Abstract
The dehalogenating performance of an anaerobic 5-amino-2,4,6-triiodoisophthalic acid (ATIA) fixed-bed reactor was evaluated. The reactor operating conditions were set for ATIA deiodination. A phylogenetic survey for a stable anaerobic ATIA-deiodinating microbial consortium was carried out using 16S rDNA restriction fragment length polymorphism, and unique clones were sequenced. Four phylotypes were identified. Two sequences were related to those of Desulfitobacterium frappieri species and another was closest to that of Desulfitobacterium hafniense, but may have represented a new Desulfitobacterium species. Desulfitobacteria were previously described as aryl-dechlorinating and debrominating bacteria. The new strains identified in this study were probably responsible for the ATIA deiodination. The fourth clone was related to the Clostridium-Flavobacterium-Bacteroides group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
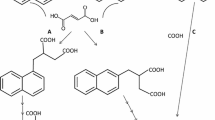
Triiodinated aromatic compounds are used as X-ray contrast agents in radiology, and so are released in hospital waste water (Erbe et al. 1998). Because of their high stability, they are not degraded in sewage treatment plants and are detected in rivers at concentrations up to 30 µg l−1 (Olesky-Frenzel et al. 1995; Putschew et al. 2000). Some studies have reported biological conversions of these compounds via deacetylation or partial deiodination (Kalsch 1999; Rode and Müller 1998; Steger-Hartmann et al. 2002). For example, 5-amino-2,4,6-triiodoisophthalic acid (ATIA) (Fig. 1), the core structure of a range of iodinated X-ray contrast agents, was completely reductively deiodinated by an anaerobic consortium (Lecouturier et al. 2002).
Several bacteria that reductively dehalogenate various chlorinated and brominated aromatic compounds have been isolated and characterized. They belong to various groups such as Desulfomonile (Shelton and Tiedje 1984), Desulfitobacterium (Bouchard et al. 1996; Breitenstein et al. 2001; Christiansen and Ahring 1996; Gerritse et al. 1996; Sanford et al. 1996; Utkin et al. 1994), Desulfovibrio (Boyle et al. 1999), and myxobacteria (Cole et al. 1994). In many cases, the bacteria use chloroaromatic compounds as terminal electron acceptors and gain energy from reductive dechlorination (Boyle et al. 1999; Christiansen and Ahring 1996; Cole et al. 1994; Dolfing 1990; Gerritse et al. 1996; Mackiewicz and Wiegel 1998; Sanford et al. 1996). As little is known about deiodinating bacteria, we set out to determine which bacteria carry out this reaction and whether they are phylogenetically related to dechlorinating organisms.
Isolation of pure cultures from anaerobic environments can often be difficult because of tight syntrophic associations between microorganisms (Dolfing and Tiedje 1986). In addition, only a small fraction of the microbial community can be cultivated using standard techniques (Amann et al. 1995; Göbel 1995), so that isolation of xenobiotic-degrading bacteria often fails (Bedard and Quensen 1995; Phelps et al. 1998). Analysis of the 16S rRNA genes thus appears to be a good alternative method to describe community constituents of xenobiotic-degrading microbial consortia, and has already yielded interesting results (Dojka et al. 1998; Knight et al. 1999; LaPara et al. 2000; Pulliam Holoman et al. 1998; von Wintgerode et al. 1999; Wagner-Döbler et al. 2000).
Here, we first evaluated ATIA deiodination in an anaerobic continuous fixed-bed reactor, and then characterized the stable ATIA-degrading bacterial population by 16S rDNA sequence analysis.
Materials and methods
Source of inoculum
The microbial consortium was enriched under anaerobic conditions from sludge obtained from an industrial waste water treatment plant of the Guerbet group (Aulnay, France) that had been receiving triiodinated compounds for a long time. The anaerobic bacteria were enriched on ATIA and ethanol by repeated transfers in liquid batch cultures as already described (Lecouturier et al. 2002). After 1 year of cultivation, 40 ml of liquid culture was used as the inoculum for the anaerobic fixed-bed reactor.
Bioreactor processing
The experiments were carried out in an upflow anaerobic biofilm reactor. The set-up is shown in Fig. 2. The bacteria were cultured in a 5-cm diameter 20-cm-long glass cylinder filled with 86 g of Rashig rings (Schott Glaswerke, Mainz, Germany). These supports are hollow porous glass cylinders (8 mm long, 2 mm thick, and 8 mm in diameter) that provide a large adsorption surface for cells. In batch experiments, the cylinders had a low adsorption capacity for ATIA. To prevent oxygen diffusion, all tubing used was either viton or glass. The liquid volume was 365 ml. The liquid phase in the reactor was recirculated at a flow rate of 5 l day−1. The reactor was thermostated at 30 °C.
The anaerobic mineral salt medium for reactor feeding was prepared as already described (Lecouturier et al. 2002). The feeding solution was kept sterile. One hundred ml of a solution containing phosphate buffer, ATIA, ethanol, Na2S and Na2CO3 was sterilized by filtration with a 0.2-µm GH Polypro filter (Gelman). The remaining medium was sterilized by autoclaving (20 min, 121 °C). The two solutions were mixed aseptically.
Before inoculation, the reactor was flushed with N2 and filled with reduced medium. The inoculum was introduced in the recirculation loop and the reactor was recycled for 6 h. It was then run in batch mode with 0.5 mM ATIA with a molar ethanol/ATIA ratio of 4 to allow the adhesion of cells and colonization of the glass supports. After complete ATIA deiodination, continuous feeding was started. The reactor was run with a hydraulic retention time of 8 days. The ATIA concentration in the feeding medium was 0.5 mM. The ethanol concentration was increased stepwise from 0.5 mM to 2 mM.
Analytical methods
Samples were analyzed as already described (Lecouturier et al. 2002). ATIA and metabolite concentrations were measured by C18 reverse-phase liquid chromatography. Ethanol and acetate were measured by liquid chromatography with an HI-plex column and 10−3 M H2SO4 eluent solution. Iodide was measured using a specific electrode Elit 281 (Bioblock, Illkirch, France) connected to a Consort multimeter (Bioblock). The iodide detection limit was 10 µM. Total organic carbon was measured with a Total Organic Carbon Analyzer model 1010 (IO Analytical, Bioritech, France). Gas production was detected with a Mariotte flask. Microbial biomass on glass cylinders was estimated by measuring proteins colorimetrically by a modified Lowry method (Peterson 1977). Recirculation was stopped and supports were taken from the bed under an N2 stream to avoid oxygen diffusion in the reactor. Cells were detached from the rings by sonication (6 min, 0.8 W/ml) with a Vibracell 72405 100 W sonicator (Bioblock Scientific, France) in 10 ml of distilled water. After sonication, the ring was transferred to another tube and the operation was repeated until the protein concentration was below 2 mg l−1.
Sampling and extraction of total genomic DNA
The bioreactor was run for 2 years before the bacterial community was analyzed. Eleven Rashig glass rings were sampled from the anaerobic reactor at approximately 5 cm from the top of the bed. The rings were centrifuged at 6,000 g for 10 min and ground in a mortar with 4 ml of 4 M guanidine thiocyanate-Tris (pH 7.5). DNA extraction was carried out as described in Godon et al. (1997) until the nucleic acids precipitation step by isopropanol. DNA was purified with a QIAamp DNA Minikit (QIAGen) according to the instructions of the manufacturer. The pellets were resuspended and pooled in 200 µl of water. Twenty µl of RNase (1 mg/ml) were added and the tube was incubated at 37 °C for 30 min. AL buffer (200 µl) was added and the tube was incubated for 10 min at 70 °C. Then, 200 µl of pure ethanol were added and the tube was vortexed. The sample was transferred to a QIAamp microcolumn and centrifuged at 14,000 g for 3 min. DNA was washed once with 500 µl of AW1 buffer, centrifuged for 3 min at 14,000 g, washed again with 500 µl AW2 buffer, centrifuged at 14,000 g, and then eluted with 50 µl of water by centrifugation for 1 min at 8,000 g.
Amplification and cloning of 16S rDNA genes
Amplification of 16S rDNA genes from purified genomic DNA was carried out with primers for conserved domains. The forward primer W018 (5′ GAGTTTCATCMTGGCTCAG 3′, [Escherichia coli, 9]), which targets the domain Bacteria, and the reverse primer W002 (5′ GNTACCTTGTTACGACTT 3′, [E. coli, 1492]), which targets all living organisms, were used to amplify bacterial 16S rDNA by PCR (Godon et al. 1997). Reaction tubes contained 1 µl of purified sample DNA, 1 U of REDTaq DNA polymerase (Sigma, Saint-Quentin-Fallavier, France), 5 µl of 10× reaction buffer containing 15 mM MgCl2, 0.2 mM of each deoxyribonucleotide triphosphate and 2 µl of each primer (100 ng/ml) in a final volume of 50 µl. Initial DNA denaturation and enzyme activation steps were done at 94 °C for 2 min in a PTC 150 thermocycler (MJ Research, Watertown, Mass., USA), followed by 25 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 1 min and elongation at 72 °C for 1 min, followed by a final elongation of 10 min at 72 °C. PCR products were purified and concentrated with a QIAquick spin PCR purification kit (QIAGen, Courtaboeuf, France). PCR fragments were eluted with 30 µl of water. Their concentration and size were estimated by agarose gel electrophoresis [0.7% (w/v) agarose and Tris borate/EDTA buffer containing 1 mg ethidium bromide ml−1]. The purified products were ligated into a pCR 4-TOPO vector (Invitrogen, Groningen, The Netherlands) as specified by the manufacturer. TOP10 competent E. coli (Invitrogen) were transformed with ligation products by heat shock (30 s at 42 °C). Recombinant cells were selected on Luria-Bertani medium with kanamycin (50 mg l−1). Colonies containing a plasmid with an insert were able to grow in the presence of kanamycin.
16S rDNA RFLP analysis and sequencing
Cloned 16S rDNA fragments were amplified directly from fresh (less than 24 h) single E. coli colonies using AmpliTaq DNA polymerase and plasmid-targeted T7 (5′ TAATACGACTCACTATAGGG 3′) and P13 (5′ GACCATGATTACGCCAA 3′) primers. The PCR reaction mix was prepared according to the manufacturer's instructions (Perkin Elmer Cetus). Reaction conditions were 10 min of initial denaturation to lyse the cells, then 30 cycles for 15 s at 94 °C, 15 s at 55 °C and 15 s at 72 °C. Reactions were ended by a 10 min elongation at 72 °C and cooling at 4 °C. Without purification, PCR products were digested with the restriction endonucleases EcoRI and HaeIII to compare clones. Restriction digests were separated by agarose gel electrophoresis (2% agarose and Tris borate/EDTA buffer containing 1 ng ethidium bromide ml−1). One clone corresponding to each group of restriction patterns was subjected to detailed sequence analysis. Relevant colony PCR products were purified as previously described. Sequencing reactions were done with a GeneAmp PCR system 9600 thermocycler (Perkin Elmer Cetus) using the Dye-Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase FS kit buffer (Applied Biosystem) and the universal primer w031 (5′ TTACCGCGGCTGCTGGCAC 3′).
16S rDNA sequence analysis
Sequences were edited to exclude the PCR primer binding sites. Newly determined sequences were compared with those available in public databases in order to ascertain their closest relatives. The presence of chimeric sequences was checked with the RDP chimera check program (Michigan State University, USA). Phylogenetic analyses were carried out with AliBee software (Belozersky 1995), and construction of the phylogenetic tree by the UPGMA method.
Reference bacteria used for phylogenetic analysis and their GenBank accession numbers are listed below. D. frappieri PCP-1 (U40078), D. frappieri TCE1 (X95742), D. frappieri TCP-A (AJ404686), D. hafniense (X94975), D. dehalogenans (L28946), D. chlororespirans (U68528), Dehalobacter restrictus (Y10164). The sequences obtained in this study have been deposited in GenBank with the following accession numbers: ATIA-3, AY223537; ATIA-6, AY223534; ATIA-8, AY223536; ATIA-12, AY223535. Their length was respectively 451 bases for ATIA-3, 595 bases for ATIA-6, 453 bases for ATIA-8 and 355 bases for ATIA-12.
Results and discussion
Fixed-bed reactor colonization and stabilization
In previous work, we obtained the complete reductive deiodination of ATIA, the core structure of a range of iodinated X-ray contrast agents, to 5-aminoisophthalic acid (AIA) in batch liquid cultures with ethanol as an auxiliary substrate (Lecouturier et al. 2002). In this work, deiodination of ATIA was carried out in a continuous-flow anaerobic biofilm reactor. Such anaerobic bioreactors have been used for the biodegradation of a wide variety of chlorinated aromatic compounds such as chlorophenols (Hendriksen et al. 1992; Juteau et al. 1995; Tartakovsky et al. 1999; Wu et al. 1993), chlorobenzoates (Ascon and Lebeault 1999; Ahring et al. 1992), polychlorobiphenyls (Pagano et al. 1995; Tartakovsky et al. 2000). In our experiments, cultivating in a bioreactor had two advantages: (1) it was a good way to ensure conservation of the active deiodinating consortium, (2) While ATIA deiodination occurs in batch liquid cultures through a sequential process, i.e. each iodinated intermediate metabolite is degraded only after the previous compound has disappeared (Lecouturier et al. 2002), in bioreactors, operating parameters were set up so that all deiodination steps occurred simultaneously.
The biofilm reactor was driven for colonization as a batch culture for 21 days with ATIA at a concentration of 0.5 mM and ethanol at 2 mM. There was a 14-day lag period before ATIA deiodination started. ATIA was dehalogenated within 7 days. On day 21, AIA accumulated in the culture medium indicating that ATIA was completely deiodinated (Fig. 3). At this time, 77% of the expected iodide was released. Continuous feeding was then started with 0.5 mM ATIA. To optimize the load of the auxiliary substrate needed to obtain complete deiodination, the concentration of ethanol was increased gradually from 0.5 to 2 mM. During the first period, the iodide concentration dropped due to wash-out and the decrease in the ATIA deiodination rate. Diiodinated and monoiodinated intermediates were present in the effluent (data not shown). When the ethanol/ATIA ratio was increased to 2, the iodide concentration rose to 0.92±0.05 mM. The diiodinated compound almost disappeared and the monoiodinated compound was the main intermediate in the effluent. When the ethanol/ATIA ratio was set at 3, the iodide concentration increased to 1.14±0.07 mM. Finally, when the ethanol/ATIA ratio was set at 4, the iodide concentration in the effluent was 1.45±0.05 mM and ATIA deiodination was complete. The operating parameters of the anaerobic bioreactor with an ethanol/ATIA ratio of 4 are presented in Table 1. ATIA was converted to AIA, ATIA was not detected, and the concentration of iodinated intermediates remained below 0.015 mM. The AIA concentration reached 0.37 mM in the effluent. The release of the three iodide atoms required the oxidation of four ethanol molecules to acetate. Neither AIA nor acetate were oxidized. Organic loads were the same in the inlet and the effluent flow and no gas production was observed, indicating that there was no methanisation in the fixed-bed reactor.
The specific deiodination rate of the consortium in the fixed-bed reactor was 13.7±2.3 mmol (g of volatile suspended solid, VSS)−1 day−1. This rate was higher than those reported for other chloroaromatic compounds degraded in anaerobic bioreactors. Ahring et al. (1992) reported a 3-chlorobenzoate dechlorination rate of 54 µmol (g VSS)−1 day−1 in a UASB reactor. Tartakovsky et al. (1999) reported a maximal dechlorination rate of 128 µmol (g VSS)−1 day−1 in a pentachlorophenol-degrading bioreactor. Both reactors were supplemented with high levels of extra carbon sources, which could explain a higher biomass content in UASB reactors. We had already found that the growth yield was quite low for the ATIA-deiodinating consortium (Lecouturier et al. 2002), and the protein concentrations remained low in our fixed-bed reactor.
The biofilm reactor was continuously fed for 2 years. Degradation parameters remained unchanged throughout.
Phylogenetic analysis
The composition of the ATIA deiodinating consortium from the stable fixed-bed reactor was explored by comparative sequence analysis of 16S rRNA genes after direct PCR amplification and cloning in E. coli from community DNA. Nineteen clones were grouped in four phylotypes by restriction fragment length polymorphism (RFLP) analysis (Fig. 4), and unique clones were sequenced (Table 2). One chimera was found and removed from further analysis. The ATIA-degrading culture yielded four clonal types. This low diversity agrees with the results of molecular community analysis of a 2-bromophenol-dehalogenating consortium (Knight et al. 1999). However, other authors have found a higher diversity in halogenated-xenobiotic-degrading anaerobic consortia (Breitenstein et al. 2001; von Wintzingerode et al. 1999). The low number of sequences in our consortium may result from the use of a mineral salt medium depleted of any complex addition and a long-term enrichment. In the reactor, ATIA deiodination represents the main energy-producing metabolism, as already shown in batch experiments (Lecouturier et al. 2002). Hence, bacteria implicated in deiodination benefit from a consistent advantage. Several parameters may also affect our ability to detect the actual species distribution, particularly cell lysis (Muyzer and Smalla 1998). Therefore, to minimize DNA extraction bias, a physical cell lysis method was used to effectively lyse all cell types. Using biomass from a continuously fed reactor enabled us to start with a good level of all the active bacterial population, because all the metabolic reactions occurred simultaneously.
Three clones were placed within the Desulfitobacterium genus. These bacteria usually form spores (Bouchard et al. 1996; Breitenstein et al. 2001; Madsen and Licht 1992). This result agrees with the maintenance of ATIA deiodination after pasteurization (80 °C, 40 min) and the microscopic observation of terminal spore-forming rod-shaped bacteria (data not shown). Two clones were related to the species Desulfitobacterium frappieri. Clones ATIA-3 and ATIA-6 corresponded to D. frappieri TCE1 (98% sequence identity from the GenBank for each clone) (Gerritse et al. 1999). Clone ATIA-3 represented 56% of clones analyzed. D. frappieri TCE1 was isolated from a soil sample from a chloroethene polluted location. It was found to grow by reductive dechlorination of tetrachloroethene and trichloroethene. This strain was able to use ethanol, hydrogen, formate, lactate and butyrate as electron donors. All of these substrates could also be used for ATIA deiodination (Lecouturier et al. 2002). However, no endospore was observed with this strain. Clone ATIA-12 resembled the strains Desulfitobacterium hafniense and Desulfitobacterium frappieri TCP-A (Breitenstein et al. 2001; Christiansen and Ahring 1996; Madsen and Licht 1992). These spore-forming bacteria are capable of dechlorinating 3-chloro-4-hydroxyphenylacetate and chlorophenols at ortho and sometimes meta positions. D. hafniense was isolated from waste water sludge. D. frappieri TCP-A was isolated from a river sediment. This strain uses pyruvate, lactate, butyrate, formate and hydrogen as electron donors but is not able to use ethanol or acetate. However, after comparison with the sequences of other aryl-dehalogenating Desulfitobacterium species, clone ATIA-12 was placed in the phylogenetic tree between Desulfitobacterium chlororespirans, a polychlorophenol ortho-dechlorinating bacterium (Sanford et al. 1996), and D. dehalogenans, which ortho-dechlorinates 2,4-dichlorophenol (Utkin et al. 1994) (Fig. 5). It may belong to a new Desulfitobacterium species.
Desulfitobacterium species represented 83% of the clones from the ATIA degrading consortium and were probably responsible for deiodination. This genus groups several species that dehalogenate a number of chlorinated and brominated compounds such as chlorophenols, 3-chloro-4-hydroxyphenylacetic acid, chloroethenes and bromophenols. Desulfitobacterium dehalogenans dechlorinates 2,4-dichlorophenol and 3-chloro-4-hydroxyphenylacetate at the ortho position (Utkin et al. 1994). D. frappieri PCE-1 possesses two dehalogenating activities on chloroethenes and chlorophenols (Gerritse et al. 1996). Strain D. frappieri PCP-1, which was isolated from a methanogenic consortium on pentachlorophenol (Bouchard et al. 1996), is able to utilize a wide spectrum of halogenated substrates (Dennie et al. 1998). The strain has ortho, meta and para dechlorinating activities. In the presence of inducers, it is able to dehalogenate a variety of chlorinated aromatic compounds including pentachlorophenol and 2,3,5,6-tetrachloroaniline. It also dehalogenates 2,4,6-tribromophenol but had no activity on fluorinated phenols or aromatic compounds substituted with carboxyl groups. To date, no activity of desulfitobacteria has ever been found on iodinated compounds.
The last clone, ATIA-8, was placed in the Cytophaga-Flavobacterium-Bacteroides group. This clone represented 17% of the clones analyzed. Its sequence was related to those of two clone isolated from contaminated environments: clone TDC-S1:26, from an anaerobic consortium capable of degrading tetrachloroethene (Dennis et al., GenBank, unpublished data), and WCHB1-69, from a hydrocarbon- and chlorinated-solvent-contaminated aquifer (Djoka et al. 1998). However, the members of this group do not have the ability to sporulate. Since ATIA deiodination was resistant to pasteurization, this strain was not directly implicated in the deiodination. The presence of this species may be explained by a close interrelationship with deiodinating bacteria.
We have characterized for the first time the bacterial community from a steady-state anaerobic ATIA-deiodinating bioreactor. Most of the 16S rDNA sequences amplified from this consortium belonged to the genus Desulfitobacterium. Interestingly, identified bacteria were closely related to known aryl-dechlorinating bacteria, but represented new strains or new species. They were highly representative of the total bacterial population and their physiological characteristics corresponded to the properties of the ATIA-degrading consortium. These results suggest that desulfitobacteria were responsible for ATIA reductive deiodination. This work confirms the large contribution of these bacteria to the elimination of a variety of chlorinated, brominated, and iodinated xenobiotic compounds.
References
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Ahring BK, Christiansen N, Mathrani I, Hendriksen HV, Macario AJL, Conway de Macario E (1992) Introduction of a de novo bioremediation ability, aryl reductive dechlorination, into anaerobic granular sludge by inoculation of sludge with Desulfomonile tiedjei. Appl Environ Microbiol 58:3677–3682
Ascon MA, Lebeault J-M (1999) High efficiency of coupled aerobic-anaerobic recycling biofilm reactor system in the degradation of recalcitrant chloroaromatic xenobiotic compounds. Appl Microbiol Biotechnol 52:592–599
Bedard DL, Quensen IJF (1995) Microbial reductive dechlorination of polychlorinated biphenyls. In: Microbial transformation and degradation of toxic organic chemicals. Wiley, New York, N. Y., pp 127–216
Belozersky, AN (1995) GeneBee: multiple alignement. Institute of Physico-Chemicaal Biology, Moscow State University
Bouchard B, Beaudet R, Villemur R, McSween G, Lepine F, Bisaillon, JG (1996) Isolation and characterization of Desulfitobacterium frappieri sp. nov., an anaerobic bacterium which reductively dechlorinates pentachlorophenol to 3-chlorophenol. Int J Syst Bacteriol 46:1010–1015
Boyle AW, Phelps CD, Young LY (1999) Isolation from estuarine sediments of a Desulfovibrio strain which can grow on lactate coupled to the reductive dehalogenation of 2,4,6-tribromophenol. Appl Environ Microbiol 65:1133–1140
Breitenstein A, Saano A, Salkinoja-Salonen M, Andreesen JR, Lechner U (2001) Analysis of a 2,4,6-trichlorophenol-dehalogenating enrichment culture and isolation of the dehalogenating member Desulfitobacterium frappieri strain TCP-A. Arch Microbiol 175:133–142
Christiansen N, Ahring BK (1996) Desulfitobacterium hafniense sp. nov., an anaerobic reductively dechlorinating bacterium. Int J Syst Bacteriol 46:442–448
Cole JR, Cascarelli AL, Mohn WW, Tiedje JM (1994) Isolation and characterization of a novel bacterium growing via reductive dehalogenation of 2-chlorophenol. Appl Environ Microbiol 60:3536–3542
Dennie D, Gladu I, Lépine F, Villemur R, Bisaillon J-G, Beaudet R (1998) Spectrum of the reductive dehalogenation activity of Desulfitobacterium frappieri PCP-1. Appl Environ Microbiol 64:4603–4606
Dojka MA, Hugenholtz P, Haack SK, Pace NR (1998) Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol 64:3869–3877
Dolfing J (1990) Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in an anaerobic bacterium, strain DCB-1. Arch Microbiol 153:264–266
Dolfing J, Tiedje JM (1986) Hydrogen cycling in a three-tiered food web growing on the methanogenic conversion of 3-chlorobenzoate. FEMS Microbiol Ecol 38:293–298
Erbe T, Kümmerer K, Gartiser S, Brinker L (1998) Röntgenkontrastmittel, Quelle für die AOX-Belastung des Abwassers durch Krankenhäuser. Fortschr Röntgenst 169:420–423
Gerritse J, Renard V, Gomes TMP, Lawson PA, Collins MD and Gottschal JC (1996) Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol 165:132–140
Gerritse J, Drzyzga O, Kloetstra G, Keijmel M, Wiersum LP, Hutson R, Collins MD and Gottschal JC (1999) Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl Environ Microbiol 65:5212–5221
Göbel UB (1995) Phylogenetic amplification for the detection of uncultured bacteria and the analysis of complex microbiota. J Microbiol Methods 23:117–128
Godon J-J, Zumstein E, Dabert P, Habouzit F, Moletta, R (1997) Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol 63:2802–2813
Hendriksen HV, Larsen S, Ahring BK (1992) Influence of a supplemental carbon source on anaerobic dechlorination of pentachlorophenol in granular sludge. Appl Environ Microbiol 58:365–370
Juteau P, Beaudet R, McSween G, Lépine F, Milot S, Bisaillon J-G (1995) Anaerobic biodegradation of pentachlorophenol by a methanogenic consortium. Appl Microbiol Biotechnol 44:218–224
Kalsch W (1999) Biodegradation of the iodinated X-ray contrast media diatrizoate and iopromide. Sci Tot Environ 225:143–153
Knight VK, Kerkhof LJ, Häggblom MM (1999) Community analyses of sulfidogenic 2-bromophenol-dehalogenating and phenol-degrading microbial consortia. FEMS Microbiol Ecol 29:137–147
LaPara TM, Nakatsu CH, Pantea L, Alleman JE (2000) Phylogenetic analysis of bacterial communities in mesophilic and thermophilic bioreactors treating pharmaceutical wastewater. Appl Environ Microbiol 66:3951–3959
Lecouturier D, Rochex A, Lebeault J-M (2002) Enrichment and properties of an anaerobic mixed culture which reductively deiodinates 5-amino-2,4,6-triiodoisophthalic acid, an X-ray contrast agent precursor. Appl Microbiol Biotechnol (submitted)
Mackiewicz M, Wiegel J (1998) Comparison of energy and growth yields for Desulfitobacterium dehalogenans during utilization of chlorophenol and various traditional electron acceptors. Appl Environ Microbiol 64:352–355
Madsen T, Licht D (1992) Isolation and characterization of an anaerobic chlorophenol-transforming bacterium. Appl Environ Microbiol 58:2874–2878
Muyzer G and Smalla K (1998)Application of denaturating gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek 1:127–41
Oleksy-Frenzel J, Wischnack S, Jekel M (1995) Bestimmung der organischen Gruppenparameter AOCl, AOBr und AOI in Kommunalabwasser. Vom Wasser 85:59–67
Pagano JJ, Scrudato RJ, Roberts R, Bemis JC (1995) Reductive dechlorination of PCB-contaminated sediments in an anaerobic bioreactor system. Environ Sci Technol 29:2584–2589
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Chem 83:346–356
Phelps CD, Kerkhof LJ, Young LY (1998) Molecular characterization of a sulfate-reducing consortium which mineralizes benzene. FEMS Microbiol Ecol 27:269–279
Pulliam Holoman TR, Elberson MA, Cutter LA, May HD, Sowers KR (1998) Characterization of a defined 2,3,5,6-tetrachlorobiphenyl-ortho-dechlorinating microbial community by comparative sequence analysis of genes coding for 16S rRNA. Appl Environ Microbiol 64:3359–3367
Putschew A, Wischnack S, Jekel M (2000) Occurence of triiodinated X-ray contrast agents in the aquatic environment. Sci Tot Environ 255:129–134
Rode U, Müller R (1998) Transformation of ionic X-ray contrast agent diatrizoate and related triiodinated benzoates by Trametes versicolor. Appl Environ Microbiol 64:3114–3117
Sanford RA, Cole JR, Löffler FE, Tiedje, JM (1996) Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl Environ Microbiol 62:3800–3808
Shelton DR, Tiedje JM (1984) General method for determining anaerobic biodegradation potential. Appl Environ Microbiol 47:850–857
Steger-Hartmann T, Länge R, Schweinfurth H, Tschampel M, Rehmann I (2002) Investigations into the environmental fate and effects of iopromide (ultravist), a widely used iodinated X-ray contrast medium. Wat Res 36:266–274
Tartakovsky B, Levesque M-J, Dumortier R, Beaudet R, Guiot SR (1999) Biodegradation of pentachlorophenol in a continuous anaerobic reactor augmented with Desulfitobacterium frappieri PCP-1. Appl Environ Microbiol 65:4357–4362
Tartakovsky B, Hawari J, Guiot SR (2000) Enhanced dechlorination of Arochlor 1242 in an anaerobic continuous bioreactor. Water Res 34:85–92
Utkin I, Woese C, Wiegel J (1994) Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol 44:612–619
Wagner-Döbler I, Lünsdorf H, Lübbehüsen T, von Canstein HF, Li Y (2000) Structure and species composition of mercury-reducing biofilms. Appl Environ Microbiol 66:4559–4563
von Witzingerode F, Selent B, Hegemann W, Göbel UB (1999) Phylogenetic analysis of an anaerobic, trichlorobenzene-transforming microbial consortium. Appl Environ Microbiol 65:283–286
Wu W-M, Bhatnagar L, Zeikus JG (1993) Performance of anaerobic granules for degradation of pentachlorophenol. Appl Environ Microbiol 59:389–397
Acknowledgements
We are grateful to Valérie Bru for technical assistance. We thank Alice Rochex for critical review of the manuscript. This work was supported financially by Guerbet Group (Aulnay, France). The experiments comply with the current laws of France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lecouturier, D., Godon, JJ. & Lebeault, JM. Phylogenetic analysis of an anaerobic microbial consortium deiodinating 5-amino-2,4,6-triiodoisophthalic acid. Appl Microbiol Biotechnol 62, 400–406 (2003). https://doi.org/10.1007/s00253-003-1278-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1278-7