Abstract
Triiodinated benzoic acid derivatives are widely used as contrast media for medical examinations and are found at high concentrations in urban aquatic environments. During bank filtration, deiodination of iodinated contrast media has been observed under anoxic/anaerobic conditions. While several bacterial strains capable of dechlorination and debromination have been isolated and characterized, deiodination has not yet been shown for an isolated strain. Here, we investigate dehalogenation of iodinated contrast media (ICM), triiodobenzoic acids (TIBA), and analogous chlorinated compounds by Dehalococcoides mccartyi strain CBDB1 and its corrinoid co-factor vitamin B12. No cell growth of CBDB1 was observed using iodinated compounds as electron acceptor. Only negligible deiodination occurred for ICM, whereas 2,3,5-TIBA was nearly completely deiodinated by CBDB1 without showing cell growth. Furthermore, TIBA inhibited growth with hexachlorobenzene which is usually a well-suited electron acceptor for strain CBDB1, indicating that TIBA is toxic for CBDB1. The involvement of CBDB1 enzymes in the deiodination of TIBA was verified by the absence of deiodination activity after heat inactivation. Adding iodopropane also inhibited the deiodination of TIBA by CBDB1 cells, indicating the involvement of a corrinoid-enzyme in the reductive TIBA deiodination. The results further suggest that the involved electron transport is decoupled from proton translocation and therefore growth.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iodinated contrast media (ICM) are triiodinated benzoic acid derivatives used for medical X-ray examinations to enhance the contrast of organs and vessels. They are designed to be polar and stable in order to be excreted unmetabolized from the human body within hours after application (Oberdisse et al. 1999). The benzene ring is substituted with iodine atoms at positions 2, 4, and 6 and with three side-chains whose structures vary with the type of ICM. The concentration of ICM and their iodinated transformation products can be determined by differential adsorbable organic halides (AOX) analysis as adsorbable organically bound iodine (AOI) (Oleksy-Frenzel et al. 2000). Due to the stability of the carbon–iodine bond under aerobic conditions and the high polarity of ICM, they are only poorly retained in wastewater treatment plants. Consequently, high concentrations of organic bound iodine in the μg L−1 range are frequently measured in urban surface waters (Kormos et al. 2011). At lower concentrations, ICM have been detected in anthropogenically influenced groundwater and drinking water (Putschew and Jekel 2006; Schittko et al. 2004; Ternes and Hirsch 2000).
In aerobic water–soil systems, biotic transformation of ICM has been reported and various metabolites with modified side-chains but still substituted with three iodine atoms were identified (Batt et al. 2006; Kormos et al. 2010, 2011; Schulz et al. 2008). In contrast, deiodination associated with an AOI decrease of around 63% was observed under anoxic conditions during bank filtration (Schittko et al. 2004). Anaerobic batch tests revealed microbial deiodination of the ICM diatrizoate (Redeker et al. 2014), of the ICM precursor 5-amino-2,4,6-triiodoisophthalic acid (Lecouturier et al. 2003a, b) and of 2,4,6-triiodophenol in the presence of anaerobic microbial consortia (Oba et al. 2014). Hapeshi et al. (2013) observed deiodination of the ICM iohexol and diatrizoate in a moving bed biofilm reactor and identified several microorganisms in the biofilm. To our knowledge, no isolated and characterized bacterial strain capable of ICM deiodination has been described to date. It therefore remains to be elucidated, whether deiodination occurring under anoxic/anaerobic conditions in nature is mainly caused by biotic or by abiotic processes.
Over the last decades, intensive research has been carried out on bacteria that dehalogenate diverse toxic and highly nonpolar chlorinated and brominated organic compounds. Most of these organohalide-respiring bacteria were strictly anaerobic and used halogenated compounds as a respiratory electron acceptor for energy conservation (Atashgahi et al. 2016). The breakage of the carbon–halogen bond is catalyzed by reductive dehalogenase enzymes of which most use corrinoids as a prosthetic group. Corrinoids are either synthesized de novo by the organism itself or they are required as vitamins from the medium or from a supporting microbial community (Atashgahi et al. 2016). To date, several strains of the species Dehalococcoides mccartyi have been isolated. All of them can use hydrogen as electron donor and organohalides as electron acceptor for growth (Zinder 2016). Furthermore, Dehalococcoides mccartyi strains for which genome sequences are available share the incapability of de novo corrinoid synthesis (Kube et al. 2005; Seshadri et al. 2005). They can be grown in mineral medium containing a halogenated electron acceptor, acetate as carbon source and vitamins, including a corrinoid (Zinder 2016). The electrons released by the oxidation of hydrogen are used for the reduction of CoIII to CoI in the corrinoid co-factors of the reductive dehalogenases (Zinder 2016). Genome sequencing of Dehalococcoides mccartyi strain CBDB1 revealed the high number of 32 reductive dehalogenase genes (Kube et al. 2005) encoding various reductive dehalogenases which are able to dechlorinate a wide range of chlorinated and brominated organic compounds (Zinder 2016).
The carbon–iodine bond is the weakest of the carbon–halogen bonds due to the lower electronegativity and higher steric repulsion of the larger iodine atom which facilitates its removal (Holleman et al. 2007) as has previously been shown for brominated and chlorinated compounds (Sadowsky et al. 2013). Since the substrate specificity of strain CBDB1 reductive dehalogenases is very broad, we hypothesized that iodinated compounds might also function as respiratory electron acceptor for strain CBDB1.
Apart from microbial dehalogenation, abiotic reductive dehalogenation catalyzed by heat-inactivated enzymes or free corrinoids has been shown for several chlorinated compounds (Gantzer and Wackett 1991; Krone et al. 1989a, b; Neumann et al. 2002) and recently for ICM and triiodobenzoic acids (El-Athman et al. 2019a). Under oxygen-free conditions in the presence of a strong reducing agent such as titanium (III) citrate and the corrinoid vitamin B12, the ICM iopromide, iopamidol, and diatrizoate are completely deiodinated (El-Athman et al. 2019a).
In this study, we investigated whether strain CBDB1 can be cultivated using ICM and other triiodo- and trichlorobenzoic acids as electron acceptors. Surprisingly, our results showed that no growth occurred when iodinated compounds were provided as the only electron acceptor despite deiodination of triiodobenzoic acid. The properties of the deiodinating enzymes and the free corrinoid co-factor vitamin B12 were compared with respect to heat inactivation and inhibition by iodopropane. This way, we verified the involvement of a corrinoid-containing enzyme in the deiodination of triiodobenzoic acid.
Materials and methods
Chemicals and stock solutions
Iopromide (IOP) and diatrizoate (DIA) were provided by Schering AG (Berlin, Germany). Hexachlorobenzene (HCB) was purchased from Campro Scientific (Berlin, Germany); hexaiodobenzene (HIB); 5-amino-2,4,6-triiodoisophthalic acid (ATIA); 2,4,6- and 2,3,5-triiodobenzoic acid (TIBA); 2,4,6- and 2,3,5-trichlorobenzoic acid (TCBA); 1,2,4-trichlorobenzene (TCB); 1,2-dibromobenzene (DBB); 1-iodopropane and cyanocobalamin (vitamin B12); methyl viologen; and l-cysteine were purchased from Sigma Aldrich (Steinheim, Germany). Titanium (III) citrate was prepared from titanium (III) chloride solution (Merck-Schuchardt, Hohenbrunn, Germany) as previously described (Zehnder and Wuhrmann 1976). All chemicals used were of analytical grade. Ultrapure water was produced from deionized water with a water purification system. For stock solutions, IOP and vitamin B12 were dissolved in ultrapure water (10 g L−1 and 0.5 g L−1); DIA, ATIA, 2,4,6-, 2,3,5-TIBA, and 2,4,6- and 2,3,5-TCBA were dissolved in methanol (10 g L−1). 1,2,4-TCB and iodopropane were diluted by methanol (8.05 M and 20.6 mM) and 1,2-DBB was diluted by acetone (10 mM). For the microtiter plate assays, all stock solutions were further diluted to 1–2 g L−1.
Cultivation and cell harvest
Dehalococcoides mccartyi strain CBDB1 was grown in a defined medium buffered with NaHCO3 (10 mM) to pH 7.3 containing acetate (5 mM) as carbon source and hydrogen (nominal concentration of 7.5 mM) as electron donor. Reducing conditions were ensured by the addition of titanium (III) citrate (0.4 mM) or l-cysteine (6 mM), and the medium was further amended with vitamins and trace elements as previously described (Adrian et al. 2007). Electron acceptors were either added from stock solutions to a final concentration of 10 μM or as crystals (HCB and HIB). For inoculation, cultures of strain CBDB1 originally grown on HCB with a cell density of about 1 × 107 cells mL−1 were added with a volume fraction of 10%, leading to a starting cell density of about 1 × 106 cells mL−1. The headspace was first pressurized with N2/CO2 80:20% to 0.3 bar and then with H2 to a final pressure of 0.44 bar. Cultivation was performed in the dark at 30 °C without shaking under strictly anoxic conditions. All cultivations were done in duplicates or triplicates. Additionally, abiotic controls without strain CBDB1 inoculation, negative growth controls without electron acceptor, and positive controls with HCB were set up.
Cells were harvested from a culture volume of 800 mL using a SandTrap, a glass tube filled with silica, for trapping the bacteria as described by Frauenstein et al. (2017). The silica was suspended in phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 12 mM phosphate) for cell detachment and subsequently centrifuged in two soft steps (4 min at 500×g and 3 min at 1000×g, at 4 °C) to remove the cell suspension as supernatant clear from silica. Cell numbers in cultures and in the harvested cell suspension were determined after staining with SYBR-green by direct cell counting on agarose-coated slides as previously described (Adrian et al. 2007). The initial cell density before the enrichment was 2.5 × 107 cells mL−1. By cell harvesting, the cell density of the suspension increased to 2.9 × 108 cells mL−1. Assuming a protein content of 3 × 10−11 mg protein per cell (Cooper et al. 2015), the total protein concentration of the enriched cell suspension amounts to 8.7 μg mL−1.
Activity assays
B12 and dehalogenase activity were analyzed with a microtiter plate assay using vitamin B12 or whole-cell suspension as catalyst and reduced methyl viologen as artificial electron donor. The reaction solution was prepared in an anaerobic chamber (95% N2, 5% H2, Coy Laboratories, Grass Lake, USA) and contained ultrapure water buffered with potassium acetate to pH 5.8, 0.33 mM titanium (III) citrate, and 1 mM methyl viologen. To ensure that titanium (III) citrate was completely oxidized by methyl viologen, the reaction solution was prepared 24 h before starting the measurement. One hundred micromolar of different halogenated compounds as electron acceptor and either vitamin B12 (50 μM) or 40 μL strain CBDB1 cell suspension (total protein concentration of 1.39 μg mL−1) as catalyst were added to the microtiter plate wells before the reactions were started by the addition of the reaction solution. The microtiter plate assays were set up in a 96 round-bottom well microtiter glass plate (Zinsser Analytic, Frankfurt, Germany). The total sample volume in each microtiter well was 250 μL. The microtiter plate was monitored with a microtiter plate reader (PowerWave HT, BioTek Instruments GmbH, Bad Friedrichshall, Germany) within the anaerobic chamber at 578 nm (methyl viologen absorbance maximum) every 2 min at 30, 40, or 50 °C. The plates contained triplicate samples as well as triplicate controls without halogenated electron acceptor and controls without catalyst. The iodide release rates were calculated from the slope of the linear part of the absorption curves (first 6 up to 20 min) and were corrected by the decolorization rates of the controls without halogenated compound and of the controls without catalyst.
Inhibition by iodopropane
Corrinoids are known to be light-reversibly inhibited by iodopropane propylation (Neumann et al. 1995; Hölscher et al. 2003). Inhibition by iodopropane was tested for both B12 and dehalogenases of CBDB1 to investigate the presence of a corrinoid in the dehalogenases. The tests were conducted in 1.5-mL vials with the same reaction mix used for the microtiter plate assays. IOP was used as electron acceptor for the vitamin B12-catalyzed reaction and 2,3,5-TIBA for the enzyme-catalyzed reaction. Iodopropane was added to a concentration of 1 mM. The vials were stored in an oxygen-free glovebox (100% N2, GS E-Line, Glovebox Systemtechnik, Maisch, Germany) in the dark. After 1 h, a part of the tests with iodopropane was exposed to the light of an LED flashlight (1300 lm) for another hour. Samples were taken after 1 and 2 h and the reaction was stopped by introducing air into the samples. The tests were conducted in triplicate with triplicate controls without iodopropane, controls without catalyst and iodopropane, and controls which were not exposed to light. Iodide was measured by ion chromatography.
Analysis
The amount of organic bound iodine (AOI) was quantified by differential AOX-analysis (Oleksy-Frenzel et al. 2000). The samples were filtrated (0.45 μm) and acidified to pH 2 with concentrated nitric acid. The organic compounds were enriched onto 80 mg activated carbon (adsorption unit EFU 1000, Thermo Instruments GmbH, Germany). To exclude an adsorption of inorganic halogens, a high concentration of nitrate was added to the samples prior to the enrichment. After adsorption, the activated carbon was combusted/oxidized (electric furnace ECS 1000, Thermo Instruments GmbH, Germany), reducing the bound halogens to halides. The dried combustion gas was trapped in water which was analyzed for iodide as described below.
Iodide was quantified by IC-UV using an ion exchange system (DX-100, Dionex, Germany) or an HPLC system (HP 1100, Agilent, Waldbronn, Germany) equipped with an anion exchange column (IonPac AS9-SC; 4 × 250 mm, Dionex). The eluent contained 2.2 mM Na2CO3 and 0.75 mM NaHCO3 and the flow rate was 1 mL min−1. The injection volume was 100 μL for AOI analysis and 10 μL for dissolved iodide. Iodide was detected by UV absorption (226 nm). The lowest calibration point was 10 μg L−1 for AOI analysis and 100 μg L−1 for the quantification of dissolved iodide. For more details, see Oleksy-Frenzel et al. (2000) and El-Athman et al. (2019a).
Results
Deiodination in titanium (III) citrate–reduced growth medium
Previously, we have shown that ICM, e.g., IOP, and several other triiodinated benzoic acid derivatives can be chemically deiodinated in the presence of a strong reducing agent such as titanium (III) citrate and vitamin B12 as electron shuttle and catalyst (El-Athman et al. 2019a). Therefore, we tested the stability of IOP in titanium (III) citrate–reduced growth medium of strain CBDB1. The growth medium contained 68.8 nM vitamin B12 which is required as co-factor for strain CBDB1. Controls contained the reducing agent but no vitamins.
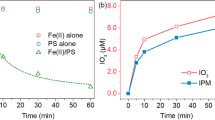
After 26 days, IOP was almost completely deiodinated in titanium (III) citrate–reduced growth medium without microorganisms when vitamin B12 was present (Fig. 1). The concentration of organic bound iodine (AOI) was close to zero and the concentration of released iodide almost reached the expected maximum of 30 μM. In comparison, tests without any vitamins in the growth medium showed an iodide release of only one-third, probably caused by direct reduction by titanium (III) citrate. Thus, IOP is not stable in the growth medium due to deiodination catalyzed by vitamin B12 and due to the strong reducing agent titanium (III) citrate. In the following, we refer to deiodination without the involvement of active enzymes as abiotic deiodination. Slower abiotic iodide release occurs when cysteine is used as reducing agent (El-Athman et al. 2019a). Hence, in the following experiments, cysteine-reduced medium was used for the cultivation of strain CBDB1 with iodinated compounds.
Deiodination of IOP with titanium (III) citrate: sum concentration of released iodide (blue) and remaining AOI (orange) after 26 days of incubation. Ten micromolar IOP (AOI, 30 μM) was used as electron acceptor in a titanium (III) citrate–reduced medium with and without vitamin B12. Shown are mean values with SD (n = 2)
Cultivation of Dehalococcoides mccartyi strain CBDB1
Strain CBDB1 was cultivated with different triiodinated benzoic acid derivatives and HIB as electron acceptors in cysteine-reduced medium. The cultures were inoculated with cells from cultures initially grown on HCB. Therefore, HCB was used as a model compound for positive controls as well as in combination with an iodinated compound.
After 160 days in cysteine-reduced medium, abiotic deiodination was around 14% for 2,3,5-TIBA (Fig. 2b) but negligible for IOP, DIA, and HIB (Fig. 2a). In the presence of CBDB1, all iodinated compounds with the exception of 2,3,5-TIBA also showed negligible iodide release. For 2,3,5-TIBA, deiodination in the CBDB1 cultures was significantly higher than in the abiotic controls. However, no cell growth was observed (Fig. 3b). Cell growth was only observed in cultures with HCB or HCB together with IOP or DIA but inhibited by the presence of TIBA (Fig. 3a).
Activity assays for reductive dehalogenases and vitamin B12
We used photometric activity assays to determine the initial dehalogenation activities of native CBDB1 dehalogenases and the free corrinoid vitamin B12. Their properties regarding substrate specificity, temperature, and inactivation were compared to provide evidence for enzyme-catalyzed deiodination. The activity, described as the amount of halide ions released per hour, was measured in the presence of different halogenated compounds. We have previously shown that B12 activity linearly depends on the vitamin B12 concentration (El-Athman et al. 2019a). While the concentration of the enzyme-bound corrinoids in the CBDB1 cell suspension is unknown, the maximum concentration that can be reached if the complete vitamin B12 amount in the medium was bound to enzymes is 68.8 nM. In contrast, the vitamin B12 concentration of the abiotic activity tests was 50 μM since lower concentrations did not result in measurable activities for some compounds like DIA and TIBA (data not shown).
For IOP, strong B12 activity, and for DIA and 2,4,6- and 2,3,5-TIBA, low B12 activity were observed, whereas no abiotic activity was recorded for the other iodinated, chlorinated, and brominated compounds (Table 1). In contrast, high activity was recorded with cells of strain CBDB1 for 2,4,6- and 2,3,5-TIBA and for 1,2-DBB as electron acceptors. With the analogous chlorinated compounds, 2,4,6- and 2,3,5-TCBA, and other iodinated compounds, no biotic activity was detected. It seems that compounds for which high enzyme activities were observed with strain CBDB1 dehalogenases induced none or low abiotic activity and vice versa. In a previous study, DIA was completely deiodinated in the presence of vitamin B12 and titanium (III) citrate, and for ATIA and 2,4,6- and 2,3,5-TIBA, at least one iodine atom was abiotically released when titanium (III) citrate was used as electron donor (El-Athman et al. 2019b). In contrast, the activity assays using reduced methyl viologen as electron donor for the dehalogenation revealed no or only low abiotic activities with DIA, ATIA, and both TIBA despite high vitamin B12 concentrations.
The activity of the free corrinoid co-factor and the corrinoid-containing reductive dehalogenases in strain CBDB1 was compared at different temperatures. For B12 and strain CBDB1 activity, IOP and 1,2-DBB were used as electron acceptors, respectively. Both activities increased with increasing temperature and showed a similar temperature dependence in the range from 30 to 50 °C (Supporting Information, Figure S1). Iodide and bromide release rates increased by factor 1.1 for a temperature increase from 30 to 40 °C and by factor 1.3 for an increase from 40 to 50 °C. Thus, the temperature dependence in the investigated range cannot be used for the distinction between abiotic and biotic dehalogenations. Furthermore, heat-inactivation of CBDB1 dehalogenases was tested by incubating the cell suspension for 10 min at 70 °C and at 100 °C. Activity assays with 2,3,5-TIBA showed no activity for the cell suspensions incubated at either 70 or 100 °C (Supporting Information, Figure S2). Control tests with 2,3,5-TIBA and vitamin B12 solution heated at 70 and 100 °C showed approximately the same activity for non-heated as for heated vitamin B12, confirming that vitamin B12 is a heat-stable catalyst.
Inhibition of reductive dehalogenases and vitamin B12 by iodopropane
All known reductive dehalogenases contain a corrinoid which is responsible for their catalytic capability by changing the oxidation state of the central cobalt ion (Atashgahi et al. 2016). We investigated the presence of a corrinoid in the dehalogenases of strain CBDB1 by the addition of iodopropane. Iodopropane light reversibly propylates and thereby inhibits corrinoid-containing enzymes (Neumann et al. 1995; Hölscher et al. 2003). To compare the influence of iodopropane on co-factor B12 with that on dehalogenases, deiodination of IOP by vitamin B12 in the presence of iodopropane was tested as well. Both vitamin B12 and dehalogenases of strain CBDB1 showed lower activity but no complete inactivation in the presence of iodopropane (Table 2). CBDB1 activity was more strongly inhibited by iodopropane than B12 activity. By exposing the samples to light for 1 hour, the activity was partly restored.
Discussion
The strictly anaerobic organism Dehalococcoides mccartyi CBDB1 is an organohalide-respiring bacterial strain that can utilize a very broad spectrum of halogenated electron acceptors. While dechlorination and debromination of different compounds by strain CBDB1 have been shown in numerous studies, deiodination has not yet been reported. Here, we investigated the deiodination of ICM and other halogenated compounds by strain CBDB1 cultivation and activity assays with whole-cell suspension and co-factor B12. Previously, we have shown that ICM and other iodinated compounds can be abiotically deiodinated under reducing conditions using electron shuttles such as corrinoids (e.g., vitamin B12) (El-Athman et al. 2019a). For that reason, we now tested the stability of iodinated compounds with IOP in titanium (III) citrate–reduced growth medium (standard redox potential of titanium (III) citrate: − 480 mV; Zehnder and Wuhrmann 1976). Due to almost complete deiodination of IOP within a few weeks, the weaker reducing agent cysteine (− 220 mV; Jocelyn 1967) was used for the cultivation of strain CBDB1 to minimize abiotic deiodination. Abiotic controls with cysteine-reduced medium showed only negligible reductive deiodination.
When using iodinated compounds as the only electron acceptor, no cell growth of CBDB1 was observed over a period of 7.5 months. This time period is expected to be sufficient for an acclimation to iodinated compounds according to previous studies which reported acclimation on iodinated aromatics within a period of several weeks (Horowitz et al. 1983; Lecouturier et al. 2003b). While the ICM showed no deiodination in CBDB1 cultures, 2,3,5-TIBA was nearly completely deiodinated. The involvement of dehalogenases in the deiodination of TIBA was verified by heat inactivation. This enzymatic TIBA dehalogenation showed very high deiodination activities with 2,3,5- and 2,4,6-TIBA, with the activity with 2,3,5-TIBA being approximately three times higher than with 2,4,6-TIBA. Thus, adjacent iodine atoms, as found in 2,3,5-TIBA, are preferentially released by dehalogenases of CBDB1 which is in line with previous results on polychlorinated biphenyls (Adrian et al. 2009). However, it could not be determined whether the carboxyl substituent has an accelerating or an inhibiting effect on the biotic deiodination by CBDB1. For the chlorinated compounds 2,3,5-TCBA and 1,2,4-TCB which only differ in the carboxyl substituent, we observed no CBDB1 activity in contrast to previous reports on CBDB1 activity with 1,2,4-TCB (Hölscher et al. 2003; Adrian et al. 2000). Abiotic tests on the deiodination of different TIBA and monoiodobenzoic acids provided evidence that the vitamin B12–catalyzed deiodination is faster in case of adjacent iodine atoms and iodine atoms in ortho-position to a carboxyl group (El-Athman et al. 2019b).
The presence of iodopropane decreased TIBA dehalogenase activity to about 67% compared with control samples without inhibitor. Activity could be partly restored by exposure to light. These results suggest the presence of a corrinoid co-factor in TIBA dehalogenases of strain CBDB1. The concentrations of the enzyme-bound corrinoids are unknown but based on the growth medium, the maximum concentration in the whole-cell suspension is 70 nM, assuming that all vitamin B12 was bound to enzymes. Therefore, B12 activity with both TIBA congeners was significantly lower than dehalogenase activity despite the higher vitamin B12 concentration of 50 μM in the abiotic tests. Thus, biotic TIBA deiodination required a much lower corrinoid concentration than abiotic deiodination with free corrinoids, indicating a strongly activating role of the protein moiety. Furthermore, it seems likely that a very low redox potential is more important for abiotic TIBA deiodination than for biotic deiodination. While our previous study showed a very high B12 activity with 2,3,5-TIBA compared with B12 activity with IOP when using titanium (III) citrate as reducing agent (El-Athman et al. 2019a), here, B12 activity was much lower with 2,3,5-TIBA than with IOP when using reduced methyl viologen. In cysteine-reduced CBDB1 cultures, 2,3,5-TIBA was nearly completely deiodinated, while abiotic deiodination has recently been shown to be very low when using cysteine as reducing agent (El-Athman et al. 2019a).
Although 2,3,5-TIBA was deiodinated by strain CBDB1, no cell growth occurred, indicating that no energy was conserved by the iodination. Since the addition of HCB together with 2,3,5-TIBA as electron acceptors also did not result in a growing cell number, it seems likely that 2,3,5-TIBA is toxic to strain CBDB1 and inhibits cell growth in the applied concentration. However, no cell lysis, recognizable by a decreasing cell number, was observed. The carbon–iodine bond has the weakest bond-dissociation energy of all carbon–halogen bonds (C–F, 489; C–Cl, 327; C–Br, 272; and C–I, 214 kJ mol−1; Holleman et al. 2007). Thus, taking thermodynamic considerations into account, the cleavage of the carbon–iodine bond is expected to provide more energy due to the high value of the change in Gibbs free energy. From a biochemical point of view, it can be hypothesized that the deiodination reaction might occur too fast, causing the reduction of the halogen substituent to be uncoupled from the transport of protons across the cell membrane, ultimately leading to a decoupling of deiodination and growth. A similar effect has been previously observed with 1,2,3,4-trichlorobenzene (Jayachandran et al. 2003).
The two investigated ICM, IOP and DIA, showed no or only negligible deiodination with cells of strain CBDB1 but clear deiodination in the presence of vitamin B12. It is conceivable that CBDB1 dehalogenases are sterically hindered by the complex side-chains of the ICM molecules and thus not able to deiodinate ICM. Furthermore, ICM are very polar compounds in contrast to the known chlorinated and brominated CBDB1 electron acceptors and to TIBA. Thus, the hydration shell of the ICM molecules might shield them from entering the reductive dehalogenases of strain CBDB1.
If the structure of ICM molecules hinders microbiological deiodination, side-chain transformation processes prior to deiodination would be of great importance in the aquatic environment. In aerobic water–soil systems, biotic transformation, including the cleavage of carbon–nitrogen bonds, of hydroxyl propanoic acids and of acetyl groups, led to various iodinated metabolites with smaller side-chains (Kormos et al. 2010). Since these processes occur typically under oxic conditions, it is important to ensure sufficiently long residence times in oxic zones during bank filtration or managed aquifer recharge. Advantages of biotic deiodination over abiotic deiodination catalyzed by free corrinoids arise from lower corrinoid concentrations and less negative redox potentials required for dehalogenase activity. AOI concentrations in surface water are 1000 times lower than the concentrations of iodinated compounds used in our experiments. Thus, potential negative effects on cell growth which result from high concentrations may not occur in nature. In future studies, it could also be investigated whether strain CBDB1 or other organohalide-respiring bacteria species are capable of deiodinating aerobic transformation products of ICM. In nature, it is difficult to distinguish between biotic enzyme-catalyzed and abiotic corrinoid-catalyzed deiodination. A differentiation between enzymes and free corrinoids could possibly be achieved by using different isotope effects as observed in compound-specific stable isotope analysis (Renpenning et al. 2014).
Conclusion
In this study, we investigated the biotic deiodination of ICM and other iodinated organic compounds by Dehalococcoides mccartyi strain CBDB1 and its co-factor B12. Our results suggest that free corrinoids and dehalogenase enzymes are both involved in the deiodination process observed in nature. Since no cell growth of CBDB1 occurred when an undefined amount of HIB (crystals added) or 10 μM of iopromide, diatrizoate or 2,3,5-TIBA were used as the only electron acceptor; it remains to be elucidated whether CBDB1 or other organohalide-respiring bacteria are able to gain energy from deiodination. While no biotic ICM deiodination by strain CBDB1 was observed, TIBA, which has a similar basic structure to ICM, could be deiodinated by corrinoid-containing CBDB1 dehalogenases. Thus, it seems likely that the dehalogenases are sterically hindered by the side-chains of the ICM, necessitating prior aerobic transformation and breakdown of the side-chains for biotic ICM deiodination.
References
Adrian L, Szewzyk U, Wecke J, Görisch H (2000) Bacterial dehalorespiration with chlorinated benzenes. Nature 408(6812):580–583. https://doi.org/10.1038/35046063
Adrian L, Hansen SK, Fung JM, Görisch H, Zinder SH (2007) Growth of Dehalococcoides strains with chlorophenols as electron acceptors. Environ Sci Technol 41(7):2318–2323. https://doi.org/10.1021/es062076m
Adrian L, Dudková V, Demnerová K, Bedard DL (2009) “Dehalococcoides” sp. strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl mixture aroclor 1260. Appl Environ Microbiol 75(13):4516–4524. https://doi.org/10.1128/AEM.00102-09
Atashgahi S, Lu Y, Smidt H (2016) Overview of known organohalide-respiring bacteria – phylogenetic diversity and environmental distribution. In: Adrian L, Löffler FE (eds) Organohalide-respiring bacteria. Springer, Berlin, pp 63–105
Batt AL, Kim S, Aga DS (2006) Enhanced biodegradation of iopromide and trimethoprim in nitrifying activated sludge. Environ Sci Technol 40(23):7367–7373. https://doi.org/10.1021/es060835v
Cooper M, Wagner A, Wondrousch D, Sonntag F, Sonnabend A, Brehm M, Schüürmann G, Adrian L (2015) Anaerobic microbial transformation of halogenated aromatics and fate prediction using electron density modeling. Environ Sci Technol 49(10):6018–6028. https://doi.org/10.1021/acs.est.5b00303
El-Athman F, Adrian L, Jekel M, Putschew A (2019a) Abiotic reductive deiodination of iodinated organic compounds and X-ray contrast media catalyzed by free corrinoids. Chemosphere 221:212–218. https://doi.org/10.1016/j.chemosphere.2019.01.003
El-Athman F, Jekel M, Putschew A (2019b) Reaction kinetics of corrinoid-mediated deiodination of iodinated X-ray contrast media and other iodinated organic compounds. Chemosphere 234:971–977. https://doi.org/10.1016/j.chemosphere.2019.06.135
Frauenstein D, Seidel K, Adrian L (2017) SandTraps are efficient, scalable, and mild systems for harvesting, washing and concentrating cells. J Microbiol Methods 132:106–111. https://doi.org/10.1016/j.mimet.2016.11.018
Gantzer CJ, Wackett LP (1991) Reductive dechlorination catalyzed by bacterial transition-metal coenzymes. Environ Sci Technol 25(4):715–722. https://doi.org/10.1021/es00016a017
Hapeshi E, Lambrianides A, Koutsoftas P, Kastanos E, Michael C, Fatta-Kassinos D (2013) Investigating the fate of iodinated X-ray contrast media iohexol and diatrizoate during microbial degradation in an MBBR system treating urban wastewater. Environ Sci Pollut Res Int 20(6):3592–3606. https://doi.org/10.1007/s11356-013-1605-1
Holleman AF, Wiberg E, Wiberg N (2007) Lehrbuch der anorganischen Chemie, 102nd edn. de Gruyter, Berlin
Hölscher T, Gorisch H, Adrian L (2003) Reductive dehalogenation of chlorobenzene congeners in cell extracts of Dehalococcoides sp. Strain CBDB1. Appl Environ Microbiol 69(5):2999–3001. https://doi.org/10.1128/AEM.69.5.2999-3001.2003
Horowitz A, Suflita JM, Tiedje JM (1983) Reductive dehalogenations of halobenzoates by anaerobic lake sediment microorganisms. Appl Environ Microbiol 45(5):1459–1465
Jayachandran G, Görisch H, Adrian L (2003) Dehalorespiration with hexachlorobenzene and pentachlorobenzene by Dehalococcoides sp. strain CBDB1. Arch Microbiol 180(6):411–416. https://doi.org/10.1007/s00203-003-0607-7
Jocelyn PC (1967) The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur J Biochem 2(3):327–331. https://doi.org/10.1111/j.1432-1033.1967.tb00142.x
Kormos JL, Schulz M, Kohler H-PE, Ternes TA (2010) Biotransformation of selected iodinated X-ray contrast media and characterization of microbial transformation pathways. Environ Sci Technol 44(13):4998–5007. https://doi.org/10.1021/es1007214
Kormos JL, Schulz M, Ternes TA (2011) Occurrence of iodinated X-ray contrast media and their biotransformation products in the urban water cycle. Environ Sci Technol 45(20):8723–8732. https://doi.org/10.1021/es2018187
Krone UE, Laufer K, Thauer RK, Hogenkamp HPC (1989a) Coenzyme F430 as a possible catalyst for the reductive dehalogenation of chlorinated C1 hydrocarbons in methanogenic bacteria. Biochemistry 28(26):10061–10065. https://doi.org/10.1021/bi00452a027
Krone UE, Thauer RK, Hogenkamp HPC (1989b) Reductive dehalogenation of chlorinated C1-hydrocarbons mediated by corrinoids. Biochemistry 28(11):4908–4914. https://doi.org/10.1021/bi00437a057
Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L (2005) Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol 23(10):1269–1273. https://doi.org/10.1038/nbt1131
Lecouturier D, Godon J-J, Lebeault J-M (2003a) Phylogenetic analysis of an anaerobic microbial consortium deiodinating 5-amino-2,4,6-triiodoisophthalic acid. Appl Microbiol Biotechnol 62(4):400–406. https://doi.org/10.1007/s00253-003-1278-7
Lecouturier D, Rochex A, Lebeault J-M (2003b) Enrichment and properties of an anaerobic mixed culture that reductively deiodinates 5-amino-2, 4, 6-triiodoisophthalic acid, an X-ray contrast agent precursor. Appl Microbiol Biotechnol 62(5–6):550–556. https://doi.org/10.1007/s00253-003-1296-5
Neumann A, Wohlfarth G, Diekert G (1995) Properties of tetrachloroethene and trichloroethene dehalogenase of Dehalospirillum multivorans. Arch Microbiol 163(4):276–281. https://doi.org/10.1007/BF00393380
Neumann A, Siebert A, Trescher T, Reinhardt S, Wohlfarth G, Diekert G (2002) Tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans: substrate specificity of the native enzyme and its corrinoid cofactor. Arch Microbiol 177(5):420–426. https://doi.org/10.1007/s00203-002-0409-3
Oba Y, Futagami T, Amachi S (2014) Enrichment of a microbial consortium capable of reductive deiodination of 2,4,6-triiodophenol. J Biosci Bioeng 117(3):310–317. https://doi.org/10.1016/j.jbiosc.2013.08.011
Oberdisse E, Hackenthal E, Kuschinsky K (1999) Pharmakologie und Toxikologie. Springer, Berlin, Heidelberg
Oleksy-Frenzel J, Wischnack S, Jekel M (2000) Application of ion-chromatography for the determination of the organic-group parameters AOCl, AOBr, and AOI in water. Fresenius J Anal Chem 366:89–94. https://doi.org/10.1007/s002160050016
Putschew A, Jekel M (2006) Iodinated X-ray contrast media. In: Jekel M, Reemtsma T (eds) Organic pollutants in the water cycle. Properties, occurrence, analysis and environmental relevance of polar compounds. John Wiley distributor. Weinheim, Chichester, pp 87–98
Redeker M, Wick A, Meermann B, Ternes TA (2014) Removal of the iodinated X-ray contrast medium diatrizoate by anaerobic transformation. Environ Sci Technol 48(17):10145–10154. https://doi.org/10.1021/es5014714
Renpenning J, Keller S, Cretnik S, Shouakar-Stash O, Elsner M, Schubert T, Nijenhuis I (2014) Combined C and Cl isotope effects indicate differences between corrinoids and enzyme (Sulfurospirillum multivorans PceA) in reductive dehalogenation of tetrachloroethene, but not trichloroethene. Environ Sci Technol 48(20):11837–11845. https://doi.org/10.1021/es503306g
Sadowsky D, McNeill K, Cramer CJ (2013) Thermochemical factors affecting the dehalogenation of aromatics. Environ Sci Technol 47(24):14194–14203. https://doi.org/10.1021/es404033y
Schittko S, Putschew A, Jekel M (2004) Bank filtration: a suitable process for the removal of iodinated X-ray contrast media? Water Sci Technol 50(5):261–268
Schulz M, Löffler D, Wagner M, Ternes TA (2008) Transformation of the X-ray contrast medium iopromide in soil and biological wastewater treatment. Environ Sci Technol 42(19):7207–7217. https://doi.org/10.1021/es800789r
Seshadri R, Adrian L, Fouts DE, Eisen JA, Phillippy AM, Methe BA, Ward NL, Nelson WC, Deboy RT, Khouri HM, Kolonay JF, Dodson RJ, Daugherty SC, Brinkac LM, Sullivan SA, Madupu R, Nelson KE, Kang KH, Impraim M, Tran K, Robinson JM, Forberger HA, Fraser CM, Zinder SH, Heidelberg JF (2005) Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science (New York, NY) 307(5706):105–108. https://doi.org/10.1126/science.1102226
Ternes TA, Hirsch R (2000) Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment. Environ Sci Technol 34(13):2741–2748. https://doi.org/10.1021/es991118m
Zehnder A, Wuhrmann K (1976) Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science 194(4270):1165–1166. https://doi.org/10.1126/science.793008
Zinder SH (2016) The genus Dehalococcoides. In: Adrian L, Löffler FE (eds) Organohalide-respiring bacteria. Springer, Berlin, pp 107–136
Acknowledgments
We thank Corinna Schröder and Benjamin Scheer for laboratory assistance.
Funding
Financial support was provided by the German Research Foundation (DFG) as part of the Research Training Group “Urban Water Interfaces (UWI)” (GRK 2032/1) subproject “Deiodination of iodinated contrast media in bank filtration of urban waters.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerald Thouand
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
El-Athman, F., Adrian, L., Jekel, M. et al. Deiodination in the presence of Dehalococcoides mccartyi strain CBDB1: comparison of the native enzyme and co-factor vitamin B12. Environ Sci Pollut Res 26, 32636–32644 (2019). https://doi.org/10.1007/s11356-019-06505-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06505-z