Abstract
Phthalate esters (PAEs) are widely used industrial raw materials that are well known for their environmental contamination and toxicological effects as “endocrine disruptors.” In this study, environmental levels of PAEs and eco-toxicological risk assessments were determined in the eight estuaries of the Pearl River (Estuaries), main upstream tributary (Xijiang River), urban river network (River network), and nature reserve reservoir (Reservoirs). Water and sediment samples from the above water systems were collected during the low-water period (May) and the high-water period (August) between 2012 and 2014. Solid phase and ultrasonic methods were used to extract 14 different PAEs that were analyzed by gas chromatography. The analytical average recovery of PAEs in water and sediment was 75.4% ± 4.9% and 121.5% ± 8.9%, respectively. The results showed that PAEs were detected in all of the samples, and the di-n-butyl phtalate (DBP) and benzyl butyl phthalate (BBP) monomers had a detection rate of 100% in water. Similarly, in sediment samples, the detection rates of diisobutyl phthalate (DiBP), DBP, dimethoxyethyl phthalate (DMEP), BBP, di-n-octyl phthalate (DnOP), and DNP ranged from 66.7 to 100%. Among these, in sediment samples, di(2-ethylhexyl) phthalate (DEHP) and phthalic acid bis(2-butoxyethyl) ester (DBEP) had detection rates of 95.8% to 100% in the Estuaries, Xijiang River, and River network. The concentrations of Σ14PAEs in water samples and sediments ranged from 12.95 ± 1.97 to 6717.29 ± 112.37 ng/L and 71.99 ± 8.72 to 17,340.04 ± 227.83 ng/g-dw, respectively. During the low-water period, the average concentration of Σ14PAEs in water and sediment was 1159.58 ± 97.22 ng/L and 2842.50 ± 178.21 ng/g-dw, respectively, and during the high-water period, 822.83 ± 53.19 ng/L and 1936.42 ± 111.31 ng/g-dw, respectively. In water, the average concentration of Σ14PAEs in 2013 and 2014 was 963.39 ± 19.55 ng/L and 2815.35 ± 176.32 ng/L, respectively. In sediment, the average concentrations of Σ14PAEs in 2012 to 2014 were 990.10 ± 23.33 ng/g-dw, 1084.20 ± 112.12 ng/g-dw, and 1816.89 ± 79.97 ng/g-dw, respectively, with concentrations showing an increasing trend year after year (2014 > 2013 > 2012). Potential risk assessment of water ecological, the results show that exceeding environmental risk level (ERL) value in higher molecular weight plasticizer (DEHP, DMEP, DNOP, DNP) was mainly distributed in water, the lower molecular weight plasticizer (BMP, DiBP) was mainly distributed in sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters (PAEs) are man-made chemical compounds, additives used to increase a material’s transparency, strength, plasticity, and endurance, with a broad range of applications in many different products due to their varied physical and chemical properties (Kolarik et al. 2008; Serrano et al. 2014). They are primarily used as plasticizers to enhance the plasticity of industrial polymers (Sears and Darby 1982), which are used in a great deal of products such as cosmetics, tool handles, toys, recreational gear, office supplies, paints, gloves, adhesives, lubricants, personal care items, emollients, medical devices, safety glasses, packaging and building materials, coated fabrics and paper, electronics, wires and cables, wall coverings and floor tiles, humectants, shower curtains, antifoaming agents, soft polyvinyl chloride (PVC) floor coverings, mattresses, footwear, and so on, most of which are an unavoidable part of modern life (Chen et al. 2013; Horn et al. 2004; Kavlock et al. 2002; Net et al. 2015; Shea 2003; Shore 1995). The world’s PAE production increased from 6.2 million tons in 2009 to more than 8 million tons in 2011, and research has shown that more than 11 billion pounds of phthalates are produced every year worldwide (Gao and Wen 2016; Meng et al. 2014). In China, the production exceeds 0.87 million tons per year, due to the use of dibutyl phthalate (DnBP), di(2-ethylhexyl) phthalate (DEHP), and dibutyl phthalate (DBP) in a large number of plastic products. From 2003 through 2007, Taiwan, on average, produced more than 20,000 tons of DBP and 200,000 tons of DEHP each year making these the most widely used plasticizers worldwide. Although PAEs are able to improve the performance of plastic, they are not chemically bound to the PVC structure through a covalent linkage, and for this reason, they are constantly being leached from the matrix and emitted to the environment through migration and evaporation resulting in the exposure of those that come in contact with the PAEs (Fromme et al. 2002; Wang et al. 2006; Zota et al. 2014).
Since the development of PAEs in the 1920s, they have been used extensively in industry and consumer products. Previous investigations have shown that PAEs can exist in the environment during all stages of mass production, utilization, and waste disposal, and they can be distributed in the atmosphere, storm water, natural waters, bottled water, sewage sediment, soil, aquatic biota, as well as homes and more (Casalscasas and Desvergne 2011; Kolarik et al. 2008; Larcinese and Testa 2013; Liu et al. 2014; Martine et al. 2013; Net et al. 2015; Sun et al. 2013; Teil et al. 2013; Yang et al. 2013). Over the past few decades, the wide-ranging production and utilization of consumer products with PAE contamination have become a very important issue as it has resulted in the ubiquitous presence of these novel organic contaminants in the environment. Human and wildlife exposure to elevated levels of PAEs is thus unavoidable resulting in the observation of endocrine-mediated adverse effects (Casalscasas and Desvergne 2011; Kolarik et al. 2008; Larcinese and Testa 2013; Liu et al. 2014; Martine et al. 2013; Net et al. 2015; Sun et al. 2013; Teil et al. 2013; Yang et al. 2013). Studies showed that fruit Σ4PAEs 0.69–1.8 μg/g, aquatic Σ2 PAEs 6426.0–7519.0 ng/g, meat/egg Σ7PAEs ND-11460.0 ng/g, nut Σ2PAEs 230.0–3950.0 ng/g, fish Σ4PAEs 190.0–650.0 ng/g (Chen et al. 2012), daily intake of Σ7 PAEs and DEHP were 128.63 and 61.29 ng/g BW/day for adults in the Pearl River Delta (Chen et al. 2012). In the urine of children and adults and senior citizens, Σ2 PAEs at a maximum concentration of 2,105,000 ng/L (Koch et al. 2011)
Phthalate esters are typical endocrine-disrupting compounds (EDCs), where prolonged periods of intake can induce serious health issues, including reproductive effects, birth defects, decreased testosterone levels, proliferation of testicular Leydig cells, altered semen quality, decreased serum estradiol levels, thyroid-disrupting effects, hormonal and endocrine disruptions, prolonged estrus and ovulation, children growth-disrupting effects, asthma, and breast cancer, (Casalscasas and Desvergne 2011; Colón et al. 2000; Foster et al. 2015; Lovekampswan and Davis 2003; Martino-Andrade and Chahoud 2010). Furthermore, PAEs effect the cellular targets of other receptors such as the aryl hydrocarbon receptor, androgen receptor, and insulin receptor (Boas et al. 2010; Colacino et al. 2010; Krüger et al. 2008; Li et al. 2010; Mankidy et al. 2013; Rengarajan et al. 2007).
Owing to the potential risks associated with PAEs, the United Nations Environment Programme (UNEP) and the International Maritime Organization are currently assessing these compounds. The use of BBP, DEHP, DBP, and DiBP has also been restricted or banned in childcare toys and articles sold in the United States and Canada, CPSIA (US Consumer Product Safety Improvement Act 2008), Hazardous Product Act (2010).
The Pearl River Delta (PRD) is situated in South China with an annual average precipitation and air temperature of about 1500 to 1690 mm and 21.8 to 23 °C, respectively. The delta falls within a tropical and subtropical region and has a humid subtropical climate with occasional typhoon and thunderstorm activity due to its location adjacent to the South China Sea (Chen et al. 2006). The PRD has a triangular shape and was formed from the alluvial soils of the Pearl River estuary and three major rivers, the East River, West River, and North River and their tributaries. The delta covers an area of 461,000 km2 (Hills et al. 1998), with a population of over 56 million (Wei et al. 2017). According to the World Bank Group (2014), the PRD has become the largest urban area in the world in both size and population.
The PRD has experienced rapid economic development since the 1970s and includes several big cities, namely Guangzhou, Shenzhen, Zhuhai, Dongguan, Zhongshan, Foshan, Zhaoqing, Hong Kong, and Macao. Many factories and industries are based in the region, among them Guangzhou is the largest light industrial production base in China. The large population, fast economic growth, rapid commercialization, and massive industrialization and urbanization have resulted in numerous environmental concerns in the PRD, such as pollutant emissions in the region (Hills et al. 1998), especially excessive releases of domestic sewage, industrial, and municipal wastewater (Wong and Wong 2004), which have caused significant impacts on the local environment (Hills et al. 1998; Li et al. 2000). As river water represents an important habitat for a variety of aquatic life species, as well as an important source for drinking water, irrigation, production, and so on, river water contamination has been a subject of many research efforts (Ho and Hui 2001). Extensive anthropogenic activities, coupled with the lack of pollution controls, inevitably increase the fluxes of PAEs from terrestrial and atmospheric sources to river water (Chen 1992; Florig 1997).
The objectives of the present study were to determine the levels and distributions (spatial and temporal) of dimethyl phthalate (DMP), diethyl phthalate (DEP), di-n-propyl phthalate (DPRP), DBP, BBP, di-n-pentyl phthalate (DPP), DnOP, DiBP, DEHP, di-n-hexyl phthalate (DNHP), di-n-nonyl phthalate (DNP), DMEP, diethoxyethyl phthalate (DEEP), and DBEP 14 PAEs in water and sediment from freshwater systems in the PRD and to discuss their contamination profiles and potential sources. Additionally, in order to assess the environmental safety of rivers in the PRD, a preliminary water ecological risk assessment was performed assessing the risk from exposure to PAEs. This study could provide a valuable and insightful data set for use risk assessments of the PRD water environment. Moreover, the findings may provide reliable data to inform decisions for future sustainable development in China.
Materials and methods
Instrumentation and reagents
Instruments and equipment used in the preparation and analysis of samples included the following: freeze dryer (FD-1A-50, Beijing Bo Yikang Experimental Instrument Co., Ltd.); rotary evaporator (RE-2000, Shanghai Yarong Biochemical Co., Ltd.); ultrasonic cleaner (SB-5200DT, Heidolph, Germany); muffle furnace (FD-40, Shanghai Hegong Scientific Instrument Co., Ltd.); gas chromatograph (HP6890, Agilent Technologies, Inc.); chromatography column (DB-5 quartz capillary column, Agilent Technologies, Inc.); electronic balance (FA1104, Shanghai Tianping Instrument Factory); pure water meter (UNIQUE-R20, Shanghai Hegong Scientific Instrument Co., Ltd.); solid-phase extraction manifold (MULTI-SPE M08, Bonna-Agela Technologies); circulating water vacuum pump (SHZ-III, Shanghai Yarong Biochemical Co., Ltd.); and C18-E cartridge (1000 mg/6 mL; Phenomenex, Torrance, CA, USA).
Chromatography-grade sodium sulfate (dried in a muffle oven at 550 °C for 4 h), hexane, acetone, and dichloromethane were purchased from Guangzhou Chemical Reagent Factory. High purity nitrogen (99.99%) was produced by Beijing Nitrogen Plant. Phthalate esters were purchased from German DR: DMP (99.99%), DEP (99.99%), DPRP (99.87%), DBP (99.99%), BBP (99.93%), DPP (99.99%), DnOP (99.89%), DiBP (99.99%), DEHP (99.99%), DNHP (99.99%), DNP (99.99%), DMEP (99.95%), DEEP (99.99%), and DBEP (99.95%). Purchase the reference materials (RM) for 14 PAEs, respectively, and formulate a mixture of 14 PAEs, the mixture was used in the recovery calculations of the compound. Table S3 summarizes the main environmental parameters of the 14 PAEs.
Study area and sample collection
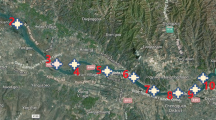
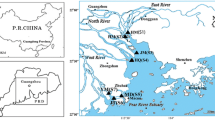
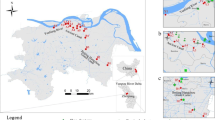
Water and sediment samples were collected from 2012 to 2014 in May (low-water period) and August (high-water period) from eight Pearl River estuaries (Estuaries), the main upstream tributary (Xijiang River), the urban river network (River Network), and the nature reserve reservoir (Reservoirs). A total of 27 sites were sampled and their locations are shown in Fig. 1, while details of each sampling site are included in Table S1.
Surface water grab samples were collected (mid-stream) into 1-L brown glass bottles and brought back to the laboratory for testing. Surface sediment grab samples were collected with a grab-type mud collector and subsampled into 1-L wide-mouth glass bottles. These were transported back to the laboratory where they were freeze-dried. After being sieved with 100 mesh, dry sediment samples were transferred to 0.5-L wide-mouth glass bottles, which were then sealed and stored for testing. Samples were collected in triplicate.
Sample preparation
The extraction of phthalates from surface water and sediment samples followed the method of Wang et al. (2005) with minor modifications.
Water samples
(1) Filtration: 1 L of water sample was filtered through a 0.45-μm filter (mixed cellulose, CA-CN) to collect the filtrate; (2) activation of C18 column: 5.0 mL of dichloromethane was added to the C18 column, stayed for 5 min, and let go; 5 mL of methanol was then added and stayed for 5 min and discarded; finally, 2 mL of double distilled water (water does not stay) was added, which slowly passed through the extraction column to achieve the wet column, activate the filler, and remove the interference impurities; (3) C18 cartridge purification: the solid phase column was dried by sequentially passing 5.0 mL each of ultrapure water and methanol/ultrapure water (v/v = 6/4) through the column to remove most of the residual moisture; (4) sample extraction: the filtered water sample was passed through the small column at a pressure of about 50 kPa and a flow rate of approximately 1.0 mL/min allowing the analyte to adsorb to the column while the water flowed out through the column and was discarded; (5) elution: the eluent, n-hexane/acetone (v/v = 9/1), was added to the column and after about 1 min, the target was eluted at a flow rate of 2.0 mL/min and 10 mL was collected in the collection tube; (6) drying: 2.0 g of anhydrous sodium sulfate was added to the collection tube for drying; (7) concentration and constant volume: the solution in the collection tube was rotated on the evaporator (50 r/s, 80 °C, 0.01 Mpa) to a volume of approximately 1 mL and transferred into a gas phase vial where it was slowly dried with high purity nitrogen and made up to 1.0 mL with n-hexane for the analysis.
Sediment samples
(1) Ultrasonic extraction: the collected sediment samples were freeze-dried, milled, passed through a 0.25 mm sieve, and stored for testing; 10.0 g of the treated sediment sample was accurately weighed and placed into a 50.0-mL Erlenmeyer flask; addition of 30 mL of a methyl chloride-acetone mixture (v/v = 1/1) was used as the solvent, and the extract was collected by ultrasonication in an ultrasonic generator (40 kHz, 20 °C) for 20 min, repeated thrice; collect the above extract mixture and keep it still for 24 h, the supernatant was taken and passed through a packed column of 20 g anhydrous sodium sulfate, collect the filtered extract for further processing; the next steps are the same as those described for the water sample treatments (2 to 7).
Analytical determination
Instrument settings
A gas chromatograph (HP6890) with an 63Ni electron capture detector (μECD) was used. The column was a DB-5 quartz capillary column with a length of 30 m, an inner diameter of 250 μm, and a stationary phase film thickness of 0.25 μm. The column flow was 2.0 mL/min, constant current, and the carrier gas was high purity nitrogen. The column temperature program was initiated at 50 °C for 2.0 min, increased to 200 °C at a rate of 25 °C/min, held for 2.0 min, and finally at 40 °C /min ramped up to 280 °C and held for 5.0 min. The inlet and detector temperatures were 280 °C and 300 °C, respectively. The extracts (1.0 μL) were injected onto the gas chromatograph in a constant current splitless mode (Wang et al. 2005).
The standard solutions that were prepared for the 14 different phthalates were detected by the gas chromatograph. The concentration of each standard was plotted on the abscissa and the peak area was plotted on the ordinate. The peak area external standard method was used to obtain the regression equation and the coefficient of determination (R2 > 0.99) and retention time. Chromatograms for each of the 14 different PAEs are shown in Fig. S1.
Quality assurance and quality control
All samples were collected as replicates and analyzed in triplicate. The data were subject to strict quality control procedures. For each batch of 10 field samples, analyzed, a procedural blank, a spiked blank, a spiked matrix sample, a spiked matrix duplicate, and a sample duplicate were run to check for the interference, contamination, and quantification (in Table S2). The instruments were calibrated daily with calibration standards. Each sample was repeatedly tested five times to calculate the average recovery of each PAE.
The average recovery of the 14 PAEs in Table S3, in the water samples, ranged from 75.4% ± 4.9% to 121.5 ± 8.9%, and the method detection limit ranged from 0.10 to 75.00 ng/L. In sediments, the percent recovery ranged from 79.6% ± 5.1% to 116.5% ± 6.9%, the method detection limit from 0.1 to 75.00 ng/g-dw. A five-point standard curve was used to quantitatively determine the PAE content in the samples (R2 > 0.99). A quality control sample was included in each batch of samples, and the standard curve was corrected to reduce the experimental error.
Statistical treatment
Statistical analysis was performed using Origin Pro 9.0 software for Windows (Origin Lab Northampton, MA, USA). One-way analysis of variance, the significant level was set to α = 0.05, the significant difference was considered when p < 0.05.
Results and discussion
PAE concentrations in water and sediment from the Pearl River Delta
Comparison of PAE concentrations in different water types
Over the past few decades, PAE pollution has attracted much attention and many investigations on the contamination of plasticizers in different water environments such as rivers, lakes, drinking water, and bottled water have been conducted. Individual concentrations of the 14 PAEs that were measured in river water samples are shown in Table S4. The results indicated that PAEs are ubiquitous environmental pollutants, and the concentrations of Σ14PAEs in water samples ranged from 465.53 ± 52.61 (W6) to 2825.35 ± 144.31 ng/L (W2). The concentrations of DEHP in the W2 were 1700.71 ± 78.11 ng/L. The distribution and concentrations of PAEs in the environment have been well documented in many regions such as the Klang River Basin, Malaysia, the Rieti District, Italy, the Urdaibai Estuary of Gernika, Spain, and the False Creek Harbor of Vancouver, Canada (Bartolomé et al. 2005; Mackintosh et al. 2006; Tan 1995; Vitali et al. 1997). Also, the Environmental Protection Agency of Japan surveyed 45 major domestic rivers that were part of a monitoring network, EPAJ (1993). Around the world, the majority of detected concentrations have ranged from 100 to 300,000 ng/L in waters (Fatoki and Noma 2010; Giam et al. 1984; Hashizume et al. 2002). As the Xijiang River is a major tributary of the Pearl River, cities such as Zhaoqing and Deqing are distributed along the river while some small tributaries flow into the Xijiang River, resulting in a high concentration of Σ14PAEs in the water. Although the Estuaries area contains many polluted water bodies, the influence of ocean tides on these water bodies has diluted the PAE concentrations to a certain extent, so that PAE levels are slightly lower than in the Xijiang River. The environment surrounding the Reservoirs of the nature reserve is less disturbed by humans and less polluted, so the concentration of Σ14PAEs in this area is also the lowest.
The detection rate of Σ14PAEs in the above three types of water bodies was 100%. The detection rates of DBP and BBP in the Xijiang River and the Estuaries were 100%, while DPRP and DnHP were only detected in the Estuaries, and DPP was only detected in the Xijiang River. Among the monomers detected, the DPP concentration was the lowest due to the limited use of this substance and its low solubility (Table S3). The highest concentration of Σ14PAEs in water was measured at W2 (6717.29 ± 112.37 ng/L, date May 2014). At the time of measurement, several cargo ships were parked on the water near the W2 site for maintenance. The paint, leather, ceramic tiles, synthetic materials, and other materials used in ship maintenance contain plasticizers (Chen et al. 2013; Horn et al. 2004; Kavlock et al. 2002), which increased the concentration of plasticizers in the water. The Σ14PAEs had the same high solubility at W12 (2224.66 ± 93.13 ng/L, date May 2014), which is surrounded by hospitals, restaurants, and commercial streets, and where residential sewage flows into the river and where a lot of garbage occur, including plastic bags, packaging materials, and used clothing. These substances contain PAEs (Net et al. 2015; Shea 2003; Shore 1995); the PAEs are constantly being leached from the matrix and emitted to the environment through migration resulting in relatively high concentrations of Σ14PAEs (Fromme et al. 2002; Wang et al. 2006; Zota et al. 2014). W9 (2190.38 ± 102.01 ng/L, date May 2014) is located near the Zhaoqing Fishery Wharf, where dense residential areas occur on the side of the river bank. W8 (2028.73 ± 78.99 ng/L, date August 2013) has a higher concentration of PAEs during the rainy periods. Studies have shown that PAEs can distribute in rainwater (Liu et al. 2014; Martine et al. 2013), automobile tires (Serrano et al. 2014; Sears and Darby 1982), the dust generated by automobile tires wear on the highways which will be washed into the water, result a higher concentration of PAEs. The concentrations of Σ14PAEs at W13 (12.95 ± 1.97 ng/L, date May 2014) and W14 (14.26 ± 2.45 ng/L, date May 2014) were relatively low. These sites are located in the nature reserve, surrounded by mountains on three sides, with few residents nearby and limited human activities. As shown in Table 1, the PAE concentrations that were measured in water in the current study area are moderate when compared to data from the Ronnebyan Svartan River in Canada (Mackintosh et al. 2006), Klang River in Germany, Ogun River in Nigeria, (Adeniyi et al. 2011), and Yongding River in Beijing (Wang et al. 2006).
Table S5 shows the spatial distribution of 14 PAEs in surface sediments that were detected in 110 samples from 17 stations, in areas that included the Estuaries, Xijiang River, River Network, and Reservoirs. The detection rate of Σ14PAEs in these areas was 100%, which indicates that PAEs are ubiquitous in the sediments. The concentration of Σ14PAEs ranged from 330.72 ± 41.07 to 7315.38 ± 140.69 ng/g-dw. The concentration ranges of Σ14PAEs in the Estuaries, Xijiang River, Reservoirs, and River Network were 971.25 ± 70.49 to 3190.43 ± 224.79 ng/g-dw, 771.79 ± 76.03 to 1146.67 ± 84.01 ng/g-dw, 330.72 ± 41.07 to 716.38 ± 38.55 ng/g-dw, and 801.43 ± 34.5 to 3844.82 ± 301.86 ng/g-dw, respectively. Around the world, the majority of detected concentrations have ranged from 100 to 100,000 ng/g-dw in sediment (Peijnenburg and Struijs 2006; Serôdio P 2006; Staples et al. 2000; Sung et al. 2003).
The urban River Network flows into a city where the river banks are populated with residents, buildings such as shops, industries, and small workshops (Chen et al. 2013). Domestic sewage and production wastewater are discharged into the River Network, and due to the high rate of urban hardening, the rain washes pollutants from the road into the River Network (Chen et al. 2016). In addition, urban rivers are with relatively slow flow rates that conducive to debris deposition and accumulation of PAEs (Fernandez et al. 2007; Fan et al. 2008).
In the above sites, the highest concentration of Σ14PAEs was measured at the Pearl River Bridge, site H3 (17,340.04 ± 227.83 ng/g-dw, date May 2012). Near the Pearl River Bridge, there are many small factories (Zeng et al. 2008). Factories manufacturing adhesives and other compounds emit large amounts of PAEs (Zeng et al. 2009). Moreover, the Pearl River Bridge connects the two sides of the Pearl River, and the daily traffic volume is huge. The friction between car tires and the road produces dust. The dust is brought into the water by rain or wind and transferred to the sediment (Serrano et al. 2014; Sears and Darby 1982). The above factors account for the higher sediment concentration of Σ14PAEs at H3. Similar high concentrations of Σ14PAEs were measured at H12 (5743.88 ± 219.05 ng/g-dw, date May 2012) and H8 (5511.88 ± 177.83 ng/g-dw, date August 2012), which are also located in the city center where household garbage such as plastic bags, used clothing, toys, mineral water bottles, and other items float in the water. PAEs are leached from household garbage (Fromme et al. 2002; Wang et al. 2006; Zota et al. 2014). W10 (5741.56 ± 203.22 ng/L, date May 2012) is located at the corner of the river, where sediments are easily accumulated resulting in a higher Σ14PAEs sediment concentration (Chen et al. 2013). W13 (71.99 ± 8.72 ng/g-dw, date May 2013) and W14 (106.34 ± 9.95 ng/g-dw, date May 2012) had the lowest concentrations. The detection rates of DiBP, DBP, DMEP, BBP, DnOP, and DNP in sediments were all high, ranging from 66.67 to 100%. The detection rates of DEHP and DBEP in the Estuaries, Xijiang River, and River Network ranged from 95.8 to100%. This suggests that the distribution of the above PAE monomers is extensive.
Comparison of water and sediment Σ14PAEs in different time periods
Figure 2a shows the concentrations of Σ14PAEs measured in the water bodies in May and August. During the low- and high-water periods, the concentrations in the water samples ranged from 12.95 ± 1.97 to 6717.29 ± 112.37 ng/L and from 68.64 ± 8.23 to 1477.33 ± 152.31 ng/L, respectively, with average values of 1159.58 ± 97.22 ng/L and 822.83 ± 53.19 ng/L. In Wuhan section of the Yangtze River, the concentration ranges of 5 PAEs in water phase were 34.0 to 1259.0 and 54.0 to 1321.2 ng/L in high- and low-water periods, respectively (Fan et al. 2008). The Σ14PAE concentrations at W1-W3, W5-W7, and W9-W12 were high during the low-water period. During the high-water period, the volume of water was larger, and the concentration of Σ14PAEs in the water was diluted. The pollution contributed by the PAEs was greater than that contributed by dilution. The concentrations of Σ14PAEs at W4, W8, W13, and W14 during the high-water period were higher than those during the low-water period. Due to the small tributaries associated with W4 and W8 are mainly surrounded by rural residential areas. In August, when there was more rain, more pollutants were washed into the tributaries from terrestrial sources (Zeng et al. 2008), resulting in more pollution than can be diluted by the increased water volume. The surrounding environment at W13 and W14 mostly consists of mountains and forest. During the high-water period, pollutants are mainly contributed from the upstream water bodies. Runoff from urban surfaces during rainfall will affect the water quality of rivers during both high- and low-water periods. Similar observations have been reported for DEHP in the Rieti River in Italy (Vitali et al. 1997) and the Gomti River in India (Srivastava et al. 2010).
Comparison of water and sediment Σ14PAE concentrations from different time periods. a Concentrations of Σ14PAEs in the water bodies in May and August. b Concentrations of Σ14PAEs in sediments in May and August. c Concentrations of Σ14PAEs in water during 2013–2014. d Concentrations of Σ14PAEs in sediments during 2012–2014
Concentrations of Σ14PAEs measured in sediments during the low-water period in May and the high-water period in August are compared in Fig. 2b. The Σ14PAEs concentrations ranged from 167.55 ± 12.11 to 17,340.04 ± 99.87 ng/g-dw and 60.32 ± 10.19 to 13,981.54 ± 127.87 ng/g-dw, during the low- and high-water periods, respectively, with average values of 2842.50 ± 178.21 ng/g-dw and 1936.42 ± 111.31 ng/g-dw. In Wuhan section of the Yangtze River, the PAE concentrations in sediment phase ranged from 6300.0 to 478,900.0 ng/g (Fan et al. 2008). In Qiantang river, the mean concentration in low flow areas (sites 1–9, 13, n = 10) was 2850.0 ± 20.3 ng/g-dw, while that in high flow areas (sites 10–12, 14–23, n = 13) was 1390.0 ± 7.1 ng/g-dw (Sun et al. 2013), while Fromme et al. (2002) reported concentrations in the range of 210 to 8440 ng/g-dw in sediment of the Klang River estuary in Germany.
Sediments from W1, W4, W7, W8, and W10-W12 had higher Σ14PAE concentrations in May. In August, the rapid water flow washes and disturbs surface sediments resulting in lower sediment concentrations. The Σ14PAE sediment concentrations were higher at W2, W3, W5, W6, and W9 in August because of the increased rainwater, which introduced more PAE pollution into the upstream water bodies. When the water flow slows down, the PAEs precipitate with the suspended matter in the water into the sediment, resulting in a higher concentration of Σ14PAEs in the sediment. Owing to the deep water in the Reservoir, environmental factors such as the bottom temperature and pH are stable, and the sediment is less influenced by the surface water. Therefore, W13 and W14 have little change between the low-water and high-water periods. In the urban River Network, H2, H8, and H9 had high Σ14PAE concentrations during the high-water period. There are many villages near H2 such as Jiangmei Village, where the infrastructure is relatively primitive, and the rainwater during the high-water period flushes more domestic sewage into the water body. Companies such as Xiadonghua Industrial Zone, Yuanfeng Chuangzhan Hardware Furniture, Yuanchao Furniture Co., Ltd., Xiadong Garment Factory are located near H8. H9 is surrounded by companies such as Lianbai Steel Structure Factory, Xinyong Industrial Zone, Shanyou Shopping Mall, Lam Nuclear Retirement Home, Fengyi Electronics Co., Ltd., and Ruiying Electronic Processing Factory. Rainwater washes away dust on the ground in industrial areas (Zeng et al. 2008), resulting in more pollutants entering H8 and H9 during the high-water period, which then deposit into the sediment. H10 is located near Lianxi Fengshan Park, and a small tributary with good water quality from the upstream park is combined to dilute the concentration of pollutants entering the mainstream river. This results in a small change in the Σ14PAE concentration at H10. Other locations are characterized by high levels of Σ14PAEs during the low-water period. In sediment from the Kaohsiung Harbor in Taiwan, the total concentration of 10 PAEs ranged from 8713.0 to 11,454.0 ng/g-dw (Chen et al. 2016). Municipal sludge from Beijing was found to contain DMP, DEP, di-n-octyl phthalate (DOP), DBP, DEHP, and BBP with a total concentration of 114,166.0 ng/ g-dw (Mo et al. 2001).
The measured concentrations of Σ14PAEs in water are compared between years in Fig. 4c. The concentrations in 2013 and 2014 ranged from 23.79 ± 3.66 to 2028.73 ± 112.07 ng/L and 63.77 ± 10.21 to 3577.36 ± 117.23 ng/L, respectively, with average values of 1119.52 ± 99.87 ng/L and 1060.85 ± 109.87 ng/L. Based on data from the Guangdong Provincial Meteorological Yearbook, the average precipitation recorded in the province in 2013 and 2014 was 2124.5 mm and 1652.5 mm, respectively. The volume of water entering the river system has a direct effect on the overall concentration of Σ14PAEs in the water. When the increase of pollutants is greater than the dilution capacity of the water, the concentration as in 2013 is high, otherwise the concentration is low.
The concentrations of Σ14PAEs that were measured in sediments in different years are shown in Fig. 4d. The concentrations from 2012, 2013, and 2014 ranged from 91.39 ± 11.38 to 3837.69 ± 122.31 ng/g-dw, 136.65 ± 12.17 to 3214.01 ± 39.97 ng/g-dw, and 228.13 ± 12.89 to 5040.70 ± 145.76 ng/g-dw, respectively, with average values of 990.10 ± 23.33 ng/g-dw, 1084.20 ± 112.12 ng/g-dw, and 1816.89 ± 79.97 ng/g-dw. The Σ14PAE concentrations in the sediments showed an upward trend as they increased each year from 2012 to 2014. The low solubility and slow degradation of PAEs (Table S1), for example, the solubility of DnOP is only 3,000,000 ng/L and the long half-life of water of 107 years make it easy for PAEs to accumulate in surface sediments from settling suspended matter. Sediments are more stable than water, therefore, the concentration of PAEs in sediments is a better indicator of the overall PAE status and pollution trends in the region’s water systems. The change in the PAE sediment concentrations is related to the specific location of the sampling site and the local conditions including water flow, depth, and water volume. If the water is deep and hot with a small volume, contaminant levels in the sediment are relatively low and vice versa.
PAE congener profiles in water and sediments of the Pearl River Delta
The concentrations of PAEs that were measured in surface water are shown in Fig. S2A. The concentrations of several monomers, namely DMP, DiBP, DBP, DMEP, DEHP, DnOP, and DNP, were relatively higher with average values of 224.20 ± 20.11 ng/L, 76.87 ± 10.71 ng/L, 144.36 ± 11.42 ng/L, 60.03 ± 5.36 ng/L, 328.74 ± 18.69 ng/L, 118.88 ± 9.17 ng/L, and 61.64 ± 5.66 ng/L, respectively. Of all the monomers, DEHP had the highest concentration. This result is consistent with other studies where DiBP and DEHP have been commonly reported as the major components of PAEs in water (Fernandez et al. 2007; Fromme et al. 2002; Peijnenburg and Struijs 2006; Xie et al. 2005; Yuan et al. 2002). DEHP has a wide range of uses and is characterized by strong adsorption and low degradation in sediments. Its solubility is higher than the solubility of the other PAEs in the study (Table S1), while its density is lower than that of water (0.985 g/cm3), resulting in higher PAE concentrations in water. The highest DEHP level was measured at W2 (1700.71 ± 107.52 ng/L). In Malaysia, Tan (1995) reported sediment concentrations of DEHP in the range of 490 to 15,000 ng/g-dw. Huang et al. (2005) analyzed 35 different river sediments from northern Taiwan and found the DEHP concentration to range from nondetectable levels to 8970 ng/g-dw. Over the last decade in China, DEHP levels in drinking water source have been measured with a median value of 1300 ng/L (Wang et al. 2015). In Guangzhou, (Zeng et al. 2008), Taiwan, (Chen et al. 2013), and China’s Yellow River (Xie et al. 2005; Sha et al. 2007), environmental samples also contained high concentrations of DiBP and DnOP. Maximum levels of DBP and DEHP were found to range from 17,000 to 76,000 ng/L in water samples from water treatment plants in Hangzhou, China (Ling L et al. 2010). In rivers and dams of the Venda region, South Africa, PAEs were reported in the range of 160.0 to 10,170.0 ng/L in water samples and 20.0 to 890.0 ng/g-dw in sediment samples (Fatoki et al. 2010). In addition, high concentrations of DEHP, DnBP, and DiBP were also detected in the Yongding River (Peijnenburg and Struijs 2006) and Taihu Lake (Mai et al. 2005).
The measured concentrations of PAEs in sediment are shown in Fig. S2B. The concentrations of DMP, DEP, DiBP, DBP, DMEP, DPP, and DNP were relatively high, with average values of 277.72 ± 19.01 ng/g-dw, 168.64 ± 12.56 ng/g-dw, 449.33 ± 23.17 ng/g-dw, 178.55 ± 11.37 ng/g-dw, 658.55 ± 28.99 ng/g-dw, 178.55 ± 12.33 ng/g-dw, and 165.96 ± 11.69 ng/g-dw, respectively. The highest concentration was measured for DMEP (9587.68 ± 139.91 ng/g-dw), which is consistent with observations reported in other studies (Klamer et al. 2005; Lin et al. 2003; Yuan et al. 2002). The applications of EMEP include toughening agent, solvent, rubber, and polyurethane plasticizer. Three factories producing oil-resistant plastics and rubber are distributed near H3. In addition, the vehicular tire debris on the Pearl River Bridge contains DMEP. The solubility of DMEP is also relatively high among the 14 PAEs that were studied allowing it to easily dissolve in river water, while the density of DMEP is greater than the density of water (1.264 g/cm3). It is thus easily transferred to sediments when the external conditions such as temperature and suspended matter content change.
The composition of PAEs in the various water samples is shown in Fig. 3a. The main PAEs that were measured in water at W1 were DEHP, DnOP, and DBP; DEHP at W2; DMP and DEHP at W3; DMP and DEHP at W4; DEHP at W5; DEHP and DBP at W6; DEHP at W7; DnOP at W8; DMP and DEHP at W9; DMP at W10; DMP and DEHP at W11; DMP and DnOP at W12; DMP, DnOP, and DEHP at W13; and, DiBP, DnOP, and DEHP at W14. DEHP has a proportionately higher fraction of up to 60%, which is consistent with the proportion of about 50% reported in other studies (Chen et al. 2016).
The composition of PAEs in the various sediment samples is shown in Fig. 3b. The main PAEs that were measured in sediment at W1 were DEP and DEEP; DiBP and DBP at W2; DPP, DnOP, and DEP at W3; DMEP and DiBP at W4; DEP and DMEP at W5; DiBP at W6; DiBP and DBP at W7; DiBP and DPRP at W8; DiBP at W9; DMP at W10; DMP and DBP at W11; DMP and DPP at W12; DEEP, DiBP, and DnOP at W13; and, DMP, DiBP, and DBP at W14. Sediment at H1 was mainly composed of DMP, DBP, and DiBP; at H2 DMPand DBEP; at H3 DMEP and DiBP; at H4 DEEP and DPP; at H5 DBEP, DnOP, and DNP; at H6 DMEP; at H7 DMEP and DPP; at H8 DMEP; at H9 DEEP; at H10 DEP and DPP; at H11 DMP and DBEP; at H12 DEEP and DiBP; and, at H13 DMEP and DMP. The concentrations of DMP, DiBP, DnOP, and DMEP in the sediment are relatively high, indicating that among the 14 PAEs, these monomeric substances are more widespread.
By comparing the composition of PAE in analogous water and sediment samples, the results showed that there were differences in the types and contents of PAEs in different locations and matrices. The variability in PAE concentrations is caused by factors such as the characteristics of the local environment, water quantity, temperature, pH, and other conditions, as well as the composition of pollutants, sedimentation dispersion/accumulation processes, environmental degradation, or metabolism of aquatic organisms. This has resulted in the prevalence of PAEs in this basin and a wide range of pollution. Phthalate esters are prevalent because they are important part of our lives and are widely used. Small-molecular weight PAEs such as DMP, DiBP, DEP, and DPRP are commonly used in cosmetics and personal care products. Moreover, DiBP is used in epoxy resins, cellulose esters, and special adhesive formulations. Some PAEs with high-molecular weights or longer alkyl groups such as BBP, DNP, DnOP, DEHP, and DMEP are widely used as plasticizers in the polymer industry to improve flexibility (Chen et al. 2013; Gao and Wen 2016; Net et al. 2015).
It has been suggested that lower pH could reduce anoxic degradation rates and contribute to organic matter accumulation in sediment (Enríquez et al. 1993; Shi et al. 2007). The pH in the study area ranged from 6.87 to 8.37 with an average pH of 7.65 in water and from 6.89 to 8.85 with an average pH of 7.88 in sediment. Moreover, dissolved aqueous and sediment Σ14PAEs concentrations were also negatively correlated with water pH and sediment pH, respectively (Fig. 4). pH was not a significant parameter controlling the behavior of dissolved and sediment-associated PAEs in this study. This area of PAE pollution may be more prone to local input. Owing to the physical and chemical properties of PAEs, namely low-water solubility, high organic carbon–water partition coefficients (Koc), and hydrophobicity (Table S1), PAEs tend to adsorb onto suspended particles and eventually accumulate in the sediment as particles settle out of solution.
Factors affecting the concentration and distribution of different forms of PAEs in water and sediment include adsorption, particle aggregation, degradation, dilution, and dissolution (Shi et al. 2005). As PAEs are hydrophobic with low water solubilities and high Koc values (e.g., DEHP), they tend to adsorb to particles and dissolved materials at low flow rates (Chen and Zhu 1999). Low flow rates also promote finer suspended particles to bind organic contaminants (Chen et al. 2006). High flow rates not only have greater dilution effects but also disturb surface deposits, resulting in reduced PAE contaminants in sediments (Fan et al. 2008). Phthalate esters have longer degradation half-lives in sediment than in water, resulting in slower degradation, longer residence times, and higher concentrations in sediments (Doong and Lin 2004).
Figure 5 shows that PAEs in both water and sediment are normally distributed. The Σ14PAE concentrations in water and sediment are mainly distributed between 1000 and 2000 ng/L and 0 and 5000 ng/g-dw, respectively. PAE monomers and concentrations vary widely between different places and different water types. This is influenced by many factors such as the population of the region, the development model, the industrial level, and the type of industry.
Potential risk assessment of water ecological
Increases in pollutant emissions can have a significant impact on the local water environment, which may increase the risk of PAE contamination in the regional food chain. Continuous exposure of resident aquatic organisms to low doses of endocrine-disrupting compounds (EDCs) is unavoidable (Ramaswamy et al. 2011). Although PAEs are present at levels ranging from ng to μg, they may pose a threat to aquatic organisms. Studies have shown that some PAEs (such as DMP, DEP, BBP, DnBP, and DEHP) have bioaccumulation, toxicity, and estrogen effects on aquatic organisms, which in turn affect biological reproduction and induce genetic aberrations (Kambia et al. 2001; Oehlmann et al. 2009; Ye et al. 2016). It has been reported that PAEs (such as DEHP and DBP) at concentrations above 100,000 ng/L can damage blood cells and affect the defense mechanism of Macrobrachium rosenbergii (Sun et al. 2013; Sung et al. 2003). Therefore, risk assessment studies are needed to assess the health of rivers.
ERLs are derived using data on eco-toxicology and environmental chemistry and represent the potential risk of the substances to the ecosystem. ERLs that are derived in The Netherlands until 1997 are summarized by De Bruijn et al. (van der Pas et al. 1999). ERLs are determined for chronic exposure, in which organisms apply ERL to fish (Hypophthalmichthys molitrix, Ctenopharyngodon idellus, Carassius auratus, et al.), Misgurnus miolepis, Corbicula fluminea, Penaeus monodon Fabricius, Zooplankton, and so on (Van Wezel et al. 2000; Sun et al. 2013; Ye et al. 2016). They are recommended as a basis for environmental quality standards: the maximum permissible concentration (MPC) and negligible concentration (NC = MPC/100), and the derived ERLs are 10,000 and 190 ng/L for DBP and DEHP in water, respectively, and are 700 and 1000 ng/g-dw in sediment, respectively. The ERLs of substances such as DMP, DMEP, DiBP, and DnOP have not been established. Therefore, in this study, the ERLs of higher molecular weight (such as DnOP and DNP) and lower molecular weight (such as DMP, DMEP) PAEs were evaluated by DEHP and DBP, respectively. The results show that (Table 2) exceeding ERL value in higher molecular weight plasticizer (DEHP, DMEP, DNOP, DNP) was mainly distributed in water, for example, the DEHP in water is W1 (1.42), W2 (8.95), W3 (1.49), W4 (1.09), W5 (2.72), W10 (1.45), W11 (2.30), W12 (1.99), W14 (1.77); DnOP was W1 (1.35), W4 (1.29), W8 (2.65), W10 (1.35); DNP was W2 (1.87), W4 (1.93), W10 (1.06); DMEP was W9 (1.99). In addition, DMEP was H3 (4.50) and H11 (1.41) in sediment. The lower molecular weight plasticizer (BMP, DiBP) was mainly distributed in sediment, BMP was W12 (2.45), H4 (2.15); DiBP was W8 (1.62). Others were below ERL value.
Owing to the potential impacts on the environment and human health, environmental risk management standards and regulations have been established for PAEs, such as the maximum contaminant level (MCL) for drinking water, Environmental Quality Standards (EQS) for seawater and freshwater, ERLs for soil and sediment, and minor adverse effect concentration (MAEC) for marine sediment (Net et al. 2015). Interestingly, PAE standards for water have been formulated. For example, in China, DEHP limits of 3000 and 8000 ng/L in source water have been recommended by the Ministry of Environmental Protection (MEP) (Library 2013) and General Administration of Quality Supervision, Inspection and Quarantine (GAQSIQ) (Lin 2003), respectively, while the World Health Organization (WHO) has established a limit for DEHP in drinking water of 8000 ng/L (WHO 2011).
Conclusion
The purpose of this study was to evaluate the degree of PAE contamination in the waters of the Pearl River Delta by studying the distribution and assessing the environmental risk of 14 PAEs in surface waters and sediments during different periods (low and high water) and years. This study provided the first comprehensive overview of the distribution of PAEs in the waters of the Pearl River Delta’s eight major estuaries (Estuaries), upstream tributaries (Xijiang River), urban river networks (River Networks), and natural reservoirs (Reservoirs). Phthalate esters were present in all of the samples that were analyzed, which indicates that PAEs are already ubiquitous in the above regions. This study showed that the degree of pollution in the water bodies that were examined decreased in the order of River Networks > Xijiang River > Estuaries > Reservoirs. The average concentrations of PAEs in surface water and sediment during the low-water period were higher than those measured during the high-water period. Phthalate ester concentrations in surface water are affected by regional precipitation. The total concentration of PAEs in the sediment is increasing year after year.
This study found that the concentration of PAEs is very high in the vicinity of industrial parks, residential areas, and roadside drains, which shows that the concentration and distribution of PAEs in water are more affected by human activities. The study of the 14 PAEs showed that DEHP, DMEP DiBP, DNP, DnOP, DBP, and DBEP are abundant. The results are similar to those reported in other research studies. Compared to data collected in other studies, although the total PAE concentration in this area is at a medium to low level, some places in the study area have been seriously polluted. At present, we do not have a comprehensive understanding of the mechanisms by which PAEs enter these water systems or their pathways of degradation and migration. So, more research is needed. Furthermore, a better understanding of the ecotoxicity and fate of PAE compounds within the water bodies is needed in order to provide a better risk assessment and to establish standards to control PAEs in the future.
References
Bartolomé L, Cortazar E, Raposo JC, Usobiaga A, Zuloaga O, Etxebarria N et al (2005) Simultaneous microwave-assisted extraction of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, phthalate esters and nonylphenols in sediments. J Chromatogr A 1068(2):229–236
Boas M, Frederiksen H, Feldtrasmussen U, Skakkebæk NE, Hegedüs L, Hilsted L (2010) Childhood Exposure to Phthalates: Associations with Thyroid Function, Insulin-like Growth Factor I, and Growth. Environ Health Persp 118:1458–1464
Casalscasas C, Desvergne B (2011) Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73:135–162
Chen ZS (1992): Metal contamination of flooded soils, rice plants, and surface waters in Asia
Chen H, Zhu Y (1999) Heavy Metal Pollution in Soils in China: Status and Countermeasures. Ambio 28:130–134
Chen SJ, Gao XJ, Mai BX, Chen ZM, Luo XJ, Sheng GY, Fu JM, Zeng EY (2006) Polybrominated diphenyl ethers in surface sediments of the Yangtze River Delta: Levels, distribution and potential hydrodynamic influence. Environ Pollut 144:951
Chen L, Zhao Y Li L, Chen B, & Zhang Y (2012) Exposure assessment of phthalates in non-occupational populations in China. Sci Total Environ 427–428
Chen CW, Chen CF, Dong CD (2013) Distribution of Phthalate Esters in Sediments of Kaohsiung Harbor, Taiwan. J Soil Contam 22:119–131
Chen CF, Chen CW, Ju YR, Dong CD (2016) Determination and assessment of phthalate esters content in sediments from Kaohsiung Harbor, Taiwan. Mar Pollut Bull 124
Colacino JA, Harris TR, Schecter A (2010) Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Persp 118:998–1003
Colón I, Caro D, Bourdony CJ, Rosario O (2000) Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Persp 108:895–900
Doong RA, Lin YT (2004) Characterization and distribution of polycyclic aromatic hydrocarbon contaminations in surface sediment and water from Gao-ping River, Taiwan. Water Res 38:1733–1744
Enríquez S, Duarte CM, Sandjensen K (1993) Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia 94:457–471
EPAJ (Environmental Protection Agency of Japan) (1993) Chemicals in the environment, Report on Environmental Survey and Wildlife Monitoring of Chemicals in F.Y
Fan W, Xia X, Sha Y (2008) Distribution of Phthalic Acid Esters in Wuhan section of the Yangtze River, China. J Hazard Mater 154:317
Fatoki OS, Bornman M, Ravandhalala L, Chimuka L, Genthe B, Adeniyi A (2010) Phthalate ester plasticizers in freshwater systems of Venda, South Africa and potential health effects. Water SA 36(1)
Fernandez MP, Ikonomou MG, Buchanan I (2007) An assessment of estrogenic organic contaminants in Canadian wastewaters. Sci Total Environ 373:250–269
Flaherty E (2008) Consumer product safety improvement act of 2008. Loyola Consumer Law Review 21
Florig HK (1997) China's air pollution risks. Environ Sci Technol 31:274A–275A
Foster PMD, Mylchreest E, Gaido KW, Sar M (2015) Effects of phthalate esters on the developing reproductive tract of male rats. APMIS 109:S272–S277
Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A (2002) Occurrence of phthalates and bisphenol A and F in the environment. Water Res 36:1429
Gao DW, Wen ZD (2016) Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ 541:986–1001
Giam CS, Atlas E, Powers MA, Leonard JE (1984) Phthalic acid esters. In: Handbook of Environmental Chemistry, Anthropogenic Compounds, vol 3, pp 67–142
Gopal A, Aarti J, Chetna G, Arvinder D, Cooper HL (2010) A practical method to extract and dechlorinate pcbs in soils. Practice Periodical of Hazardous Toxic & Radioactive Waste Management 14(2)
Group W B (2014) World bank group and world bank corporate scorecards, October 2015
Hadjmohammadi MR, Fatemi MH, Taneh T (2011) Coacervative extraction of phthalates from water and their determination by high performance liquid chromatography. J Iran Chem Soc 8:100–106
Hashizume K, Nanya J, Toda C, Yasui T, Nagano H, Kojima N (2002) Phthalate esters detected in various water samples and biodegradation of the phthalates by microbes isolated from river water. Biol Pharm Bull 25(2):209–214
He H, Hu GJ, Sun C, Chen SL, Yang MN, Li J, Zhao Y, Wang H (2011) Trace analysis of persistent toxic substances in the main stream of Jiangsu section of the Yangtze River, China. Environ Sci Pollut Res 18:638–648
Hills P, Zhang L, Liu J (1998) Transboundary Pollution between Guangdong Province and Hong Kong: Threats to Water Quality in the Pearl River Estuary and Their Implications for Environmental Policy and Planning. J Environ Plan Manag 41:375–396
Ho KC, Hui KCC (2001) Chemical contamination of the East River (Dongjiang) and its implication on sustainable development in the Pearl River Delta. Environ Int 26:303–308
Horn O, Nalli S, Cooper D, Nicell J (2004) Plasticizer metabolites in the environment. Water Res 38:3693–3698
Kambia K, Dine T, Gressier B, Germe AF, Luyckx M, Brunet C, Michaud L, Gottrand F (2001) High-performance liquid chromatographic method for the determination of di(2-ethylhexyl) phthalate in total parenteral nutrition and in plasma. J Chromatogr B Biomed Sci Appl 755:297–303
Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R (2002) NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of butyl benzyl phthalate. Reprod Toxicol 16:451
Klamer HJ, Leonards PE, Lamoree MH, Villerius LA, Åkerman JE, Bakker JF (2005) A chemical and toxicological profile of Dutch North Sea surface sediments. Chemosphere 58:1579–1587
Koch HM, Wittassek M, Brüning T, Angerer J, Heudorf U (2011) Exposure to phthalates in 5-6 years old primary school starters in Germany-a human biomonitoring study and a cumulative risk assessment. Int J Hyg Environ Health 214(3):188–195
Kolarik B, Bornehag CG, Naydenov K, Sundell J, Stavova P, Nielsen OF (2008) The concentrations of phthalates in settled dust in Bulgarian homes in relation to building characteristic and cleaning habits in the family. Atmos Environ 42:8553–8559
Krüger T, Long M, Bonefeld-Jørgensen EC (2008) Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 246:112–123
Larcinese V, Testa C (2013) Meta-analysis of environmental contamination by phthalates. Environ Sci Pollut Res 20:8057–8076
Li X, Wai OWH, Li YS, Coles BJ, Ramsey MH, Thornton I (2000) Heavy metal distribution in sediment profiles of the Pearl River estuary, South China. Appl Geochem 15:567–581
Li N, Wang D, Zhou Y, Ma M, Li J, Wang Z (2010) Dibutyl Phthalate Contributes to the Thyroid Receptor Antagonistic Activity in Drinking Water Processes. Environ Sci Technol 44:6863
Lin G (2003) China's compliance management system. Electromagnetic compatibility, 2003, IEEE International Symposium on. IEEE
Lin ZP, Ikonomou MG, Jing H, Mackintosh C, Gobas FA (2003) Determination of phthalate ester congeners and mixtures by LC/ESI-MS in sediments and biota of an urbanized marine inlet. Environ Sci Technol 37:2100
Liu H, Cui K, Zeng F, Chen L, Cheng Y, Li H, Li S, Zhou X, Zhu F, Ouyang G (2014) Occurrence and distribution of phthalate esters in riverine sediments from the Pearl River Delta region, South China. Mar Pollut Bull 83:358–365
Library WP (2013) Ministry of environmental protection of the People's Republic of China
Lovekampswan T, Davis BJ (2003) Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Persp 111:139
Mackintosh CE, Maldonado JA, Ikonomou MG, Gobas FAPC (2006) Sorption of phthalate esters and pcbs in a marine ecosystem. Environ Scie Technol 40(11):3481–3488
Mai B, Chen S, Luo X, Chen L, Yang Q, Sheng G, Peng P, Fu J, Zeng Y (2005) Distribution of Polybrominated Diphenyl Ethers in Sediments of the Pearl River Delta and Adjacent South China Sea. Environ Sci Technol 39:3521–3527
Mankidy R, Wiseman S, Ma H, Giesy JP (2013) Biological impact of phthalates. Toxicol Lett 217:50–58
Martine B, Marie-Jeanne T, Cendrine D, Fabrice A, Marc C (2013) A ssessment of Adult Human Exposure to Phthalate Esters in the Urban Centre of Paris (France). Bull Environ Contam Toxicol 90:91–96
Martino-Andrade AJ, Chahoud I (2010) Reproductive toxicity of phthalate esters. Mol Nutr Food Res 54:148–157
Meng XZ, Wang Y, Xiang N, Chen L, Liu Z, Wu B, Dai X, Zhang YH, Xie Z, Ebinghaus R (2014) Flow of sewage sludge-borne phthalate esters (PAEs) from human release to human intake: implication for risk assessment of sludge applied to soil. Sci Total Environ 476-477:242–249
Mo C, Cai Q, Wu Q, Wang B, Wang JWC, Zhou L (2001) A study of phthalic acid esters (PAEs) in the municipal sludges of China. China Environ Sci 21:362–366
Net S, Sempéré R, Delmont A, Paluselli A, Ouddane B (2015) Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ Sci Technol 49:4019–4035
Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ (2009) A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans Biol Sci 364:2047
Peijnenburg WJ, Struijs J (2006) Occurrence of phthalate esters in the environment of The Netherlands. Ecotoxicol Environ Saf 63:204–215
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DG (2011) GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186:1586–1593
Rengarajan S, Parthasarathy C, Anitha M, Balasubramanian K (2007) Diethylhexyl phthalate impairs insulin binding and glucose oxidation in Chang liver cells. Toxicol in Vitro 21:99–102
Santhi VA, Mustafa AM (2013) Assessment of organochlorine pesticides and plasticisers in the Selangor River basin and possible pollution sources. Environ Monit Assess 185:1541–1554
Sears JK, Darby JR (1982) The technology of plasticizers
Selvaraj KK, Sundaramoorthy G, Ravichandran PK, Girijan GK, Sampath S, Ramaswamy BR (2015) Phthalate esters in water and sediments of the Kaveri River, India: environmental levels and ecotoxicological evaluations. Environ Geochem Health 37:83–96
Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S (2014) Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13(1):43
Sha Y, Xia X, Yang Z, Huang GH (2007) Distribution of PAEs in the middle and lower reaches of the Yellow River, China. Environ Monit Assess 124:277–287
Shea KM (2003) Pediatric exposure and potential toxicity of phthalate plasticizers. PEDIATRICS 111:1467
Shi Z, Tao S, Pan B, Fan W, He XC, Zuo Q, Wu SP, Li BG, Cao J, Liu WX, Xu FL, Wang XJ, Shen WR, Wong PK (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Shi Z, Tao S, Pan B, Liu WX, Shen WR (2007) Partitioning and source diagnostics of polycyclic aromatic hydrocarbons in rivers in Tianjin, China. Environ Pollut 146:492–500
Shore MWC (1995) Toxicological profile for diethyl phthalate. Agency for Toxic Substances and Disease Registry
Srivastava A, Sharma VP, Tripathi R, Kumar R, Patel DK, Mathur PK (2010) Occurrence of phthalic acid esters in Gomti River Sediment, India. Environ Monit Assess 169:397–406
Sun J, Huang J, Zhang A, Liu W, Cheng W (2013) Occurrence of phthalate esters in sediments in Qiantang River, China and inference with urbanization and river flow regime. J Hazard Mater 248-249:142–149
Sung HH, Kao WY, Su YJ (2003) Effects and toxicity of phthalate esters to hemocytes of giant freshwater prawn, macrobrachium rosenbergii. Aquat Toxicol (Amsterdam), 64(1), 0–37
Tan GH (1995) Residue levels of phthalate esters in water and sediment samples from the klang river basin. Bull Environ Contam Toxicol 54(2):171–176
Teil MJ, Blanchard M, Moreau-Guigon E, Dargnat C, Alliot F, Bourges C, Desportes A, Chevreuil M (2013) Phthalate Fate in the Hydrographic Network of the River Seine Basin (France) Under Contrasted Hydrological Conditions. Water Air Soil Pollut 224:1–14
van der Pas LJT, Matser ANM, Boesten JJT, Leistra M (1999) Behaviour of metamitron and hydroxy-chlorothalonil in low-humic sandy soils. Pestic Sci 99:923–934
Van Wezel AP, Van VP, Posthumus R, Crommentuijn GH, Sijm DT (2000) Environmental risk limits for two phthalates, with special emphasis on endocrine disruptive properties. Ecotoxicol Environ Saf 46:305–321
Vethaak AD, Lahr J, Schrap SM, Belfroid AC, Rijs GB, Gerritsen A, De BJ, Bulder AS, Grinwis GC, Kuiper RV (2005) An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere 59:511
Vitali M, Guidotti M, Macilenti G, Cremisini C (1997) Phthalate esters in freshwaters as markers of contamination sources – a site study in Italy. Environ Int 23(3):337–347
Wang Y, Hu W, Cao Z, Fu X, Zhu T (2005) Occurrence of endocrine-disrupting compounds in reclaimed water from Tianjin, China. Anal Bioanal Chem 383:857–863
Wang XT, Ma LL, Sun YZ, Xu XB (2006) Phthalate Esters in Sediments from Guanting Reservoir and the Yongding River, Beijing, People's Republic of China. Bull Environ Contam Toxicol 76:799–806
Wang WL, Wu QY, Wang C, He T, Hu HY (2015) Health risk assessment of phthalate esters (PAEs) in drinking water sources of China. Environ Sci Pollut Res 22:3620–3630
Wei C, Taubenböck H, Blaschke T (2017) Measuring urban agglomeration using a city-scale dasymetric population map: A study in the Pearl River Delta, China. Habitat Int 59:32–43
World Health Organization (2011) Recent highlights-hazards
Wong AWM, Wong MH (2004) Recent socio-economic changes in relation to environmental quality of the Pearl River delta. Reg Environ Chang 4:28–38
Xie Z, Ebinghaus R, Temme C, Caba A, Ruck W (2005) Atmospheric concentrations and air–sea exchanges of phthalates in the North Sea (German Bight). Atmos Environ 39:3209–3219
Yang H, Xie W, Liu Q, Liu J, Yu H, Lu Z (2013) Distribution of phthalate esters in topsoil: a case study in the Yellow River Delta, China. Environ Monit Assess 185:8489
Ye T, Kang M, Huang Q, Fang C, Chen Y, Liu L, Dong S (2016) Accumulation of di(2-ethylhexyl) phthalate causes endocrine-disruptive effects in marine medaka (Oryzias melastigma) embryos. Environ Toxicol 31:116–127
Yuan SY, Liu C, Liao CS, Chang BV (2002) Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. CHEMOSPHERE 49:1295–1299
Zeng F, Cui K, Xie Z, Liu M, Li Y, Lin Y, Zeng Z, Li F (2008) Occurrence of phthalate esters in water and sediment of urban lakes in a subtropical city, Guangzhou, South China. Environ Int 34:372
Zeng Z, Cui K, Xie Z, Wu L, Luo D, Chen L, Lin Y, Min L, Sun G (2009) Distribution of phthalate esters in urban soils of subtropical city, Guangzhou, China. J Hazard Mater 164:1171–1178
Zhang Q, Lu XM, Zhang XL, Sun YG, Zhu DM, Wang BL, Zhao RZ, Zhang ZD (2013) Levels of phthalate esters in settled house dust from urban dwellings with young children in Nanjing, China. Atmos Environ 69:258–264
Zota AR, Calafat AM, Woodruff TJ (2014) Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect 122:235–241
Acknowledgments
We thank the anonymous reviewers for their valuable comments and suggestions. Thanks to Yu Hongliang, Wang Chao, Yang Wanling, and Li Haiyan from the plateau for their help with this experiment, and thanks to Ms. Zhang Pengying for modifying the images in this article. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This study was financially supported by the Guangdong Provincial Marine Fisheries Science and Technology Promotion Project (A201101I02) and the Guangxi Natural Science Major Project (2013GXNSFEA053003). The work is supported by the Foundation of Key Laboratory of Urban Agriculture in South China, Ministry of Agriculture, People’s Republic of China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hongwen Sun
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The concentrations of detected PAEs in sediment increased over the study period.
• The average concentrations of PAEs in water and sediment were higher during the low-water period.
• The degree of pollution within the study area decreased in the order of River network > Xijiang River > Estuaries > Reservoirs.
• Some waters have serious PAE pollution and ecological risks.
Electronic supplementary material
ESM 1
(DOCX 1750 kb)
Rights and permissions
About this article
Cite this article
Weizhen, Z., Xiaowei, Z., Peng, G. et al. Distribution and risk assessment of phthalates in water and sediment of the Pearl River Delta. Environ Sci Pollut Res 27, 12550–12565 (2020). https://doi.org/10.1007/s11356-019-06819-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06819-y