Abstract
Nickel (Ni) is a fundamental micronutrient in plants but hampers plant growth and metabolism at elevated levels in the soil by inducing oxidative stress. In the recent years, use of polyamines (PAs) and arbuscular mycorrhiza (AM) have gained importance for their roles in enabling plants to withstand Ni toxicity. However, information about their comparative effectiveness in alleviating Ni stress is scanty. Therefore, the current study was designed to evaluate relative impacts of three PAs (Put, Spd, and Spm) and AM (Rhizoglomus intraradices) in reducing Ni uptake, ROS generation, and modulating antioxidant defense machinery in two pigeonpea genotypes (Pusa 2001-tolerant and AL 201-sensitive). Roots of Ni supplied plants accumulated significantly more Ni than the leaves, more in AL 201 than Pusa 2001, which was proportionate to reduced dry weights and enhanced oxidative burst. Although all the three PAs as well as AM inoculations upsurge plant growth by remarkably lowering Ni transport as well as the sequential oxidative burden, AM was most effective, followed by Put, Spd with least positive impact of Spm. The combined applications of AM and Put were able to strengthen antioxidant defense mechanisms, including those of ascorbate-glutathione cycle, most strongly when compared with + Spd + AM and + Spm + AM. Pusa 2001 was more responsive to PAs priming because of its proficiency to develop better effective mycorrhizal symbiosis with R. intraradices when compared with AL201. Hence, the results suggest use of combined applications of PAs (mainly Put) and R. intraradices as an effective strategy for mitigating Ni toxicity in pigeonpea genotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nickel (Ni) belongs to group VIII B of the transition series and ranks 28th in the periodic table. It is a vital micronutrient for plants growth and development because it is required in essential metabolic processes (Liu 2001; Sachan and Lal 2017; Patra et al. 2019). However, at raised concentrations in soil and water, it is considered as wide-scale pollutant (Sreekanth et al. 2013) and is discharged into the soil by numerous natural and human-induced sources (Bhalerao et al. 2015; Soares et al. 2016). High concentrations of Ni in the soil adversely affect plant growth by increasing membrane permeability which causes electrolyte leakage, decrease photosynthesis, N2-fixing efficiency, nutrient status, and yield of the plants (Saad et al. 2016; Garg and Saroy 2019). In addition to this, high concentration of Ni also generates reactive oxygen species (ROS) as well as induce oxidative injury as reported in Solanum nigrum (Soares et al. 2016), Glycine max (Sirhindi et al. 2016), soybean (Barcelos et al. 2018; Mir et al. 2018), etc.
In order to detoxify ROS, plant tissues upregulate the activities of enzymatic antioxidants like superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPOX) and enzymes of ascorbate-glutathione (AsA-GSH) cycle—ascorbate peroxidase (APOX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and non-enzymatic glutathione reductase (GR), phytochelatins (PCs) along with total glutathione (Gajewska and Skłodowska 2007; Bhalerao et al. 2015). Reports on the activation of defense mechanisms under Ni stress are diverse with some observing an enhancement in SOD, CAT along with APOX activities, and peroxidase in soybean (Sirhindi et al. 2016; Barcelos et al. 2018) while others recording a slight upsurge in SOD activity with a decrease in CAT activity as well as enzymes of AsA-GSH cycle in pigeonpea and soybean under Ni toxicity (Rao and Sresty 2000; Mir et al. 2018). Moreover a decrease in SOD and CAT activities has also been observed under Ni stress in Hydrocharis dubia leaves (Zhao et al. 2008). In addition, the activities of these antioxidant enzymes vary among the genotypes of a plant species (Garg and Bhandari 2016; Garg and Kashyap 2019), with tolerant genotypes displaying stronger antioxidant defense mechanisms than the sensitive ones. GSH is a precursor of PCs that attaches to metal for transport and sequestration inside the vacuole, thus maintaining cellular redox status (Hasanuzzaman et al. 2017). Reports about the involvement of enzymatic and non-enzymatic antioxidants in reducing Ni toxicity in plants are scarce especially in legumes and require investigations.
PAs are low molecular weight (LMW) polycations with three main plant PAs namely spermidine (Spd), spermine (Spm) along with their precursor putrescine (Put) (Tiburcio et al. 2014; Chen et al. 2019). They are well recognized for their good metal chelation properties, safeguarding ROS, and protecting plant cells against metal-induced oxidative injuries (Nahar et al. 2016; Paul et al. 2018). In a study, exogenous Put supplementation lessened the toxic effects of Ni in Brassica napus by enhancing nutrient status and endogenous PAs (mainly Put) levels (Shevyakova et al. 2011). Besides, exogenous Spm application scavenged ROS via upgrading the activity of enzymes and non-enzymatic antioxidants under Cd stress in mungbean (Nahar et al. 2016). Similarly, Put and Spd pretreatment promoted metal chelation, antioxidant defense system, and conferred Cd tolerance in wheat (Szalai et al. 2020). Rady et al. (2016) observed Put to be more effective than Spd and Spm in Triticum aestivum plants under Cd stress while Spd was better than Put and Spm in cucumber under water stress (Kubiś et al. 2014).
Inoculation of plants with arbuscular mycorrhiza (AM) is considered another important and cost effective strategy in ameliorating heavy metal (HM) stress (Gamalero et al. 2009; Begum et al. 2019; Dhalaria et al. 2020). A study found that AM inoculation with Glomus mosseae could improve plant growth by minimizing Ni uptake in Glycine max and Lens culinaris (Jamal et al. 2002). In addition, Glomus etunicatum inoculation to Sorghum vulgare, Alphitonia neocaledonica and Cloezia artensis, under Ni stress, upgraded plant growth by increasing mycorrhizal colonization (MC) and sporulation of the fungal isolates since the symbionts acted as a barricade to Ni uptake by the plants (Amir et al. 2013). Moreover, PAs have been observed to upgrade MC and infection in plants roots namely pea and soybean (El Ghachtouli et al. 1995; Salloum et al. 2018). Furthermore, exogenic supply of three PAs (Spm, Put, and Spd) has been recognized to upsurge plant growth, mycorrhizal colonization, and biomass production of Poncirus trifoliata seedlings, with Glomus versiforme inoculation (Wu et al. 2010). Moreover, Put enhanced mycorrhizal development and colonization in Freesia hybrida when inoculated with Rhizophagus intraradices (Rezvanypour et al. 2015). However, no information is available on the functional complementarity between PAs and AM in reducing Ni induced ROS generation and activation of antioxidative defense mechanisms in plants which require elaborate research.
Pigeonpea (Cajanus cajan L.) is a perennial crop of tropics and sub-tropics (Ghosh et al. 2014), grown on 7.03 million hectares of land worldwide. India is the major producer with 5.6 million hectares and 3.29 MMT produced annually (FAOSTAT 2017). It is a rich source of proteins, carbohydrates, calcium, manganese, minerals as well as vitamins (Saxena et al. 2010). It is moderately tolerant to metals and is considered ideal for research studies. Till date, as per our knowledge, there are no reports on the relative roles of PAs and AM in reducing Ni induced stress responses in terms of ROS generation and antioxidant defense in crop plants especially pigeonpea.
Therefore, this work was aimed to determine the comparative effects of exogenous applications of PAs (Put, Spm, Spd) and/or arbuscular mycorrhizal (Rhizoglomus intraradices) in imparting Ni tolerance in two differentially tolerant pigeonpea genotypes. The objectives of the present research was to (i) evaluate the effect of Ni toxicity on growth, mycorrhizal symbiosis, Ni uptake, and ROS generation; (ii) assess the relative impacts of PAs seed priming and AM inoculation in reducing metal induced stress responses through the activation of ROS scavenging enzymes as well as those of ascorbate-glutathione cycle; and (iii) analyze the functional complementarity between PAs and AM in strengthening redox homeostasis as well as modulating the activities of non-protein thiols and PC contents.
Materials and methods
Present research is an extension of our preceding work (Garg and Saroy 2019) in which we investigated the interactive impacts of PAs and AM in modulating rhizobial symbiosis, trehalose, and ureide metabolisms in two differentially tolerant pigeonpea genotypes under Ni stress. This research addresses the impact of PAs/+AM applications in modulating oxidative stress through the activation of various antioxidant defense mechanisms in the two genotypes of pigeonpea subjected to two Ni concentrations (Ni100 = 100 mg/kg soil and Ni200 = 200 mg/kg soil).
Procurement of biological materials and research set-up
Seeds of pigeonpea (8 genotypes) were collected from agricultural institutes of India (Indian Agricultural Research institute-IARI, New Delhi, Panjab Agricultural University-PAU, Ludhiana, and Chaudhary Charan Singh Haryana Agricultural University-CCSHAU, Hisar). The genotypes were raised under a range of NiSO4 (50–300 mg/kg) and two genotype (Ni-tolerant—Pusa 2001 and relatively sensitive—AL 201) were selected with two Ni dosages (Ni100—100 mg/kg and Ni200—200 mg/kg of soil) for comprehensive and comparative investigations. Spores of Rhizoglomus intraradices from The Energy and Resource Institute-TERI and the inoculum of Sinorhizobium fredii AR-4 were procured from IARI, New Delhi. Experiments were performed in the Botany Department of Panjab University, Chandigarh (30°45′N, 76°45′E and elevation 305–348 m above sea level) with a relative humidity 45–57% (morning) and 36–50% (afternoon), minimum temperature 22–29 °C, and maximum 34–44 °C. Experiment soil (obtained from agricultural lands) comprised of sand and loam (1:1), which was autoclaved for 1 h at 121 °C twice at an interval of 48 h in order to remove native micro-flora. Soil had pH 7.4, ECe 0.825 dS m−1, total N = 0.42% (Nelson and Sommers 1973), organic C = 0.669% (Estefan et al. 2013), P = 10.11 mg kg−1 (Olsen and Sommers 1982), available K = 0.17 meq/100 g (Mehlich 1953), and Ni content = 5.87 μg g−1 (Marguí et al. 2007). The soil with Ni200 had total N (0.28%), P (5.09 mg/kg−1), organic C (0.523%), and K (0.08 meq/100g).
Experimental layout and nickel dosages
Earthenware pots, disinfected with ethanol (70%), were lined with polybags and then filled with autoclaved soil (8 kg/pot). Seeds were disinfected with hydrogen peroxide (10% v/v) solution and primed with 0.5 mM Put, Spd, and Spm for 12h and then coated with the inoculum of S. fredii. AM inoculum was prepared by growing the spores with Coriandrum sativum, Zea mays, and Sorghum bicolor and consisted of a mixture of soil, spores, and root fragments. The inoculum (50 g per pot containing approximately 45 spores/g soil) was kept beneath (1.5 cm) the seeds under each AM treatment and in order to maintain consistency, non-AM sets were supplemented with uniform sterilized inoculum. After 15 days of emergence (DAE), three plants per pot were maintained and treated with 100 and 200 mg/kg NiSO4, with/without PAs treatments and AM inoculations (six replicates each). The experiment set up were arranged in 3 × 4 × 2 × 2 factorial combination (completely randomized design), where three Ni dosages (0, 100, and 200 mg/kg); two PAs (Spm, Spd, and Put—0 and 0.5 mM); two AM inoculations [(+), (−)]; and two genotype (Pusa 2001-tolerant and AL 201-sensitive). The plants were harvested at 80 DAE, segregated into shoots and roots for physiological and biochemical analysis. For dry weight experiments, roots and shoots were oven dried at 70 °C for 72 h till they attained constant weight.
Mycorrhizal colonization and responsiveness
Percentage of mycorrhizal colonization (MC) in pigeonpea roots was analyzed microscopically in all AM inoculated stained roots as per the protocol of Phillips and Hayman (1970) and Giovannetti and Mosse (1980). Mycorrhizal responsiveness (MR) was calculated according to the formula given by Hetrick et al. (1992).
MC (%) = 100 (Total count of colonized segments / Total count of segments seen)
MR (%) = 100 [Weight of AM plants (oven dried) − Weight of non AM plants (oven dried)] / Weight of non AM plants (oven dried)
Electrolyte leakage (EL) and membrane stability index (MSI)
Fresh roots and leaves (2.5 g) were placed in 25 ml deionized water and electrical conductivity (EC) of the solution was noted by digital conductivity meter, 611 E. Samples have been autoclaved, cooled, and EC measured (Zwiazek and Blake 1991). EL was calculated by the equation: EL = 100 × (EC of solution before heat up / EC of solution after heat up).
Membrane stability index (MSI) was measured according to the methodology of Sairam et al. (1997) where EC of plant sample (500 mg) was analyzed in two groups and their corresponding EC1 and EC2 observed through digital conductivity meter, calculated as MSI = 100 [1 − (EC1 / EC2)] × 100.
ROS generation/oxidative stress indicators (O2˙−, LPO, H2O2)
For assessing the superoxide radical (O2˙−), fresh roots and leaves (100 mg) were dipped in potassium-phosphate buffer (10 mmol), pH 7.8, having NBT and NaN3. Two-milliliter reaction solution was heated for 15 min (85 °C), cooled, and read at 580 nm according to the method of Doke (1983). According to the procedure given by Velikova et al. (2000), amount of H2O2 was evaluated and OD of supernatant read spectrophotometrically at 390 nm. Estimation of lipid peroxidation (LPO) was done on the basis of quantity of MDA released through thiobarbituric acid (TBA) reaction according to Heath and Packer (1968). The absorbance (Abs) was noted at 532 and 600 nm, Abs at 600 nm were deducted from Abs at 532 nm, and MDA content evaluated with the help of an extinction coefficient (€ = 155 mM−1 cm−1).
Enzymatic antioxidants (SOD, CAT, and GPOX)
Preparation of enzyme extract
Five hundred-milligram plant material (roots and leaves) was crushed (with liquid N2) in potassium phosphate buffer (50 mmol L−1, pH 7.8) containing EDTA (1 mmol L−1), 2-mercaptoethanol (3 mmol L−1) along with polyvinylpolypyrrolidone (2% w/v). The homogenate material was centrifuged and the supernatant served for antioxidant enzymatic activities (nkat mg−1 protein).
SOD activity was evaluated at 560 nm by checking its capacity to hamper the photochemical diminution of NBT (Dhindsa et al. 1981). CAT activity was analyzed through the reduction in Abs of H2O2 at 240 nm as stated in procedure of Aebi (1984). GPOX activity was examined by the protocol given by Castillo et al. (1984). Upsurge in OD was noted at 470 nm because of the oxidation of guaiacol to tetra-guaiacol and enzyme activity was estimated by using extinction coefficient tetra-guaiacol (€ = 26.6 mM−1 cm−1).
Ascorbate-glutathione (AsA-GSH) cycle
Ascorbate pool (APOX, MDHAR, DHAR, AsA, DHA, total AsA)
Extraction procedure was similar to that of SOD, CAT, and GPOX.
Ascorbate peroxidase (APOX) activity was examined as the decline in OD as a result of oxidation of ascorbic acid to MDHA and dehydroascorbate (DHA). The decrease in Abs was noted at 290 nm by UV-visible spectrophotometer (Nakano and Asada 1981). The quantity of AsA oxidized by APOX activity was evaluated through extinction coefficient (ε = 2.8 mmol−1 cm−1). Dehydroascorbate reductase (DHAR) activity was measured according to the rate of upsurge in Abs at 265 nm (Asada 1984). MDHAR activity was calculated by the reduction in Abs (340 nm) as per the procedure of Nakagawara and Sagisaka (1984). Total AsA was calculated according to Arakawa et al. (1981) and Nakagawara and Sagisaka (1984). DHA content was calculated by subtracting the AsA from the total AsA (DHA = total AsA − AsA). A calibration curve was plotted using 0–10 μmol of AsA or DHA to calculate AsA, DHA, total AsA concentration and plotted as μmol g−1 FW.
Glutathione pool (GR, GSH, and GSSG)
Extraction procedure for GR was similar to SOD. The assessment of GR activity was based upon development of red-colored complex by reduced glutathione through 5,5-dithiobis-2-nitrobenzoic acid (DTNB) (Smith et al. 1988). The reaction was begun by mixing 20 mmol GSSG (0.1 ml) and the upsurge in Abs was observed spectrophotometrically at 412 nm. For GSH and GSSG content, plant material (100 mg) was homogenized in potassium phosphate buffer solution and then centrifuged. The supernatant collected was utilized for the assessment of GSH and total glutathione according to Castillo and Greppin (1988). A reduction in absorbance from the oxidation of NADPH was noted at 340 nm. GSH concentrations were analyzed by assessing the upsurge in Abs at 412 nm resulting from reduction of DTNB. GSSG was calculated by deducting GSH from total GSH.
Total non-protein thiols (NP-SH) and phytochelatin content
Non-protein thiols (NP-SH) were analyzed by the procedure of Del Longo et al. (1993). Leaves and roots were crushed in TCA (5%), incubated, and then centrifuged. SH group was analyzed by adding aliquot (100 μl) to the reaction mixture having phosphate buffer (0.1 M, pH 7.0), EDTA (0.5 mmol), and DTNB (0.5 ml of 1 mmol) and Abs noted at 412 nm. A calibration curve was drawn from different concentrations of cysteine to evaluate the NP-SH content. To carry out theoretical determination of PCs, the difference between total NP-SH and GSH was assessed to signify PCs (Bhargava et al. 2005).
Statistical analysis
The data was statistically analyzes using SPSS 25.0 (Chicago, USA). All figures and tables constituted the mean values of six replicates ± standard error (SE) for each treatment. Data were examined by ANOVA for the major impact (Ni, Spm, Spd, Put, AM, G) and interactions between them. One-way ANOVA was used and further Duncan multiple range test (DMRT at p < 0.05) applied to assess the variations among the treatments. Regression analysis was applied to compare the separate impacts of six independent factors (Ni, Spm, Spd, Put, AM, G) on a specific parameter and presented as standardized coefficient (β). Pearson’s correlation coefficient (r) was conducted to draw the correlations among relevant dependent factors for individual parameters.
Results
Growth parameters
Both concentrations of Ni had a negative correlation with root and shoot dry weights (RDW, SDW) in the two genotypes with higher concentration more toxic than the lower one (Table 1). Pusa 2001 was able to tolerate Ni stress more strongly than compared with AL 201, there 35.72% and 72.7% decline in roots over control were recorded in Pusa 2001 and AL 201 individually under Ni200 treatments. On the other hand, SDW declined in Pusa 2001 by 29.12% and in AL 201 by 54.30% respectively under Ni200 treatments. The decline was higher in terms of RDW than SDW, as evidenced by standardized β coefficients using regression analysis (ESM Table 1) [RDW β(Ni) = −0.702, SDW β(Ni) = −0.638] thus, disturbing the root to shoot ratio in a genotype dependent manner (Table 1). Seed priming of both the genotypes with three PAs along with AM inoculations with R. intraradices had a positive impact in improving the plant biomass significantly. Pusa 2001 was more responsive to these amendments when compared with AL 201. Relative comparison of the data indicated that AM had a much stronger influence in improving both RDW and SDW, followed by Put, Spd, and Spm as indicated by β coefficient values (ESM Table 1). AM and Put was able to ameliorate the negative impacts of Ni200 completely and the data was significantly better than even the control set of unstressed Pusa 2001 genotype, with significant beneficial effects recorded with Spd. Spm priming was least effective with no significant improvement in both the genotypes. The co-treatments of AM and Put were extremely beneficial in enhancing RDW, SDW as well as root to shoot ratio than + Spd + AM and + Spm + AM treatment and data was even higher Ni dosages than the control in Pusa 2001. However, AL 201 was relatively less responsive to all the combined treatments and displayed significantly improved plant biomass, especially with + Put + AM.
Mycorrhizal symbiosis
Microscopic assessment of the grid sections of roots indicated no root colonization in uninoculated controls. Both genotypes displayed a strong ability and efficiency to establish symbiosis with R. intraradices, with Pusa 2001 (81.2%) recording higher percent root colonization than AL 201 (77.6%) (Table 1). Although, no significant difference was observed in terms of percent MC in the unstressed control plants and both the genotypes equally responsive to all the PAs treatments. However, the differences in percent root colonization became significant in the two genotypes with greater decline in AL 201 than Pusa 2001, when the soil were supplemented with the two Ni concentrations as authenticated by correlation values [Pusa 2001 r(MC-Ni) = −0.869; AL 201 r(MC-Ni) = −0.951 at p = 0.01]. However, even at Ni200, 59.03% was recorded in tolerant Pusa 2001 and 41.38% in sensitive AL 201 respectively, indicating relatively less negative effects on colonization potential than the growth parameters. A significantly high MR was observed in Pusa 2001 when compared with AL 201, which further increased with increasing Ni concentrations in rooting medium. All the three PAs were beneficial in strengthening percent root colonization, while reducing MR in a genotype dependent manner, Put followed by Spd and then Spm, there Put was able to completely nullify the negative effects of even at Ni200.
Total Ni concentrations
The total Ni concentrations estimated by WD-XRF in both leaves and roots indicated significant increase in Ni uptake, more AL 201 compare to Pusa 2001 (Table 1). Roots absorbed and retained higher amount of Ni than leaves [roots β(Ni) = 0.846, leaves β(Ni) = 0.808]. PAs priming and AM inoculation help in reducing Ni take up in root as well as their movement to leaves. AM was relatively highly effective in minimizing the metal concentrations when compared with the three PAs as indicated by regression analysis (ESM Table 1). Among the three PAs, maximum benefits were recorded with Put seed priming along with AM inoculations in genotype related manner. Whereas, the beneficial impacts of + Spm + AM were lowest under both Ni concentrations in the two genotypes.
Electrolyte leakage (EL) and membrane stability index (MSI)
Ni stress damaged the plasma membranes more negatively in roots than leaves of AL 201 than Pusa 2001, which was equivalent to the increased Ni concentrations (Table 2—roots and ESM Table 2—leaves) [EL roots β(Ni) = 0.676, leaves β(Ni) = 0.484]. The increased EL revealed an inverse relation with the MSI under Ni toxicity in an organ dependent mode with higher in AL 201 than Pusa 2001 [MSI roots β(Ni) = −0.755, leaves β(Ni) = −0.718]. Among the various amendments, AM was most effective in arresting membrane damage followed Put, Spd, and Spm, thus reducing EL and increasing MSI. Put seed priming complemented AM inoculations more strongly when compared with + Spd + AM and + Spm + AM.
ROS generation/oxidative stress indicators (O2˙−, LPO, H2O2)
A substantial increase in ROS generation in terms of in O2˙−, H2O2 accumulation, and MDA was observed with increasing concentrations of Ni, more in AL 201 than Pusa 2001 (Table 2—roots and ESM Table 2—leaves). The increase in H2O2 and O2˙− was much higher in root than leaves in the two genotypes as authenticated with regression analysis (ESM Table 1). In addition a significant enhancement in MDA content was observed, with was proportionate to Ni concentration as well as genotype (Table 2 and ESM Table 2). Exogenous supplementation of PAs (mainly Put) and AM (R. intraradices) lowered the concentrations of oxidative stress markers, proportionate to the improved MSI of roots and leaves under Ni stress. Regression analysis confirmed that AM and Put supply was more efficient in lessening ROS generation in the two genotypes when compared with Spd and Spm (ESM Table 1). The dual supplementations of Ni stress soil further lowered O2˙− and H2O2 level in an organ dependent manner. Higher benefits were recorded in Pusa 2001 where the quantum of ROS generation even lower than the unstressed controls, with partial amelioration in AL 201. Put and AM were the most effective in arresting ROS generation when given individually as well as in combination.
Antioxidant defense mechanisms
SOD, CAT, and GPOX
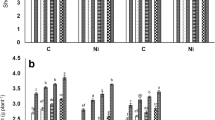
Significant enhancements were recorded in the activities of SOD, CAT, and GPOX under Ni stress (Fig. 1—roots; ESM Fig. 1—leaves) and the extent of escalation was greater in leaves in contrast to roots, more in Pusa 2001 than AL 201 as evident from regression analysis of statistics (ESM Table 1). SOD activity (Fig. 1a; ESM Fig. 1a) was comparatively higher than GPOX and CAT in roots as well as leaves which could be positively correlated with the generation of ROS (H2O2, O2˙− and MDA). Significant Ni × Put × AM and Put × AM × G interactions (ESM Table 3) of leaves and roots of SOD, CAT and GPOX activities indicated that exogenic Put and AM lowered Ni content, higher in Pusa 2001 comparison AL 201. The treatments of PAs and R. intraradices further boosted the antioxidative enzyme activity with + Put + AM more effective than + Spd + AM and + Spm + AM. The co-application of AM and PAs indicated that + Put + AM complemented each other which resulted in further enhancement of these antioxidant enzymes at both Ni concentrations.
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM-Rhizoglomus intraradices) inoculation on a SOD, b CAT, c GPOX, d APOX, e MDHAR, and f DHAR activities in roots (nkat mg−1 protein) of Pusa 2001 and AL 201 pigeonpea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni100 = 100 mg/kg Ni added; Ni200 = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put + AM = Put and AM added
Ascorbate pool (APOX, DHAR, MDHAR, AsA, and DHA)
Both the genotypes displayed enhancement in the activities of APOX, MDHAR, and DHAR (Fig. 1—roots; ESM Fig. 1—leaves) together with AsA and DHA amount in roots and leaves (Fig. 2—roots; ESM Fig. 2—leaves). An increased H2O2 accumulation under Ni stress strongly correlated with escalated APOX activity and this correlation was stronger in Pusa 2001 [roots, Pusa 2001 r(H2O2-APOX) = 0.945, AL 201 r(H2O2-APOX) = −0.860 at p = 0.01]. Interestingly, MDHAR and DHAR activities were greater in roots compared with leaves under Ni stress which subsequently results in higher AsA and DHA content in a genotype and concentration dependent manner, with Pusa 2001 exhibiting higher activity than AL 201, which thus, results in diminution in AsA/DHA ratio (Table 3). APOX, MDHAR, and DHAR activities further increased when the genotypes were pretreated with all the three PAs (Fig. 1; ESM Fig. 1), “especially Put” and improved ASA/DHA ratio. As compare PAs, AM inoculated plants indicated remarkable elevations in APOX, MDHAR, and DHAR activities in comparison with corresponding non-AM stressed plants. The combined treatment were much more efficacious in maintaining redox balance in terms of the metabolites of ascorbate pool, most effective being + Put + AM treatments, especially in Pusa 2001.
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM-Rhizoglomus intraradices) inoculation on a total ascorbate, b ascorbate, c dehydroascorbate in roots (μmole g−1 FW), d reduced glutathione (nmoles g−1 FW), e oxidized glutathione (nmoles g−1 FW), and f GR activity in roots (nkat mg−1 protein), of Pusa 2001 and AL 201 pigeonpea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni100 = 100 mg/kg Ni added; Ni200 = 200mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put + AM = Put and AM added
Glutathione pool (GR, GSH, and GSSG)
GR activity along with GSH and GSSG and total glutathione increased under both Ni100 and Ni200 levels, the increment being genotype and concentration dependent manner, compared to control series (Fig. 2—roots; ESM Fig. 2—leaves). GR activity under Ni stress was relatively lower than DHAR that further elicits into greater accumulation of GSSG (oxidized form) compare to reduced GSH, hence unbalancing the GSH redox homeostasis (higher GSSG/GSH) in a genotype dependent manner (Table 3). A significant induction of redox homeostasis was observed under AM and PAs treatment which results in relatively greater accumulation GSH than GSSG, thus strengthening the reformative enzymes of glutathione cycle and improving GSH/GSSG ratio under both Ni concentrations. Regression coefficients verified better proficiency of AM applications as compared to PAs pretreatment (ESM Table 1). Moreover, tolerant Pusa 2001 genotype revealed larger GSH/GSSG ratio than AL 201 and + Put + AM application provided maximum redox stability as compared to other PAs and AM combinations.
Thiol derivatives (NP-SH, total glutathione, and PCs)
Significantly higher total GSH content was observed with Ni treatments which subsequently led to elevated level of NP-SH and PCs (Fig. 3—roots; ESM Fig. 3—leaves), as a result signifying stronger tendency of Ni to stimulate PC biogenesis as mentioned by beta coefficient values [root total GSH β(Ni) = 0.787; NP-SH β(Ni) = 0.745; root PC β(Ni) = 0.704]. Further enhancement in PC synthesis was recorded when Ni stressed plants were pretreated with PAs and AM (Fig. 3c; ESM Fig. 3c). Moreover, higher negative impact of Ni concentrations in AL 201 could be correlated with lesser biosynthesis of GSH, therefore lesser accumulation of PC as well. Individually, PAs priming and R. intraradices assisted plants to deal with Ni toxicity by enhancing the activities GSH pool related enzymes and maintain better redox balance, because of this results in enhanced PC synthesis. Moreover, joint applications of individual PAs with AM (especially + Put + AM) had high beneficial effect under control as well as stress conditions in Pusa 2001 than AL 201.
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM-Rhizoglomus intraradices) inoculation on a total glutathione, b non-protein thiols, and c phytochelatins in roots (nmol g−1 FW) of Pusa 2001 and AL 201 pigeonpea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C = PAs and AM absent; Spm = 0.5 mM Spm added; Spd = 0.5 mM Spd added; Put = 0.5 mM Put added; AM = AM added; Ni100 = 100 mg/kg Ni added; Ni200 = 200 mg/kg Ni added; Spm + AM = Spm and AM added; Spd + AM = Spd and AM added; Put + AM = Put and AM added
Discussion
The present results indicated negative correlation between increasing Ni concentrations and plant biomass with higher decline in RDW than SDW, thus leading to reduced root to shoot ratio, with higher decline in AL 201 than Pusa 2001. The reduction of plant growth because of Ni stress is primarily related to the elevated osmotic potential of Ni stressed soil which results in disturbed mineral nutrient as well as water status, thereby inhibiting plant growth (Rucińska-Sobkowiak 2016). Moreover, roots are the first one organ to confront Ni induced stress, hence suffer high deleterious effects as mentioned in pigeonpea (Rao and Sresty 2000). The reduced plant growth was proportionate to the increase in Ni levels, more in roots than leaves. About 50% Ni content is normally retained in roots because of its sequestration to the interior walls of xylem parenchymatic cells, also immobilization in vacuoles of mungbean (Nahar et al. 2016); Lens culinaris (Saad et al. 2016).
The colonizing ability of pigeonpea plants with R. intraradices reduced under Ni stress with Pusa 2001 displaying better proficiency to develop mycorrhizal symbiosis than sensitive AL 201. The negative effects of Ni were significantly higher on growth parameters when compared with AM symbiotic establishment. The reduction in MC could because of the straight impact of Ni concentrations on propagation of spores, hyphal branching, and maturation or due to the negative impacts of Ni upon roots (Twanabasu et al. 2013). Moreover, ability of AM to establish root colonization even in the sensitive genotype AL 201 because of the fact that AM fungal propagules never vanish entirely even in high HM-contaminated soil sites (Vallino et al. 2006). The differential response of two genotypes could also be due to higher MR displayed by Pusa 2001 when compared with AL 201.
In the current study, a remarkable increment in the generation of ROS (O2˙−, MDA and H2O2) was recorded in pigeonpea in a dose and genotype dependent manner. Elevated levels of oxidative stress indicators in AL 201 could be because of its lower ability to hinder Ni take up into both roots as well as shoots, which led to higher membrane damage and EL. Increased H2O2 and MDA contents in pigeonpea leaves and roots under Ni stress along with increased LPO have been reported by Sirhindi et al. (2016) in Glycine max and Rao and Sresty (2000) in pea plants and in wheat (Gajewska and Skłodowska 2007). Higher detrimental effects of Ni200 on membrane could be due to its tendency to bind with –SH group and form disulfide bonds leading distortion of structure as well as working of membrane ion and channels (Gajewska and Skłodowska 2007). LPO affects the permeability of lipid membranes by means of increasing the microviscosity, probably via interconnection of lipid radicals (Stark 1991). Increased ROS production under Ni stress could also be related to elevated NADPH oxidase activity as observed in roots of wheat seedlings (Hao et al. 2006).
Even though, ROS generation was accompanied by the activation of antioxidant machinery, both genotypes experienced significant negative effects of Ni stress on growth, higher in AL 201 than Pusa 2001. Under stressful situations, equilibrium among ROS production and antioxidant machinery is extremely disturbed which inhibits many physiological and biochemical functions mandated for adequate functioning of plants (Al Mahmud et al. 2019). Our results displayed enhancement in the activities of SOD, CAT, GPOX, and APOX antioxidant enzymes in pigeonpea plants subjected to high levels of Ni signifying that SOD in Ni-induced plants might have conferred defense over oxidative harm to a certain extent. The increase in the antioxidant enzymatic activities was significantly higher in Pusa 2001 which might have been responsible for its superiority to tolerate Ni induced oxidative stress when compared with AL 201. Moreover, overproduction of ROS under Ni stress results in higher accumulation of DHA and GSSG, respectively, hence resulting in disturbed redox status in the form of reduced AsA/DHA and GSH/GSSG ratios. Equality among ROS generation and scavenging of oxidative indices by enzymatic and non-enzymatic antioxidants is important in order to negate the effects of Ni toxicity in plants (Nahar et al. 2016). Even though, an increase in the activities of non-enzymatic antioxidants in form of NP-SH, PCs along with total GSH was observed, they were not adequate enough to deal from oxidative burden mainly because of persistent formation of ROS under Ni induced stress. PCs are metal sensitive peptides, which confer tolerance by binding toxic HMs (Galli et al. 1996).
The three PAs (Put, Spd, and Spm) were able to control the Ni induced stress and could improve root, shoot biomass and reduce Ni uptake as well as generation of ROS with Put most effective. Inoculation of pigeonpea genotypes with R. intraradices outperformed the positive roles of three PAs with more beneficial impacts observed in Pusa 2001 relative to AL 201. The enhancement in plant dry weights could be because of the direct participation of PAs in cell partition, replication, and transcription (Tiburcio et al. 2014; Chen et al. 2019). Additionally, PAs minimize the aggregation of HMs (Ni, Cd, Zn, Cu, and Pb) in wheat plants and improve their tolerance for these HMs (Aldesuquy et al. 2014). The present study also observed that, among the three PAs, the stimulatory effects were higher in Put primed plants, which could be due the fact that Put is a precursor of Spd and Spm in PAs biogenesis (Sannazzaro et al. 2004). The relative performance of three PAs is variable depending upon the plant species as well the type of metal stress. Rady et al. (2016) found Put to be more effective than Spd and Spm in Triticum aestivum plants under Cd stress. In a study, exogenous Put supplementation to Brassica napus lessened the toxic effects of Ni on root growth by enhancing nutrient status (Shevyakova et al. 2011). On the other hand, in cucumber roots, Spd performed better than Put and Spm and exhibited higher membrane stability under water stress (Cucumis sativus cv. Dar) seedlings (Kubiś et al. 2014). However, in our previous study, Spm was found to be least effective in improving growth as well as rhizobial symbiosis in pigeonpea genotypes under Ni stress when compared with Put and Spd (Garg and Saroy 2019). Higher beneficial effects of R. intraradices could be awarded to its capability to minimize endogenous Ni in the roots and leaves more proficiently than PAs. The beneficial effects of AM could also be directly related to its better ability for adsorption or chelation of HMs in the soil. Numerous functional groups namely imidazole carboxyl, free hydroxyl, etc. allocate binding spots for HMs and adsorb HMs in the land soil and obstruct transport inside the plants (Dhalaria et al. 2020). Higher beneficial effects in Pusa 2001 were directly related its ability to establish a stronger percent root colonization with more extensive hyphal network in root rhizosphere which enabled the roots to explore larger soil zone, when compared with AL 201. Mycorrhizas can contribute plant species to colonize HMs polluted locations by upgrading the plant’s P take up and eventually improving their growth (Zhang et al. 2018).
Functional complementary between the three PAs and AM inoculation was recorded in both the genotypes in terms of improving plant biomass with highest positive effects under combined treatments of + Put + AM in comparison to + Spd + AM and + Spm + AM. PAs (Put, Spd, and Spm) have been reported to significantly upsurge the mycorrhizal infection along with the number of appressoria development in Pisum sativum (El Ghachtouli et al. 1995). Exogenous supply of three PAs (Put, Spd and Spm) has been identified to boost plant growth, MC, and biomass production of Poncirus trifoliata seedlings with Glomus versiforme inoculation (Wu et al. 2010), with Put most efficient in increasing MC as well as number of entry points, arbuscules, and vesicles as compared to Spm and Spd. The results clearly conveyed that exogenously supplied PAs, especially Put, had a significantly stimulating effect on mycorrhizal colonization and could be considered a necessary regulatory factor in plant AM interactions. In this research, exogenous co-supplementations of PAs and AM (mainly + Put + AM) were most effective in reducing the Ni concentrations in both root as well as shoots through their cumulative roles under Ni stress, thus resulting in highest plant biomass accumulation and restoring membrane damage along with reduced LPO. PAs (Put, Spd, and Spm) significantly reduced oxidative stress in pigeonpea plants subjected to Ni stress by reducing EL and increasing MSI. PAs priming decreased that rate of O2˙− generation in mungbean under Cd induced stress (Nahar et al. 2016), because of formation of triplet complex with Fe+2 thus, protecting the membrane (Velikova et al. 2000). AM inoculation could more efficiently diminish the ROS generation than PAs, which could either be due to its ability in maintaining better membrane stability and reducing EL than PAs.
PAs and AM boosted the activities of antioxidative enzymes (SOD, CAT, and GPOX) as well as AsA-GSH cycle under Ni stress. SOD establishes the primary line of defense against ROS by reducing O2˙− to H2O2 (Nahar et al. 2016). Attachment with antioxidant enzymes, PAs augment their ROS scavenging activity, permit them to enter the site of oxidative stress (Tang and Newton 2005). Put and Spd pretreatment has been found to promote metal chelation, antioxidant defense system, and confer Cd tolerance in wheat (Szalai et al. 2020). Besides, exogenic supply of Spm scavenged ROS by upgrading the activity of enzymes (SOD, CAT, APOX, etc.) and non-enzymatic antioxidants (ASA and GSH) under Cd stress in mungbean (Nahar et al. 2016). PAs (mainly Put) attach with CAT, GPOX and increases transmissivity to reach the spots of oxidative stress inside the cells and increases the efficiency of CAT, as observed in the present study. Inoculation of pigeonpea plants with AM was most effective in upregulating the antioxidant enzymatic activities due to its direct role in escalating the genes (GintSOD1 with R. intraradices; Cu/Zn SOD with Glomus margarita) (González-Guerrero et al. 2010). Mycorrhiza has some special and unique genes encoding for antioxidant enzymes, whose expression behavior stimulates the activities of antioxidant enzymes individually (Alqarawi et al. 2014). Exogenous PAs as well as AM significantly increased APOX, MDHAR, DHAR, and GR activities while restoring AsA and GSH, more in Pusa 2001 than AL 201. Therefore, stimulation of these antioxidant enzymes in R. intraradices inoculated plants could be due indirect outcome of improved effects of AM on host plants growth and mineral status (mainly P) than PAs. Triggering of MDHAR and DHAR (assemblage of intermediatory metabolites of AsA pool) declined DHA, hence resulted in upgraded AsA/DHA ratio with both PAs and AM treatments. Exogenous PAs (especially Put) and R. intraradices inoculation upsurge the activity of both GSH and GSSG along with improved GSH/GSSG ratio thereby conferring Ni tolerance in pigeonpea genotypes. Generally, AsA is found in reduced form and its regeneration is entirely necessary since completely oxidized DHA has a limited half-life and is lost until it is reduced again (Foyer and Noctor 2011). In the GSH pool, GR reduces the disulfide bond of GSSG as well as maintains the reduced status of GSH, thus maintaining proportion within GSH and GSSG (Chellamma and Pillai 2013).
In our study, increase in total GSH, NP-SHs, and PCs along with reduced Ni content in Ni-affected pigeonpea plants were observed, higher in Pusa 2001 than AL 201. PCs are oligomers of GSH, formed by the enzyme PC synthase. PCs bind to metals and transport them to vacuole and are effective in chelating HM ions (Zagorchev et al. 2013). PAs applications further increased the contents of total GSH, NP-SHs, and PC under Ni stress indicating upregulation of Ni chelation and sequestration capacity mainly in the range of Put > Spd > Spm. PAs were found to function as metal chelators in transgenic pear encoding PA biogenesis gene (Wen et al. 2010). In addition, PAs are capable of increasing total GSH content and escalate the production of PCs, which bind to metal as an efficient metal detoxification strategy (Nahar et al. 2016). In the current investigation, R. intraradices was more beneficial in upregulating the synthesis of GSH, PCs, and NP-SH than PAs in a genotype dependent manner. Glomalin, obtained from contaminated land or from AM hyphae, is very efficient in sequestering HMs particularly Cu, Cd, Pb, and Zn (Wright and Upadhyaya 1998; Gonzalez-Chavez et al. 2004). Therefore, AM may aggregate HMs in the land soil itself, shorten their availability, and minimize toxic effects to soil organisms and plants (Gamalero et al. 2009). In addition, alteration of metal content in AM inoculated plants with an enhancement in plant tolerance, as recorded in our study, might be due to immense alterations in gene expression together with protein anabolism triggered by the symbiosis. In the present study, dual applications of the three PAs with R. intraradices, especially + Put + AM, could completely mitigate the Ni induced ROS generation by significantly lowering metal uptake and accumulation, enhancing PC production, thereby sequestering Ni into the vacuoles.
Conclusion
In conclusion, Ni stress caused oxidative stress in pigeonpea plants by causing membrane damage, increasing metal uptake. The two PAs (Put and Spd) as well as AM pre-treatment were highly effective in imparting Ni stress tolerance in pigeonpea plants by enhancing PC synthesis, with benefits stronger in AM inoculated plants. Moreover the pigeonpea genotype having better ability to colonize with R. intraradices displayed complementarity with PAs, especially Put, thereby, displaying stronger resistance to the presence Ni in the rooting medium. Moreover, study emphasized the significance of ascorbate-glutathione cycle in maintaining redox balance, through accelerated activities of regenerative enzymes, as a vital indicator of Ni tolerance. Hence, the combined applications of PAs and AM proved to be a cost-effective and environment-friendly strategy for reducing or alleviating Ni toxicity in pigeonpea genotypes.
References
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology, 105th edn. Academic Press, Orlando, pp 121–126
Al Mahmud J, Bhuyan MB, Anee TI, Nahar K, Fujita M, Hasanuzzaman M (2019) Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In: Hasanuzzaman M, Hakeem KR, Nahar K, Alharby HF (eds) Plant abiotic stress tolerance. Springer, Cham, pp 221–257
Aldesuquy H, Haroun S, Abo-Hamed S, El-Saied AW (2014) Involvement of spermine and spermidine in the control of productivity and biochemical aspects of yielded grains of wheat plants irrigated with waste water. Egypt J Basic Appl Sci 1:16–28
Alqarawi AA, Abd Allah EF, Hashem A (2014) Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J Plant Interact 9:802–810
Amir H, Lagrange A, Hassaïne N, Cavaloc Y (2013) Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23:585–595
Arakawa N, Tsutsumi K, Sanceda NG, Kurata T, Inagaki C (1981) A rapid and sensitive method for the determination of ascorbic acid using 4, 7-diphenyl-l, 10-phenanthroline. Agric Biol Chem 45:1289–1290
Asada K (1984) Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol 105:422–429
Barcelos JPQ, Reis HPG, Godoy CV, Gratão PL, Furlani Junior E, Putti FF, Campos M, Reis AR (2018) Impact of foliar nickel application on urease activity, antioxidant metabolism and control of powdery mildew (Microsphaera diffusa) in soybean plants. Plant Pathol 67:1502–1513
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ahmed N, Ashraf M, Zhang L (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation, implications in abiotic stress tolerance. Front Plant Sci 10:1068–1083
Bhalerao SA, Sharma AS, Poojari AC (2015) Toxicity of nickel in plants. Int J Pure Appl Biosci 3:345–355
Bhargava P, Srivastava AK, Urmil S, Rai LC (2005) Phytochelatin plays a role in UV-B tolerance in N2-fixing cyanobacterium Anabaena doliolum. J Plant Physiol 162:1220–1225
Castillo FJ, Greppin H (1988) Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. leaves after ozone exposure. Environ Exp Bot 28:231–238
Castillo FJ, Penel C, Greppin H (1984) Peroxidase release induced by ozone in Sedum album leaves: involvement of Ca2+. Plant Physiol 74:846–851
Chellamma S, Pillai BV (2013) Approaches to improving salt tolerance in maize. In: Ahmad P, Azooz MM, Prasad MNV (eds) Salt stress in plants. Springer, New York, pp 261–281
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants, metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1–13
Del Longo OT, González CA, Pastori GM, Trippi VS (1993) Antioxidant defences under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol 34:1023–1028
Dhalaria R, Kumar D, Kumar H, Nepovimova E, Kuča K, Torequl Islam M, Verma R (2020) Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 10:815–837
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence, correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23:345–357
El Ghachtouli N, Paynot M, Morandi D, Martin-Tanguy J, Gianinazzi S (1995) The effect of polyamines on endomycorrhizal infection of wild-type Pisum sativum, cv. Frisson (nod+ myc+) and two mutants (nod− myc+ and nod− myc−). Mycorrhiza 5:189–192
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region:170–176
FAOSTAT (2017) http,//www.fao.org/faostat/en/#data/QC
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20:27–36
Galli U, Schüepp H, Brunold C (1996) Thiols in cadmium-and copper-treated maize (Zea mays L.). Planta 198:139–143
Gamalero E, Lingua G, Berta G, Glick BR (2009) Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress. Can J Microbiol 55:501–514
Garg N, Bhandari P (2016) Interactive effects of silicon and arbuscular mycorrhiza in modulating ascorbate-glutathione cycle and antioxidant scavenging capacity in differentially salt-tolerant Cicer arietinum L. genotypes subjected to long-term salinity. Protoplasma 253:1325–1345
Garg N, Kashyap L (2019) Joint effects of Si and mycorrhiza on the antioxidant metabolism of two pigeonpea genotypes under As (III) and (V) stress. Environ Sci Pollut Res 26:7821–7839
Garg N, Saroy K (2019) Interactive effects of polyamines and arbuscular mycorrhiza in modulating plant biomass, N2 fixation, ureide, and trehalose metabolism in Cajanus cajan (L.) Millsp. genotypes under nickel stress. Environ Sci Pollut Res 27:3043–3064
Ghosh G, Purohit A, Chaudhuri RK, Chakraborti D (2014) Advances in genetic transformation of important pulse crop pigeonpea. Agro Food Biotechnol 12:1–16
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gonzalez-Chavez MC, Carrillo-Gonzalez R, Wright SF, Nichols KA (2004) The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut 130:317–323
González-Guerrero M, Oger E, Benabdellah K, Azcón-Aguilar C, Lanfranco L, Ferrol N (2010) Characterization of a Cu-Zn superoxide dismutase gene in the arbuscular mycorrhizal fungus Glomus intraradices. Curr Genet 56:265–274
Hao F, Wang X, Chen J (2006) Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci 170:151–158
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants 23:249–268
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts, I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat cultivars and ancestors, a synthesis. Can J Bot 71:512–518
Jamal A, Ayub N, Usman M, Khan AG (2002) Arbuscular mycorrhizal fungi enhance zinc and nickel uptake from contaminated soil by soybean and lentil. Int J Phytoremediation 4:205–221
Kubiś J, Floryszak-Wieczorek J, Arasimowicz-Jelonek M (2014) Polyamines induce adaptive responses in water deficit stressed cucumber roots. J Plant Res 127:151–158
Liu GD (2001) A new essential mineral element-nickel. Plant Nutrition and Fertilizer Science 7:101–103
Marguí E, Queralt I, Carvalho ML, Hidalgo M (2007) Assessment of metal availability to vegetation (Betula pendula) in Pb-Zn ore concentrate residues with different features. Environ Pollut 145:179–184
Mehlich A (1953) Determination of P, Ca, Mg, K, Na and NH4. In: Short test methods used in soil testing division. Department of Agriculture, Raleigh, pp 23–89
Mir MA, Sirhindi G, Alyemeni MN, Alam P, Ahmad P (2018) Jasmonic acid improves growth performance of soybean under nickel toxicity by regulating nickel uptake, redox balance, and oxidative stress metabolism. J Plant Growth Regul 37:1195–1209
Nahar K, Rahman M, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ Sci Pollut Res 23:21206–21218
Nakagawara S, Sagisaka S (1984) Increase in enzyme activities related to ascorbate metabolism during cold acclimation in poplar twigs. Plant Cell Physiol 25:899–906
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nelson DW, Sommers LE (1973) Determination of total nitrogen in plant material. Agron J 65:109–112
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of soil analysis. Agron. No. 9, part 2 - chemical and microbiological properties, 2nd edn, American society agronomy, Madison, pp 403-430
Patra A, Dutta A, Jatav SS, Choudhary S, Chattopadhyay A (2019) Horizon of nickel as essential to toxic element. IJCS 7:1185–1191
Paul S, Banerjee A, Roychoudhury A (2018) Role of polyamines in mediating antioxidant defense and epigenetic regulation in plants exposed to heavy metal toxicity. In: Hasanuzzaman M, Nahar K, Fujita M, Alam MM (eds) Plants under metal and metalloid stress. Springer, Singapore, pp 229–247
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Rady MM, El-Yazal MAS, Taie HAA, Ahmed SMAM (2016) Response of Triticum aestivum (L.) plants grown under cadmium stress to polyamines pretreatments. Plant 4:29–36
Rao KM, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Rezvanypour S, Hatamzadeh A, Elahinia SA, Asghari HR (2015) Exogenous polyamines improve mycorrhizal development and growth and flowering of Freesia hybrida. J Hortic Res 23:17–25
Rucińska-Sobkowiak R (2016) Water relations in plants subjected to heavy metal stresses. Acta Physiol Plant 38:257–270
Saad R, Kobaissi A, Robin C, Echevarria G, Benizri E (2016) Nitrogen fixation and growth of Lens culinaris as affected by nickel availability, a pre-requisite for optimization of agromining. Environ Exp Bot 131:1–9
Sachan P, Lal N (2017) An overview of nickel (Ni2+) essentiality, toxicity and tolerance strategies in plants. Asian J Biol 2:1–15
Sairam RK, Deshmukh PS, Shukla DS (1997) Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci 178:171–178
Salloum MS, Menduni MF, Benavides MP, Larrauri M, Luna CM, Silvente S (2018) Polyamines and flavonoids: key compounds in mycorrhizal colonization of improved and unimproved soybean genotypes. Symbiosis 76:265–275
Sannazzaro AI, Álvarez CL, Menéndez AB, Pieckenstain FL, Albertó EO, Ruiz OA (2004) Ornithine and arginine decarboxylase activities and effect of some polyamine biosynthesis inhibitors on Gigaspora rosea germinating spores. FEMS Microbiol Lett 230:115–121
Saxena KB, Kumar RV, Sultana R (2010) Quality nutrition through pigeonpea-a review. Health 2:1335–1344
Shevyakova NI, Il'ina EN, Stetsenko LA, Kuznetsov VV (2011) Nickel accumulation in rape shoots (Brassica napus L.) increased by putrescine. Int J Phytoremediation 13:345–356
Sirhindi G, Mir MA, Abd-Allah EF, Ahmad P, Gucel S (2016) Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front Plant Sci 7:591–603
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Soares C, de Sousa A, Pinto A, Azenha M, Teixeira J, Azevedo RA, Fidalgo F (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125
Sreekanth TVM, Nagajyothi PC, Lee KD, Prasad TNVKV (2013) Occurrence, physiological responses and toxicity of nickel in plants. Int J Sci Environ Technol 10:1129–1140
Stark G (1991) The effect of ionizing radiation on lipid membranes. Biochim Biophys Acta Biomembr 1071:103–122
Szalai G, Tajti J, Hamow KÁ, Ildikó D, Khalil R, Vanková R, Dobrev P, Misheva SP, Janda T, Pál M (2020) Molecular background of cadmium tolerance in Rht dwarf wheat mutant is related to a metabolic shift from proline and polyamine to phytochelatin synthesis. Environ Sci Pollut Res 27:23664–23676
Tang W, Newton RJ (2005) Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul 46:31–43
Tiburcio AF, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants, from development to stress. Planta 240:1–18
Twanabasu BR, Stevens KJ, Venables BJ (2013) The effects of triclosan on spore germination and hyphal growth of the arbuscular mycorrhizal fungus Glomus intraradices. Sci Total Environ 454:51–60
Vallino M, Massa N, Lumini E, Bianciotto V, Berta G, Bonfante P (2006) Assessment of arbuscular mycorrhizal fungal diversity in roots of Solidago gigantea growing in a polluted soil in Northern Italy. Environ Microbiol 8:971–983
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants, protective role of exogenous polyamines. Plant Sci 151:59–66
Wen XP, Ban Y, Inoue H, Matsuda N, Moriguchi T (2010) Spermidine levels are implicated in heavy metal tolerance in a spermidine synthase overexpressing transgenic European pear by exerting antioxidant activities. Transgenic Res 19:91–103
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198:97–107
Wu Q, Zou Y, He X (2010) Exogenous putrescine, not spermine or spermidine, enhances root mycorrhizal development and plant growth of trifoliate orange (Poncirus trifoliata) seedlings. Int J Agric Biol 12:576–580
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432
Zhang Y, Hu J, Bai J, Wang J, Yin R, Wang J, Lin X (2018) Arbuscular mycorrhizal fungi alleviate the heavy metal toxicity on sunflower (Helianthus annuus L.) plants cultivated on a heavily contaminated field soil at a WEEE-recycling site. Sci Total Environ 628:282–290
Zhao J, Shi G, Yuan Q (2008) Polyamines content and physiological and biochemical responses to ladder concentration of nickel stress in Hydrocharis dubia (Bl.) Backer leaves. Biometals 21:665–674
Zwiazek JJ, Blake TJ (1991) Early detection of membrane injury in black spruce (Picea mariana). Can J For Res 21:401–404
Acknowledgements
We gratefully acknowledge the UGC and DBT for providing financial support in undertaking this research work. We are also thankful to PAU, Panjab; IARI, New Delhi, India; and The Energy and Resource Institute (TERI), New Delhi for providing the biological research materials. The authors are also thankful to Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, India for WD-XRF analysis.
Availability of data and materials
All data analyzed during this study are included in this article and supplementary materials.
Funding
We would like to thank to University Grants Commission (UGC) and the Department of Biotechnology (DBT), Government of India, for providing financial support in undertaking this research work.
Author information
Authors and Affiliations
Contributions
The corresponding author (NG) planned and designed the research experiments. The first author (KS) performed the experiments. The both authors (KS, NG) contributed to the analysis and interpretation of the results and to the writing of the manuscript as well as gave final shape to the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Both authors agreed and consented to publish this manuscript in present form.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philipp Gariguess
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•Ni stress had negative correlation with growth, mycorrhizal colonization, and ROS.
•Put seed priming was more effective in reducing oxidative stress than Spd and Spm.
•AM was more effective than PAs in modulating ascorbate-glutathione (AsA-GSH) cycle.
•Functional complementarity between AM and PAs in reducing Ni uptake was recorded.
•+Put + AM was most promising in imparting Ni tolerance to pigeonpea genotypes
Supplementary information
Supplementary Fig. 1
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM-Rhizoglomus intraradices) inoculation on a) SOD, b) CAT, c) GPOX, d) APOX, e) MDHAR and f) DHAR activities in leaves (nkat mg-1 protein) of Pusa 2001 and AL 201 pigeonpea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C=PAs and AM absent; Spm=0.5mM Spm added; Spd=0.5mM Spd added; Put=0.5mM Put added; AM=AM added; Ni100=100mg/kg Ni added; Ni200=200mg/kg Ni added; Spm+AM=Spm and AM added; Spd+AM=Spd and AM added; Put+AM=Put and AM added. (PNG 2806 kb)
Supplementary Fig. 2
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM-Rhizoglomus intraradices) inoculation on a) total ascorbate, b) ascorbate, c) dehydroascorbate in leaves (μmole g-1 FW), d) reduced glutathione (nmoles g-1 FW), e) oxidized glutathione (nmoles g-1 FW), f) GR activity in leaves (nkat mg-1 protein), of Pusa 2001 and AL 201 pigeonpea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C=PAs and AM absent; Spm=0.5mM Spm added; Spd=0.5mM Spd added; Put=0.5mM Put added; AM=AM added; Ni100=100mg/kg Ni added; Ni200=200mg/kg Ni added; Spm+AM=Spm and AM added; Spd+AM=Spd and AM added; Put+AM=Put and AM added (PNG 2915 kb)
Supplementary Fig. 3
Effect of PAs (Put, Spd, Spm) and arbuscular mycorrhiza (AM-Rhizoglomus intraradices) inoculation on a) total glutathione, b) non-protein thiols, c) phytochelatins in leaves (nmol g-1 FW), of Pusa 2001 and AL 201 pigeonpea genotypes under Ni stress. Values are the mean of six replicates ± standard error (SE). Different letters above each bar indicate significant differences among the treatments, assessed by Duncan multiple range test, at p ≤ 0.05. C=PAs and AM absent; Spm=0.5mM Spm added; Spd=0.5mM Spd added; Put=0.5mM Put added; AM=AM added; Ni100=100mg/kg Ni added; Ni200=200mg/kg Ni added; Spm+AM=Spm and AM added; Spd+AM=Spd and AM added; Put+AM=Put and AM added. (PNG 1421 kb)
ESM 1
(DOC 117 kb)
ESM 2
(DOC 71 kb)
ESM 3
(DOC 215 kb)
Rights and permissions
About this article
Cite this article
Saroy, K., Garg, N. Relative effectiveness of arbuscular mycorrhiza and polyamines in modulating ROS generation and ascorbate-glutathione cycle in Cajanus cajan under nickel stress. Environ Sci Pollut Res 28, 48872–48889 (2021). https://doi.org/10.1007/s11356-021-13878-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13878-7