooting medium decreased the urea concentration in the leaves in a genotype-dependent manner along with a greater decline in the activity of enzyme urease [urea − β(Ni) = − 0.479; urease−β(Ni) = − 0.512]. Exogenous application of PAs mainly Put and AM increased the urea synthesis more under stressed than control conditions, the effects being more prominent in Pusa 2001 than AL 201. However, AM inoculations were more effective in increasing urea synthesis (Pusa 2001–32%, AL 201–24.5%) as well as urease activity (Pusa 2001–33.04%, AL 201–20.4%) over their respective stressed counterparts when compared with all the three PAs under Ni stress [urea − β(AM) = 0.629, β(Put) = 0.458, β(Spd) = 0.318, β(Spm) = 0.156; urease − β(AM) = 0.537, β(Put) = 0.372, β(Spd) = 0.256, β(Spm) = 0.134]. The combined treatment of Put and AM inoculation was most efficient in increasing urea as well as urease activity.
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM, Rhizoglomus intraradices) inoculation on (a) urea (μ moles urea g−1 f.wt.) and (b) urease (μ moles NH4+ liberate mg−1 min−1) in Pusa 2001 and AL 201 pigeon pea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put+AM = Put and AM added
Trehalose metabolism
An increase in Tre concentration in the nodules was recorded when Ni was applied in the rooting medium (Fig. 6). This increase could be related to an upsurge in the activity of both biosynthetic enzymes, i.e.,T6PS and T6PP [Pusa 2001 r(Tre − T6PS) = 0.998, p = 0.01; r(Tre − T6PP) = 0.996, p = 0.01; AL 201 r(Tre − T6PS) = 0.989, p = 0.01; r(Tre − T6PP) = 0.985, p = 0.01]. On the other side, a negative correlation was observed between Tre synthesis and TRE activity [Pusa 2001 r(Tre − TRE) = − 0.992, p = 0.01; AL 201 r(Tre − TRE) = − 0.996, p = 0.01]. Nodules of Pusa 2001 showed better ability for osmotic adjustment by synthesizing higher amounts of Tre with greater reduction in TRE activity when compared to AL 201. AM inoculation further enhanced the activity of anabolic enzymes as well as Tre concentration in a genotype-dependent manner. However, PAs were relatively less effective in modulating Tre metabolism when compared with AM as validated through regression analysis [Tre, β(AM) = 0.376, β(Put) = 0.263, β(Spd) = 0.155, β(Spm) = 0.081; T6PP, β(AM) = 0.409, β(Put) = 0.278, β(Spd) = 0.169, β(Spm) = 0.079; T6PS, β(AM) = 0.358, β(Put) = 0.250, β(Spd) = 0.155, β(Spm) = 0.072]. Moreover, a negative correlation observed between Tre and N2ase activity indicated that nodules with reduced N2ase activity tended to synthesize more Tre [Pusa 2001 r(Tre − N2ase) = − 0.109, p = 0.01; r(Tre − N2ase) = − 0.345, p = 0.01]. Maximum quantities of Tre could be attained when both +Put+AM were given together, especially in Pusa 2001 (significant interaction Put x AM x G; Table 3).
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM, Rhizoglomus intraradices) inoculation on (a) trehalose concentration (Tre, μg g−1 f.wt.), (b) trehalase activity (TRE, nmol glucose mg−1 protein hr−1), (c) trehalose-6-phosphate synthase activity (T6PS, nmol UDP mg−1 protein hr−1), and (d) trehalose-6-phosphatase activity (T6PP, nmol Pi mg−1 protein min−1) in Pusa 2001 and AL 201 pigeon pea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put+AM = Put and AM added
Discussion
The negative impact of Ni toxicity was elicited in the form of reduced plant biomass, the effects being more severe on RDW when compared with the SDW, thus resulting in reduced root to shoot ratios. The data also clearly demonstrated the differential response of two relatively tolerant pigeon pea genotypes under Ni stressed conditions, with higher reductions recorded in AL 201 than Pusa 2001. Roots are the first organ to face Ni stress, therefore experiencing higher harmful effects than shoots as reported in pigeon pea (Rao and Sresty 2000) and chickpea (Khan and Khan 2010). Reduction in plant biomass was proportionated to increased Ni concentrations which were significantly higher in roots than shoots in a genotype-dependent manner. The negative effects of Ni could also be attributed to modification of morphological and physiological characteristics (total biomass, leaves number, plant height, and root length) as observed in Amaranthus viridis (Joseph et al. 2018). The negative effects of Ni on root growth could also be due to retardation of cell division and alteration of mitosis in root apex zone (Kozhevnikova et al. 2007) as investigated in soybean, lentil (Jamal et al. 2002), tomato (Madhaiyan et al. 2007), as well as maize (Gopal et al. 2014). Moreover, approximately 50% of the absorbed Ni is stored in the roots because of its sequestration inside walls of xylem parenchymatic cells via cation exchange and immobilization in vacuoles (Seregin and Kozhevnikova 2006; Sachan and Lal 2017).
The colonizing ability of pigeon pea roots with AM fungus Rhizoglomus intraradices had a negative correlation with the presence of Ni in the soil. Genotype Pusa 2001 had better ability to colonize and establish effective mycorrhizal symbiosis than AL 201. The symbiosis formed between plant and soil fungi varies in effectiveness based on the plant species and even the genotype of the same species (Singh et al. 2012; Bazghaleh et al. 2018). However, Ni-induced toxicity was more severe on plant growth when compared with mycorrhizal symbiosis. On the other hand, MR increased under Ni stress with Pusa 2001 exhibiting greater responsiveness toward AM than AL 201. The decline in MC could either be due to the direct effects of Ni on germination of the spore, hyphal branching, and development in soil or because of the adverse effects of Ni on root (Ker and Charest 2010; Twanabasu et al. 2013) as investigated in our study. However, ability of AM to effectively colonize even the sensitive genotype could be due to fact that AM fungal propagules never disappear completely even in highly HM-polluted soils (Vallino et al. 2006).
In addition to mycorrhizal symbiosis, the roots of both pigeon pea genotypes formed an efficient rhizobial symbiosis under unstressed control series. However, with the introduction of Ni to the rooting medium, the nodulation potential (NN, NDW) was reduced in line with the reduction in root biomass in a genotype-dependent manner. Although under control conditions, rhizobia were capable of actively infecting pigeon pea roots; the presence of HMs (such as Ni) might have prevented the root hair formation and affected the infection process, thereby limiting the number of active nodules in legume plants (Gage 2004; Ishtiaq and Mahmood 2011; Dudeja et al. 2012; de Macedo et al. 2016). In addition, the functional efficiency of nodules (ARA) also declined under Ni toxicity, with a direct correlation between LHb concentration and ARA, suggesting the determination of LHb as a good indicator of N2-fixing efficiency. Poor root nodulation in soybean plants grown in Ni-stressed soils interfered in the N2-fixing system causing direct toxic effects on rhizobia and inhibition of LHb synthesis (de Macedo et al. 2016). Inhibition of nodulation and nitrogenase activity under Ni toxicity has also been reported in Frankia-Alnus (Wheeler et al. 2001) and Lens culinaris-Rhizobium leguminosarum symbiosis (Saad et al. 2016). Haddad et al. (2015) reported significant decline in nodulation and N uptake with increasing HM concentrations (Ni, Co, Cr, Cd, Cu, and Pb) on three leguminous crops (Vicia faba, Trifolium alexandrinum, Glycine max). The observed decrease in N2 fixation could be due to the reduction in O2 flux into the nodule under environmental stress (Hunt and Layzell 1993) and also due to impaired carbon (C) metabolism which could limit for bacteroid respiration (González et al. 2001). This fact justifies the decreased atmospheric N2 fixation due to low symbiotic activity of microbes at high Ni concentrations in soil.
The present study indicated that reduced mycorrhizal and rhizobial symbioses under Ni stress had a direct effect on nutrient (P and N) concentrations in the nodules. The decline in P and N was accompanied by reduction in Zn, Fe, and Cu concentrations as well. This nutrient imbalance was proportionate to the increase in Ni uptake due to loss of nodular membrane stability more in AL 201 than Pusa 2001. Garg and Bharti (2018) reported that salinity caused membrane damage in chickpea plants that could be a major reason for reduced growth and nutrient uptake, with greater membrane damage in the sensitive genotype than tolerant one. High concentration of Ni in nodules reduces influx of nutrient (N, P, K, Cu, Fe, and Zn) due to more accumulation of Ni inside the nodules which results in nutrient imbalance as observed in different crops such as barley, soybean, Lens culinaris (Rahman et al. 2005; de Macedo et al. 2016; Saad et al. 2016). In addition, Ni strongly competes with several cations (in particular Cu, Fe, and Zn) due to the same transporters (ABC, Nramp, CTR, and ZIP families of metal transporters) preventing them from being absorbed by plants, which ultimately leads to their deficiency (Pandey and Sharma 2002; Tamayo et al. 2014; Sachan and Lal 2017) as authenticated in our study as well.
Ureides (ALN and ALA) have been known as important N-rich compounds, playing a crucial role in the assimilation, metabolism, transport, and storage of N in several plant species such as Vigna unguiculata and V. radiata (Castro et al. 2001; Todd et al. 2005; Freitas et al. 2018). In this study, the effect of Ni toxicity on ureide metabolism in nodules of pigeon pea demonstrated that TUs concentration decreased under Ni stress which was in line with a significant decline in N2-fixing efficiency and ultimate increased Ni concentration in nodules. The decreased TUs were however accompanied by an increase in ALN, ALA, and ALNase, the effects being stronger in Pusa 2001 as compared to AL 201. ALNase, a hydrolyzing enzyme, plays an important role in ureide metabolism, because this enzyme catalyzes the last step of ureide biosynthesis (i.e., conversion of ALN to ALA) in nodules (Pélissier and Tegeder 2007; Werner et al. 2010). The increased activity of ALNase under stress has been linked with a direct increase in ALA content in the nodules. Alamillo et al. (2010) has reported that induction of ureides (ALN and ALA) in nodules mitigated the negative impacts of drought stress and conferred stress resistance to Phaseolus vulgaris plants. To elucidate this, researchers proposed that ureides accumulation might be having a regulatory role and result in N-feedback inhibition of the N2ase complex (Vadez et al. 2000; King and Purcell 2005). However, in our study, the increased ALN and ALA due to increased ALNase activity did not seem to be effective in ameliorating Ni-induced toxic symptoms in the nodules of even the tolerant genotype. Ultimately, ureides get translocated to the leaves as urea (Zrenner et al. 2006; Ladrera et al. 2007). Urea is an important source of ammonia (NH3), and the efficiency of urea is decreased after its hydrolysis with the enzyme urease, therefore reducing NH3 emissions under stress leading to large N losses (Sanz-Cobena et al. 2008). Legumes require N in large quantities, and its limited availability negatively effects the plant growth (Masclaux-Daubresse et al. 2010; Takagi et al. 2018). In our study, reduction observed in urea accumulation in the leaves might have led to reduced urease activity as well as ultimate production of NH4+ ions consequently resulting in a decline in the available N source under Ni toxicity. Similar results (reduced leaf urea accumulation and urease activity) were also obtained in cucumber and soybean under Ni toxicity (Khoshgoftarmanesh et al. 2014; de Queiroz Barcelos et al. 2017).
Current study revealed that exogenous applications of both PAs (Put, Spd, and Spm) as well as AM (Rhizoglomus intraradices) led to a remarkable improvement in plant biomass, nutrient status, and nodulation potential of pigeon pea genotypes even under stressed conditions. Regression analysis displayed that comparatively AM inoculation was more effective in lowering the toxic effects of Ni when compared with PAs priming. Such beneficial effects could be direct – by adsorption or chelation of HMs – or indirect through improved nutrient status (Zhang et al. 2015). Higher improvement in plant biomass under Ni stress by AM inoculation may be attributed to its ability to decrease concentration of endogenous Ni in the nodules more efficiently than PAs. The beneficial effects were more discernible in Pusa 2001 due to its better potential to establish AM colonization than AL 201. AM associated extensive hyphal network in root rhizosphere of tolerant genotype was able to explore more soil area, thus leading to significant improvement in nutrient accumulation in the nodules, especially P and N, and the resultant higher beneficial effects on the functional efficiency of nodules. Increased P assimilation influences the N2ase activity positively, which in turn enhances root as well as mycorrhizal growth (Stancheva et al. 2006). AM fungi increase the absorptive root surface by producing profuse hyphae and by taking up nutrients from relatively remote regions (Abbott and Robson 1991; Bolan 1991; Bitterlich et al. 2018). Our results indicated that exogenous application of AM enhanced root growth and improved root rhizosphere which resulted in the uptake of N, P, Zn, Fe, and Cu in nodules under Ni stress, by reducing the Ni uptake from the soil. PAs, especially Put and Spd, were also significantly effective in enhancing biomass, N-fixing potential, and reducing Ni uptake more in Pusa 2001 than AL 201. This enhanced plant biomass could be due to the involvement of PAs in cell division, replication, and transcription (Chen et al. 2018). PAs have been found to suppress the accumulation of HMs (Ni, Cd, Zn, Cu, and Pb) which resulted in an increase in the tolerance of wheat plants against these HMs (Aldesuquy et al. 2014). Among the three PAs, Put priming was relatively more effective than AM in increasing NN which might due to greater ability of rhizobia to infect pigeon pea roots. Similar results were reported by Vassileva and Ignatov (1999) where Put application increased NN and nodular biomass as well as induced N2 fixation by enhancing the potential of Rhizobium galegae strain HAMBI540 to infect Galega roots. However, the nodules observed were small in size, suggesting that PAs were not very effective in increasing nodule growth and N-fixing potential. On the other hand, AM application was highly capable of increasing nodule development and improving nodule biomass as well as N2-fixing efficacy. In one of our previous lab studies, AM was more effective in increasing NDW, LHb concentration, and N2ase activity in which the Put treatment increases NN under salt stress in pigeon pea (Garg and Sharma 2019). In the current study, enhanced P status in AM-treated plant might have improved the energy status required for effective rhizobial symbiosis, thereby resulting in improved N2 fixation more efficiently than all three PAs.
Existing study also revealed that AM inoculated plants could increase the accumulation of ureides, namely, ALN and ALA, in the nodules significantly and were able to mitigate the negative impact of Ni more than PAs. Put was more effective as compared to Spd and Spm but less effective than AM inoculation. Moreover, the exogenous application of AM and PAs treatments improved the urea accumulation in leaves and ultimately increased the activity of urease enzyme, making more N available to the plants and protecting them against Ni stress. In our study, the increased ALN and ALA synthesis and their degradation into urea with the help of increased urease activity more in AM inoculated plants than PAs, since AM could possibly be related to elevation in N2 fixation which then resulted in high NH3 concentration in the form of increased N status in pigeon pea plants.
In the present investigation, very low levels of Tre were observed in the nodules of the two genotypes under unstressed controls due to high activity of TRE. However, presence of Ni induced decline in TRE activity and increased Tre concentration to a certain extent only. Interestingly, negative correlation between Tre and N2ase activity indicated that nodules with reduced N2ase activity under Ni supply tended to synthesize more Tre, suggesting that more Tre is synthesized because of low demand for reduced C in the bacteroids (Streeter and Salminen 1988). Tre might also protect nitrogenase from inactivation since it has antioxidant effect (González-Párraga et al. 2003). Moreover, a significant decline in TRE activity was recorded with AM inoculations which resulted in significant improved Tre concentrations in the nodules. This increment in Tre was accompanied by increased activity of both enzyme T6PS and T6PP indicating direct role of AM in upregulating Tre biosynthesis. The genotype Pusa 2001 which could establish a stronger association with AM and Rhizobium also displayed higher Tre concentration when compared with AL 201. Tre serves as energy source for the growth and survival of rhizobia in the deteriorating nodule (Zacarías et al. 2004; Iturriaga et al. 2009), and its enhanced synthesis through AM might have helped pigeon pea plants to overcome Ni stress as investigated in our study. Moreover, AM has also been reported to accumulate high concentrations of Tre in spores, extra radical mycelium, sclerotia where it functions as energy reserves, and intermediate C storage (Bécard et al. 1991; Ocón et al. 2007; Ballesteros-Almanza et al. 2010). In addition, high amount of Tre in the nodules of mycorrhizal plants was proportionate to higher P concentration, where it is present in the intra-radical hyphae of mycorrhiza which could favor phosphate production and translocation (Ocón et al. 2007). Although reports on the impact of Ni stress in Tre metabolism are lacking, exogenous supplementation of Tre has been observed to increase the endogenous Tre subjected to Cu stress in rice signifying its additional protective role in antioxidant induction (Mostofa et al. 2015). The overexpression of Tre synthesizing genes leading to greater T6PP activity as well as Tre accumulation has also been reported under Cd, Cu, and salt stress (Martins et al. 2014; Krasensky et al. 2014) stating its importance in imparting HMs stress tolerance. Increased Tre biosynthesis through the three PAs treatments (mainly Put), observed in the current study, revealed its indirect role in enhancing growth and mycorrhizal symbiosis in pigeon pea plants.
The combined application of PAs and AM inoculation was extremely beneficial in increasing root and shoot biomass, nodulation potential, and mycorrhizal effectiveness as well as boosting Tre synthesis when compared with their individual treatments. Among the various combinations of different PAs with their respective AM treatments, Put-primed AM-inoculated plants (R. intraradices) had higher MC which indicated that the combination of these two treatments was highly competent in enhancing the nodulation and N2-fixing efficiency of pigeon pea plants. Exogenous PAs might have stimulated AM spore germination, hyphal growth, and branching which led to an efficient establishment of mycorrhizal symbiosis with R. intraradices, thereby increasing Tre biosynthesis and alleviating Ni toxicity through enhanced nutrient uptake. The improved nutrient status (especially P) resulted in improved ureides synthesis (ALN and ALA) in the nodules as well as their subsequent translocation and conversion into urea in the leaves for utilization as N source to growing pigeon pea plants.
Conclusion
The present study demonstrated that Ni had deleterious effects on plant growth and rhizobial and mycorrhizal symbioses. The resultant declined nutrient status of the nodules was proportionate to increased Ni uptake in the roots as well as its translocation to nodules. These negative effects had a direct bearing on ureide and urea synthesizing capacity of plants. Put priming facilitated mycorrhizal symbiosis which, in turn, improved the colonization ability of pigeon pea plants with rhizobia. Thus, present study highlighted the importance of PAs in complementing AM symbiosis, thereby modulating Tre and ureide metabolism, which could be a determining factor for efficient nodule functioning, especially in the tolerant genotype of pigeon pea subjected to Ni stress.
References
Abbott LK, Robson AD (1991) Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric Ecosyst Environ 35:121–150
Agency for Toxic Substances and Disease Registry (ATSDR) (2017) Priority list of hazardous substances. www.atsdr.cdc.gov/spl/index
Alamillo JM, Diaz-Leal JL, Sanchez-Moran MV, Pineda M (2010) Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ 33:1828–1837
Aldesuquy H, Haroun S, Abo-Hamed S, El-Saied AW (2014) Involvement of spermine and spermidine in the control of productivity and biochemical aspects of yielded grains of wheat plants irrigated with waste water. Egypt J Basic Appl Sci 1:16–28
Alloway BJ (2012) (Ed), Heavy metals in soils: trace metals and metalloids in soils and their bioavailability (Vol. 22), Springer Science & Business Media
Amir H, Lagrange A, Hassaïne N, Cavaloc Y (2013) Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23:585–595
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Bagni N, Tassoni A (2001) Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 20:301–317
Ballesteros-Almanza L, Altamirano-Hernandez J, Pena-Cabriales JJ, Santoyo G, Sanchez-Yanez JM, Valencia-Cantero E, Macias-Rodriguez L, Lopez-Bucio J, Cardenas-Navarro R, Farias-Rodriguez R (2010) Effect of co-inoculation with mycorrhiza and rhizobia on the nodule trehalose content of different bean genotypes. Open Microbiol J 4:83–92
Barcelos JPQ, Reis HPG, Godoy CV, Gratão PL, Furlani Junior E, Putti FF, Reis AR (2018) Impact of foliar nickel application on urease activity, antioxidant metabolism and control of powdery mildew (Microsphaera diffusa) in soybean plants. Plant Pathol 67:1502–1513
Bazghaleh N, Hamel C, Gan Y, Tar’an B, Knight JD (2018) Genotypic variation in the response of chickpea to arbuscular mycorrhizal fungi and non-mycorrhizal fungal endophytes. Can J Microbiol 64:265–275
Bécard G, Doner LW, Rolin DB, Douds DD, Pfeffer PE (1991) Identification and quantification of trehalose in vesicular-arbuscular mycorrhizal fungi by in vivo13C NMR and HPLC analyses. New Phytol 118:547–552
Bhalerao SA, Sharma AS, Poojari AC (2015) Toxicity of nickel in plants. Int J Pure Appl Biosci 3:345–355
Bitterlich M, Franken P, Graefe J (2018) Arbuscular mycorrhiza improves substrate hydraulic conductivity in the plant available moisture range under root growth exclusion. Front Plant Sci 9:1–11
Bolan NS (1991) A critical review of the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134:189–207
Castro AHF, Young MC, Alvarenga AAD, Alves JD (2001) Influence of photoperiod on the accumulation of allantoin in comfrey plants. Rev Bras Fisiol Veg 13:49–54
Chapman HD, Pratt FP (1961) Ammonium vandate-molybdate method for determination of phosphorus, methods of analysis for soils. Plants Water 1:184–203
Chen D, Shao Q, Yin L, Younis A, Zheng B (2018) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1–13
Collard JM, Corbisier P, Diels L, Dong Q, Jeanthon C, Mergeay M, Wuertz S (1994) Plasmids for heavy metal resistance in Alcaligenes eutrophus CH34: mechanisms and applications. FEMS Microbiol Rev 14:405–414
Crowe JH, Hoekstra FA, Crowe LM (1992) Anhydrobiosis. Annu Rev Physiol 54:579–599
de Macedo FG, Bresolin JD, Santos EF, Furlan F, da Silva L, Wilson T, Polacco JC, Lavres J (2016) Nickel availability in soil as influenced by liming and its role in soybean nitrogen metabolism. Front Plant Sci 7:1–12
de Queiroz Barcelos JP, de Souza Osório CRW, Leal AJF, Alves CZ, Santos EF, Reis HPG, dos Reis AR (2017) Effects of foliar nickel (Ni) application on mineral nutrition status, urease activity and physiological quality of soybean seeds. Aust J Crop Sci 11:184–192
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165:1620–1635
Dudeja SS, Sheokand S, Kumari S (2012) Legume root nodule devel legume root nodule development and functioning under opment and functioning under tropics and subtropics: perspectives and challenges. Legum Res 35:85–103
Duke JM (1980) Production and uses of nickel. In: Nriagu JO (ed) Nickel in the environment. Wiley, New York, pp 51–65
Efrose RC, Flemetakis E, Sfichi L, Stedel C, Kouri ED, Udvardi MK, Kotzabasis K, Katinakis P (2008) Characterization of spermidine and spermine synthases in Lotus japonicus: induction and spatial organization of polyamine biosynthesis in nitrogen fixing nodules. Planta 228:37–49
Eisler R (1998) Nickel hazards to fish, wildlife, and invertebrates: a synoptic review. Biological science report GS/BRD/BSR- 1998-0001, Patuxent Wildlife Research Center, U.S. Geological Survey, Laurel, MD 20708
El Ghachtouli N, Paynot M, Morandi D, Martin-Tanguy J, Gianinazzi S (1995) The effect of polyamines on endomycorrhizal infection of wild-type Pisum sativum, cv. Frisson (nod+ myc+) and two mutants (nod− myc+ and nod− myc−). Mycorrhiza 5:189–192
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region. pp 170–176
FAOSTAT (2017) http://www.fao.org/faostat/en/#data/QC
Farías-Rodríguez R, Mellor RB, Arias C, Peña-Cabriales JJ (1998) The accumulation of trehalose in nodules of several cultivars of common bean (Phaseolus vulgaris) and its correlation with resistance to drought stress. Physiol Plant 102:353–359
Freitas DS, Rodak BW, dos Reis AR, de Barros RF, de Carvalho TS, Schulze J, Guilherme LRG (2018) Hidden nickel deficiency? Nickel fertilization via soil improves nitrogen metabolism and grain yield in soybean genotypes. Front Plant Sci 9:1–16
Fujihara S, Abe H, Minakawa Y, Akao S, Yoneyama T (1994) Polyamines in nodules from various plant-microbe symbiotic associations. Plant Cell Physiol 35:1127–1134
Gage DJ (2004) Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev 68:280–300
Gajewska E, Skłodowska M (2008) Differential biochemical responses of wheat shoots and roots to nickel stress: antioxidative reactions and proline accumulation. J Plant Growth Regul 54:179–188
Gajewska E, Skłodowska M, Słaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Plant 50:653–659
Garg N, Bharti A (2018) Salicylic acid improves arbuscular mycorrhizal symbiosis, and chickpea growth and yield by modulating carbohydrate metabolism under salt stress. Mycorrhiza 28:727–746
Garg N, Pandey R (2016) High effectiveness of exotic arbuscular mycorrhizal fungi is reflected in improved rhizobial symbiosis and trehalose turnover in Cajanus cajan genotypes grown under salinity stress. Fungal Ecol 21:57–67
Garg N, Sharma A (2019) Role of putrescine (Put) in imparting salt tolerance through modulation of put metabolism, mycorrhizal and rhizobial symbioses in Cajanus cajan (L.) Millsp. Symbiosis 1–16
Garg N, Singh S (2018) Mycorrhizal inoculations and silicon fortifications improve rhizobial symbiosis, antioxidant defense, trehalose turnover in pigeon pea genotypes under cadmium and zinc stress. Plant Growth Regul 86:105–119
Garg N, Singla P (2016) Stimulation of nitrogen fixation and trehalose biosynthesis by naringenin (Nar) and arbuscular mycorrhiza (AM) in chickpea under salinity stress. Plant Growth Regul 80:5–22
González EM, Gálvez L, Royuela M, Aparicio-Tejo P, Arrese-Igor C (2001) Insights into the regulation of nitrogen fixation in pea nodules: lessons from drought, abscisic acid and increased photoassimilate availability. Agronomie 21:607–613
Gonzalez-Chavez C, D'haen J, Vangronsveld J, Dodd JC (2002) Copper sorption and accumulation by the extraradical mycelium of different Glomus spp. (arbuscular mycorrhizal fungi) isolated from the same polluted soil. Plant Soil 240:287–297
González-Guerrero M, Matthiadis A, Sáez A, Long TA (2014) Fixating on metals: new insights into the role of metals in nodulation and symbiotic nitrogen fixation. Front Plant Sci 13:5–45
González-Párraga P, Hernández JA, Argüelles JC (2003) Role of antioxidant enzymatic defences against oxidative stress (H2O2) and the acquisition of oxidative tolerance in Candida albicans. Yeast 20:1161–1169
Gopal R, Neelam C, Tapan A (2014) Effect of variation in nickel concentration on growth of maize plant: a comparative over view for pot and Hoagland culture. Res J Chem Sci 4:30–32
Gray CW, Mclaren RG (2006) Soil factors affecting heavy metal solubility in some New Zealand soils. Water Air Soil Pollut 175:3–14
Gupta K, Dey A, Gupta B (2013) Plant polyamines in abiotic stress responses. Acta Physiol Plant 35:2015–2036
Haddad SA, Tabatabai MA, Abdel-Moneim AMA, Loynachan TE (2015) Inhibition of nodulation and nitrogen nutrition of leguminous crops by selected heavy metals. Air Soil Water Res 8:1–7
Hartree EF (1957) Haematin compounds. In: Paech K, Tracey MV (eds) Modern methods of plant analysis. Springer-Verlag, Germany, Berlin, pp 197–245
Herdina JA, Silsbury JH (1990) Estimating nitrogenase activity of faba bean (Vicia faba) by acetylene reduction (AR) assay. Aust J Plant Physiol 17:489–502
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hogan ME, Swift IE, Done J (1983) Urease assay and ammonia release from leaf tissues. Phytochemistry 22:663–667
Hunt S, Layzell DB (1993) Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu Rev Plant Physiol Plant Mol Biol 44:483–511
Ishtiaq S, Mahmood S (2011) Phytotoxicity of nickel and its accumulation in tissues of three Vigna species at their early growth stages. J Appl Bot Food Qual 84:223–228
Iturriaga G, Suárez R, Nova-Franco B (2009) Trehalose metabolism: from osmoprotection to signaling. Int Mol Sci 10:3793–3810
Jamal A, Ayub N, Usman M, Khan AG (2002) Arbuscular mycorrhizal fungi enhance zinc and nickel uptake from contaminated soil by soybean and lentil. Int J Phytorem 4:205–221
Jamil M, Zeb S, Anees M, Roohi A, Ahmad I, Rehman S, Rha ES (2014) Role of Bacillus licheniformis in phytoremediation of nickel contaminated soil cultivated with rice. Int J Phytorem 16:554–571
Javaid A (2010) Role of arbuscular mycorrhizal fungi in nitrogen fixation in legumes. In: Khan MS, Musarrat J, Zaidi A (eds) Microbes for legume improvement. Springer, Vienna, pp 409–426
Jiménez Bremont JF, Marina M, Guerrero-González MD, Rossi FR, Sánchez-Rangel D, Rodríguez-Kessler M, Ruiz OA, Gárriz A (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:1–14
Jorge JA, Polizeli MDLT, Thevelein JM, Terenzi HF (1997) Trehalases and trehalose hydrolysis in fungi. FEMS Microbiol Lett 154:165–171
Joseph J, Reddy J, Sayantan D (2018) Effect of nickel uptake on selected growth parameters of Amaranthus viridis L. J Appl Nat Sci 10:1011–1017
Jules M, Beltran G, François J, Parrou JL (2008) New insights into trehalose metabolism by Saccharomyces cerevisiae: NTH2 encodes a functional cytosolic trehalase, and deletion of TPS1 reveals Ath1p-dependent trehalose mobilization. Appl Environ Microbiol 74:605–614
Kasprzak KS (1987) Nickel. Adv Mod Environ Toxicol 11:145–183
Ker K, Charest C (2010) Nickel remediation by AM-colonized sunflower. Mycorrhiza 20:399–406
Khan MR, Khan MM (2010) Effect of varying concentration of nickel and cobalt on the plant growth and yield of chickpea. Aust J Basic Appl Sci 4:1036–1046
Khoshgoftarmanesh AH, Bahmanziari H, Sanaeiostovar A (2014) Responses of cucumber to deficient and toxic amounts of nickel in nutrient solution containing urea as nitrogen source. Biol Plant 58:524–530
King CA, Purcell LC (2005) Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol 137:389–1396
Kozhevnikova AD, Seregin IV, Bystrova EI, Ivanov VB (2007) Effects of heavy metals and strontium on division of root cap cells and meristem structural organization. Russ J Plant Physiol 54:257–266
Krasensky J, Broyart C, Rabanal FA, Jonak C (2014) The redox-sensitive chloroplast trehalose-6-phosphate phosphatase AtTPPD regulates salt stress tolerance. Antioxid Redox Signal 21:1289–1304
Kyllingsbæk A (1975) Extraction and colorimetric determination of urea in plants. Acta Agric Scand 25:109–112
Ladrera R, Marino D, Larrainzar E, González EM, Arrese-Igor C (2007) Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiol 145:539–546
Lagrange A, Ducousso M, Jourand P, Majorel C, Amir H (2011) New insights into the mycorrhizal status of Cyperaceae from ultramafic soils in New Caledonia. Can J Microbiol 57:21–28
Lindner RC (1944) Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol 19:76
López M, Herrera-Cervera JA, Lluch C, Tejera NA (2006) Trehalose metabolism in root nodules of the model legume Lotus japonicus in response to salt stress. Physiol Plant 128:701–709
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228
Marguí E, Queralt I, Carvalho ML, Hidalgo M (2007) Assessment of metal availability to vegetation (Betula pendula) in Pb-Zn ore concentrate residues with different features. Environ Pollut 145:179–184
Martins LL, Mourato MP, Baptista S, Reis R, Carvalheiro F, Almeida AM, Fevereiro P, Cuypers A (2014) Response to oxidative stress induced by cadmium and copper in tobacco plants (Nicotiana tabacum) engineered with the trehalose-6-phosphate synthase gene (AtTPS1). Acta Physiol Plant 36:755–765
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115:495–501
Mehlich A (1953) Determination of P, Ca, mg, K, Na and NH4. In: Short test methods used in soil testing division. Department of Agriculture, Raleigh
Miller GL (1959) Use of dinitrosulphosalicylic acid (DNSA) reagent for determination of reducing sugar. Anal Chem 31:426–428
Mostofa MG, Hossain MA, Fujita M, Tran LSP (2015) Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci Rep 5:11433
Müller J, Xie ZP, Staehelin C, Mellor RB, Boller T, Wiemken A (1994) Trehalose and trehalase in root nodules from various legumes. Physiol Plant 90:86–92
Müller J, Aeschbacher RA, Wingler A, Boller T, Wiemken A (2001) Trehalose and trehalase in Arabidopsis. Plant Physiol 125:1086–1093
Mulrooney SB, Hausinger RP (2003) Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev 27:239–261
Nahar K, Rahman M, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ Sci Pollut Res 23:21206–21218
National Academy of Sciences (NAS) (1975) Nickel, medical and biological effects of environmental pollutants. National Research Council, National Academy of Sciences, Washington
Nelson DW, Sommers LE (1973) Determination of Total nitrogen in plant material 1. Agron J 65:109–112
Ocón A, Hampp R, Requena N (2007) Trehalose turnover during abiotic stress in arbuscular mycorrhizal fungi. New Phytol 174:879–891
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) methods of soil analysis, Agron. No. 9, part 2- chemical and microbiological properties, 2nd edn, American society agronomy, Madison, pp 403-430
Padilla L, Krämer R, Stephanopoulos G, Agosin E (2004) Overproduction of trehalose: heterologous expression of Escherichia coli trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in Corynebacterium glutamicum. Appl Environ Microbiol 70:370–376
Page V, Weisskopf L, Feller U (2006) Heavy metals in white lupin: uptake, root to shoot transfer and redistribution within the plant. New Phytol 171:329–341
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163:753–758
Panwar NR, Saha JK, Adhikari T, Kundu S, Biswas AK, Rathore A, Ramana S, Srivastava S, Subba RA (2010) Soil and water pollution in India: some case studies. IISS technical bulletin. Indian Institute of Soil Science, Bhopal
Pélissier HC, Tegeder M (2007) PvUPS1 plays a role in source-sink transport of allantoin in French bean (Phaseolus vulgaris). Funct Plant Biol 34:282–291
Polacco JC, Mazzafera P, Tezotto T (2013) Opinion-nickel and urease in plants: still many knowledge gaps. Plant Sci 199:79–90
Rahman H, Sabreen S, Alam S, Kawai S (2005) Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr 28:393–404
Rao KM, Sresty T (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Rathor G, Chopra N, Adhikari T (2014) Nickel as a pollutant and its management. Int Res J Environ Sci 3:94–98
Saad R, Kobaissi A, Robin C, Echevarria G, Benizri E (2016) Nitrogen fixation and growth of Lens culinaris as affected by nickel availability: a pre-requisite for optimization of agromining. Environ Exp Bot 131:1–9
Sachan P, Lal N (2017) An overview of nickel (Ni2+) essentiality, toxicity and tolerance strategies in plants. Asian J Biol 2:1–15
Sairam RK, Deshmukh PS, Shukla DS (1997) Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci 178:171–178
Sakamoto T, Bryant DA (2001) Requirement of nickel as an essential micronutrient for the utilization of urea in the marine cyanobacterium Synechococcus sp. PCC 7002. Microbes Environ 16:177–184
Salminen SO, Streeter JG (1986) Enzymes of alpha, alpha-trehalose metabolism in soybean nodules. Plant Physiol 81:538–541
Sannazzaro AI, Álvarez CL, Menéndez AB, Pieckenstain FL, Albertó EO, Ruiz OA (2004) Ornithine and arginine decarboxylase activities and effect of some polyamine biosynthesis inhibitors on Gigasporarosea germinating spores. FEMS Microbiol Lett 230:115–121
Sanz-Cobena A, Misselbrook TH, Arce A, Mingot JI, Diez JA, Vallejo A (2008) An inhibitor of urease activity effectively reduces ammonia emissions from soil treated with urea under Mediterranean conditions. Agric Ecol Environ 126:243–249
Seregin IV, Kozhevnikova AD (2006) Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol 53:257–277
Shabani L, Sabzalian MR (2016) Arbuscular mycorrhiza affects nickel translocation and expression of ABC transporter and metallothionein genes in Festuca arundinacea. Mycorrhiza 26:67–76
Shafeeq A, Butt ZA, Muhammad S (2012) Response of nickel pollution on physiological and biochemical attributes of wheat (Triticum aestivum L.) var. Bhakar-02. Pak J Bot 44:111–116
Shahzad B, Tanveer M, Rehman A, Cheema SA, Fahad S, Rehman S, Sharma A (2018) Nickel; whether toxic or essential for plants and environment-a review. Plant Physiol Biochem 132:641–651
Shaker-Koohi S (2014) Role of arbuscular mycorrhizal (AM) fungi in phytoremediation of soils contaminated: a review. Int J Adv Biol Biomed Res 2:1854–1864
Sharma A, Dhiman A, Dhankar J (2013) Detection of total and DTPA extractable nickel and cadmium in soil and plants irrigated with industrial effluents and sewage waste water. In: Proceedings of International conference on ecological, environmental and biological sciences 31:197–201
Shevyakova NI, Il'ina EN, Stetsenko LA, Kuznetsov VV (2011) Nickel accumulation in rape shoots (Brassica napus L.) increased by putrescine. Int J Phytorem 13:345–356
Singh AK, Hamel C, DePauw RM, Knox RE (2012) Genetic variability in arbuscular mycorrhizal fungi compatibility supports the selection of durum wheat genotypes for enhancing soil ecological services and cropping systems in Canada. Can J Microbiol 58:293–302
Sprent JI, James EK (2007) Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiol 144:575–581
Sreekanth TVM, Nagajyothi PC, Lee KD, Prasad TNVKV (2013) Occurrence, physiological responses and toxicity of nickel in plants. Int J Environ Sci Technol 10:1129–1140
Stancheva I, Geneva M, Zehirov G, Tsvetkova G, Hristozkova M, Georgiev G (2006) Effects of combined inoculation of pea plants with arbuscular mycorrhizal fungi and Rhizobium on nodule formation and nitrogen fixing activity. Gen Appl Plant Physiol 4:61–66
Streeter JG, Salminen SO (1988) Carbon metabolism and the exchange of metabolites between symbionts in legume nodules. In: O’Gara F, Manian S, Drevon JJ (eds) Physiological limitations and the genetic improvement of symbiotic nitrogen fixation. Springer, Dordrecht, pp 11–20
Streeter JG, Strimbu CE (1998) Simultaneous extraction and derivatization of carbohydrates from green plant tissues for analysis by gas–liquid chromatography. Anal Biochem 259:253–257
Takagi H, Watanabe S, Tanaka S, Matsuura T, Mori IC, Hirayama T, Shimada H, Sakamoto A (2018) Disruption of ureide degradation affects plant growth and development during and after transition from vegetative to reproductive stages. BMC Plant Biol 18:1–16
Tamayo E, Gómez-Gallego T, Azcón-Aguilar C, Ferrol N (2014) Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front Plant Sci 5:1–13
Terakado J, Yoneyama T, Fujihara S (2006) Shoot-applied polyamines suppress nodule formation in soybean (Glycine max). J Plant Physiol 163:497–505
Tiburcio AF, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Plant 240:1–18
Todd CD, Tipton PA, Blevins DG, Piedras P, Pineda M, Polacco JC (2005) Update on ureide degradation in legumes. J Exp Bot 57:5–12
Twanabasu BR, Stevens KJ, Venables BJ (2013) The effects of triclosan on spore germination and hyphal growth of the arbuscular mycorrhizal fungus Glomus intraradices. Sci Total Environ 454:51–60
Vadez V, Sinclair T, Serraj R (2000) Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol Plant 11:215–223
Vallino M, Massa N, Lumini E, Bianciotto V, Berta G, Bonfante P (2006) Assessment of arbuscular mycorrhizal fungal diversity in roots of Solidago gigantea growing in a polluted soil in northern Italy. Environ Microbiol 8:971–983
Vassileva V, Ignatov G (1999) Polyamine-induced changes in symbiotic parameters of the Galegaorientalis-Rhizobium galegae nitrogen-fixing system. Plant Soil 210:83–91
Vatansever R, Ozyigit II, Filiz E (2017) Essential and beneficial trace elements in plants, and their transport in roots: a review. Appl Biochem Biotechnol 181:464–482
Vogels GD, Van der Drift C (1970) Differential analyses of glyoxylate derivatives. Anal Biochem 33:143–157
Wang X, Shi G, Xu Q, Hu J (2007) Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J Plant Physiol 164:1062–1070
Werner AK, Romeis T, Witte CP (2010) Ureide catabolism in Arabidopsis thaliana and Escherichia coli. Nat Chem Biol 6:19–21
Wheeler CT, Hughes LT, Oldroyd J, Pulford ID (2001) Effects of nickel on Frankia and its symbiosis with Alnus glutinosa (L.) Gaertn. Plant Soil 231:81–90
Wu QS, Zou YN, Liu CY, Lu T (2012) Interacted effect of arbuscular mycorrhizal fungi and polyamines on root system architecture of Citrus seedlings. J Integr Agric 11:1675–1681
Yang Y, Liang Y, Ghosh A, Song Y, Chen H, Tang M (2015) Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead-zinc mine area: potential applications for phytoremediation. Environ Sci Pollut Res 22:13179–13193
Yao Q, Wang LR, Xing QX, Chen JZ, Zhu HH (2010) Exogenous polyamines influence root morphogenesis and arbuscular mycorrhizal development of Citrus limonia seedlings. Plant Growth Regul 60:27–33
Yusuf M, Fariduddin Q, Hayat S, Ahmad A (2011) Nickel: an overview of uptake, essentiality and toxicity in plants. Bull Environ Contam Toxicol 86:1–17
Zacarías JJJ, Altamirano-Hernández J, Cabriales JJP (2004) Nitrogenase activity and trehalose content of nodules of drought-stressed common beans infected with effective (fix+) and ineffective (fix−) rhizobia. Soil Biol Biochem 36:1975–1981
Zahran HH (2010) Legumes-microbes interactions under stressed environments. In: Khan MS, Zaidi A, Musarrat J (eds) Microbes for legume improvement. Springer, Cham, pp 353–387
Zhang L, Shi Z, Zhang J, Jiang Z, Wang F, Huang X (2015) Spatial and seasonal characteristics of dissolved heavy metals in the east and West Guangdong coastal waters, South China. Mar Pollut Bull 95:419–426
Zrenner R, Stitt M, Sonnewald U, Boldt R (2006) Pyrimidine and purine biosynthesis and degradation in plants. Annu Rev Plant Biol 57:805–836
Acknowledgments
We gratefully acknowledge the University Grants Commission (UGC) and the Department of Biotechnology, Government of India, for providing financial support in undertaking this research work. We are also thankful to PAU, Panjab; IARI, New Delhi, India; and The Energy and Resource Institute (TERI), New Delhi, for providing the biological research materials. The authors are also thankful to Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, India, for WD-XRF analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Ni stress negatively affects growth and mycorrhizal and rhizobial symbioses in pigeon pea
• Among the three PAs (Put, Spd, Spm), Put is most effective in alleviating Ni stress
• AM is more effective than PAs in improving biomass, ureide, and trehalose biosynthesis
• PAs complemented AM by enhancing symbiotic efficiency and nutrient acquisition
• +Put+AM is identified as a promising approach in imparting Ni tolerance to pigeon pea
Rights and permissions
About this article
Cite this article
Garg, N., Saroy, K. Interactive effects of polyamines and arbuscular mycorrhiza in modulating plant biomass, N2 fixation, ureide, and trehalose metabolism in Cajanus cajan (L.) Millsp. genotypes under nickel stress. Environ Sci Pollut Res 27, 3043–3064 (2020). https://doi.org/10.1007/s11356-019-07300-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07300-6