Abstract
Knowledge regarding the concentration levels resulting from the use of agricultural pesticides may indicate the nature of the controls necessary to reduce environmental and human health risks to an acceptable level. Therefore, the main goal of the present work was to assess the spatial and temporal occurrence of 35 pesticides in the River Sado estuary (Portugal) in 2017 and evaluate its environmental condition, as data for estuarine ecosystems is scarce. Since pesticides are very susceptible to matrix effects promoted by environmental samples, to attain the main goal, we developed a fast and almost solvent-free environmentally friendly method with a good performance for both estuarine surface water and sediment samples. Quantified residues were determined mostly during summer, in line with the pesticide application period. Five herbicides (alachlor, bentazon, metobromuron, metribuzin and triclopyr) were measured in the water before and after the production season, suggesting a long-term aquatic exposure. Sediment samples were less contaminated, since a lower number of quantified pesticides were found in the study area, in lower frequencies and lower concentrations. No potential high adverse effects of the use of agricultural pesticides were expected on the aquatic organisms of the Sado estuary, even considering the potential combination effect of pesticide mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The assessment of the environmental condition of aquatic ecosystems is of global importance and legally binding through the EU Water Framework Directive (Directive 2000/60/EC). Pursuant to this, European Directives set limits on concentrations of priority substances as the pesticides atrazine, alachlor, diuron, isoproturon, simazine and chlorpyrifos, as well as environmental quality standards (EQSs) in surface waters (Directive 2013/39/EU). Agricultural pesticides are considered environmentally relevant pollutants, and alluvial soil along rivers usually corresponds to the greatest areas of intense agriculture practices. Thus, estuaries seem to be suitable areas to develop monitoring programmes to determine environmental levels (Papadakis et al. 2015; Rodrigues et al. 2018). In fact, the herbicide atrazine has a specific maximum concentration of 2.0 μg L−1 allowed for European estuarine waters (Annex I, Directive 2008/105/EC). Moreover, some pesticides were released years ago and, due to their persistence, are still strongly associated with estuarine sediment (Cuevas et al. 2018; Duodu et al. 2017), with possible harmful effects to benthic organisms, as sediment provides habitat for a diverse range of estuarine organisms such as burrowing or epifaunal bivalves, burrow-forming amphipod or flatfish. Accordingly, sediment contamination has been receiving increasing attention from environmental authorities since it is recognised as a major source of ecosystem health stress (EFSA 2015).
The aquatic environmental risk characterisation of pesticides depends on their environmental levels, as well as on their potential effects on aquatic organisms. Hence, the present study proposes to determine the environmental condition of the River Sado estuary, the second largest estuary in Portugal, due to the application of agricultural pesticides in the Lower Sado (agricultural area located upstream the estuary), by linking aquatic exposure (obtained in the present study) and effects (obtained in the literature). In order to attain this main goal, two specific well-determined objectives were delineated: (a) to develop and validate a multi-residue analytical method for the simultaneous determination of pesticides in complex matrices as estuarine water and sediment and (b) to determine 32 pesticide residues and three degradation products of triazine pesticides in surface water and sediments of the River Sado estuary.
The River Sado estuary was chosen since, among the eight Portuguese estuaries studied by Vasconcelos et al. (2007), it was considered the most affected by agriculture practices (particularly rice fields). To our knowledge, the occurrence of pesticide residues in the Sado estuary has not been subject to an overall assessment since 2008 (Silva and Cerejeira 2015). By contrast, a reasonable amount of the literature concerning the occurrence and effects of biphenyls, dibenzofurans and dioxins (Nunes et al. 2014); metals and metalloids (Caeiro et al. 2005; Serafim et al. 2013); nutrients (Saraiva et al. 2007); organo-tins (Díez et al. 2005); and polycyclic aromatic hydrocarbons (Serafim et al. 2013) was reported for this estuary. The River Sado estuary was also selected as a study area because it comprises a great diversity of habitats, including two Special Protection Areas for Birds and the Sado Estuary Nature Reserve (23,160 ha), as well as one of the few resident populations of bottlenose dolphins of Europe.
The 35 pesticides were selected to cover all groups (fungicides, herbicides, insecticides and nematocides), as well as three degradation products of triazine pesticides (desethyl-atrazine, desisopropyl-atrazine and desethyl-terbuthylazine). Alachlor (not approved in EU); atrazine (not approved in EU); chlorpyrifos, diuron and isoproturon (not approved in EU); and simazine (not approved in EU) were selected as they are listed as priority substances in Directive 2013/39/EU; azoxystrobin, bentazon, imidacloprid and 2-methyl-4-chlorophenoxyacetic acid (MCPA) were selected because they are approved pesticides for rice production application in Portugal (DGAV 2016); bentazon, MCPA, propanil and triclopyr were selected as its occurrence in the Sado river basin had previously been reported by Silva and Cerejeira (2015). The persistent herbicides atrazine and simazine, which had been widely used in Portugal, were removed from the Portuguese market respectively in 2007 and 2005 in the scope of Directive 91/414/EE, repealed by Regulation 1107/2009, and its persistence in the River Sado estuary was investigated in the present study. Conversely, the placement of terbuthylazine on the market was authorised, and this herbicide was recently detected in several other Portuguese estuaries (Cruzeiro et al. 2015, 2016b; Rodrigues et al. 2018). Alachlor, benalaxyl, chlorpyrifos, kresoxim-methyl, linuron, S-metolachlor, penconazole, propazine, tebuconazole, terbuthylazine and triclopyr were also selected as they have hydrophobic properties (log Kow ≥ 3), and therefore tend to bind to suspended particulate matter and to accumulate in bottom sediments once entered into the water compartment. A log Kow ≥ 3 is used as a trigger value for sediment EQS determination, according to the European Commission (2011). Finally, the degradation products of triazine pesticides were chosen as atrazine is considered a persistent herbicide (Jablonowski et al. 2010), and the desethyl-atrazine-to-atrazine ratio could be an indicator of the residence time of atrazine in the soil (Adams and Thurman 1991; Goolsby et al. 1997). Also, desethyl-terbuthylazine is frequently found in aquatic ecosystems (recently reviewed by Tasca et al. (2018)), where it constitutes a hazard to non-target organisms (Stara et al. 2016; Velisek et al. 2016).
On-line solid-phase extraction (SPE) coupled with ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) was the chosen methodology for pesticide residue determination in the collected samples (estuarine water and sediment). In sediment samples, the accelerated solvent extraction (ASE) technique was used as an additional step for simultaneous sample extraction, clean-up and concentration. The ASE is considered an advanced technique since it provides important benefits over more traditional extraction techniques such as liquid–liquid extraction or Soxhlet extraction. ASE requires less solvent volume, thereby being in compliance with green analytical practices; demands less sample manipulation; and allows for shorter extraction times, thus increasing sample throughput (Rodrigues et al. 2016).
The outcomes of the present study allowed the determination of pesticide aquatic risk characterisation for an estuarine ecosystem by linking the exposure levels determined in this study and the EQS values obtained in the literature. A limitation using this approach should be highlighted since pesticides are considered individually, whereas agricultural contamination is usually due to a mixture of pesticides, as well as of other compounds such as fertilisers. The combined toxic effects of pesticide mixtures exceeding the effect of each individual compound has already been demonstrated (Porsbring et al. 2010; Verbruggen and van den Brink 2010). According to the European Union (2012), pesticides with common modes of action seem to act jointly to produce combination effects that are larger than the effects of each mixture component applied individually, and can be described by the concentration addition method. For mixtures of independently acting pesticides, if the individual pesticide is present above its EQS level, the effects can be estimated directly from the probability of responses to the individual components (response addition) or the sum of biological responses (effects addition). Accordingly, for the water and sediment samples with the highest number of pesticides, these approaches were applied to determine the realistic toxicity of the pesticides present in the River Sado estuary.
Experimental methodology
Study area, sample collection and sediment organic matter determination
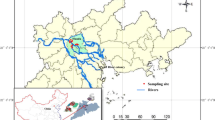
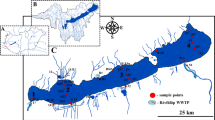
The River Sado estuary (170 km2) is located in Southern Europe, in the southwest coast of Portugal, and is considered a well-mixed mesotidal estuary with irregular river discharge (Bettencourt et al. 2004). The sampling area, along the Comporta and Alcácer do Sal channels, the main agricultural areas of the estuary, covered a total of 11 sampling points for surface water and 10 for sediments (Fig. 1), as station 5 was in a trench used for irrigation, and no sediment sample was collected. The Comporta channel is highly dynamic, and tides are the main responsible for water circulation, whereas the entrance of the Alcácer do Sal channel corresponds to a shallow area with tidal flats, and freshwater forcing conditions characterise the inner part of this channel. Pesticide seasonal and spatial occurrences were considered, as a pollutant gradient along the two channels of the estuary was expected. Seasonal variations, before and after the production season on the Lower Sado, were foreseen as well. The first sampling campaign took place a few days after the end of the rainy season but before the works for the following producing season on the Lower Sado agricultural fields started (March 14–15, 2017). The second sampling campaign occurred in July, which corresponded to the end of the producing season (July 20–21, 2017). Two different matrices, superficial water (≈ 1 L) and surface sediment (≈ 250 mg), were collected during low tide, and the samples were stored in amber glass or aluminium extrusion containers and transported to the laboratory in 12-V car refrigerators, and then immediately frozen. Several physico-chemical parameters were measured in situ in the water samples, such as dissolved oxygen concentration (mg L−1), temperature (°C), pH and salinity. Sediment samples (top 2–5 cm) were randomly collected in quadruplicate within an area of 100 m2 in each sampling station, using a stainless steel scoop. Three replicates went to the Water Institute of the Northern Region (IAREN) (Portugal) for pesticide determination, and the fourth replica was homogenised, oven-dried to constant weight at 60 °C (Raypa) to obtain dry weight and then burned at 450 °C (Nüve MF110) for 8 h to obtain organic matter content.
Pesticide standards and chemicals
Twenty four of the 35 certified standards used in the present study (Table 1) were of Pestanal© grade (Sigma-Aldrich), imidacloprid (purity 99.8%, Dr. Ehrenstorfer), and the remaining pesticides came in a pesticide mixture standard solution from Ultra (U-PPM-017-1). Ammonium acetate (purity 99%, LC grade, Sigma-Aldrich), methanol (LiChrosolv gradient grade, Riedel-de Haën, Honeywell) and ultra-pure water (Milli-Q, Millipore, Molsheim). Individual stock solutions of 2000 mg L−1 were prepared by dissolving 20 mg of each pesticide standard in methanol. An intermediate mixture stock solution was prepared from the individual stock solutions, and from this, a final work solution containing 100 μg L−1 of each pesticide was prepared. Calibration solutions were prepared daily from this 100 μg L−1 work solution in ultra-pure water.
Pesticide extraction method
The ASE technique was applied to the sediment samples, which had previously been dried for 96 h at room temperature in a hotte, and then grounded in a mortar and sifted through a 1.0-mm fine sieve. The pesticide extraction and purification steps were performed in Thermo Scientific™ Dionex™ ASE™ 350 equipment using 1.0 g of the sediment sample, diatomaceous earth (0.20 g) as a dispersant agent and methanol as a solvent. The following conditions were applied: a pressure of 1500 psi, a temperature of 80 °C, a static time of 3 min, 4 cycles, a flush volume of 60% and a purge time of 60 s. Then, final extracts (15 mL) were diluted at 1:20 ratio with ultra-pure water, filtered (in a 0.20-μm membrane filter of cellulose acetate) and treated as described for water samples.
Similar to the sediment extracts, the water samples were filtered through a 0.20-μm membrane filter of cellulose acetate and placed in 20-mL vials which were then inserted into the sampling tray of a CombiPAL autosampler. Next, a 5.0-mL sample was carried to the on-line SPE column (Oasis® HLB HP, 20 μm, 2.1 mm × 30 mm), which had previously been conditioned with eluent solution (5% methanol in ultra-pure water). After the sample was passed through the on-line SPE column, the analytes were extracted with the eluent to a final volume of 5.0 μL, providing a 1000-fold enrichment factor. The module was equipped with two SPE columns, allowing sample pre-conditioning and enriching in one column while simultaneously eluting another sample in the second column.
Analytical method
Pesticide residue determination was achieved using a UPLC-MS/MS (Waters) supplied with an Acquity UPLC® HSS T3 column of 1.8 mm, 2.1 mm × 150 mm, at 40 °C ± 1 °C, equipped with an on-line SPE module. The mobile phases comprised eluents A (5.0 mM of ammonium acetate in ultra-pure water) and B (5.0 mM of ammonium acetate in methanol), using a gradient flow and a flow rate of 0.3 mL min−1 for 12 min. The chromatographic gradient consisted at first of 5% methanol, which was then increased to 100% methanol for 5 min, and then kept at 100% for 3 min. After this period, it was quickly shifted back to 5% methanol and was kept at this value for 3 min to re-equilibrate. Methanol and a mixture of water:methanol 50:50 (v/v) were used as the strong and weak washing solvents, respectively.
The mass spectrometer detector (Waters TQD triple–quadrupole) was equipped with an electrospray interface (ESI), and the instrumental control and data acquisition and evaluation were carried out using MassLynx 4.0 software (Waters). The general conditions of the mass spectrometer were as follows: nitrogen as the desolvation gas at a flow rate of 850 L h−1, argon as the collision gas at a flow rate of 50 L h−1, a source temperature of 150 °C, a desolvation temperature of 350 °C and a capillary voltage of 3.0 kV. For mass spectrometer detection, cone voltage and collision energy were optimised by infusing 0.5 mg L−1 of individual pesticide solutions. Using IntelliStart (Waters™) software, two transition ions were obtained for each pesticide, which was then analysed by multiple reaction monitoring (MRM). This optimisation was carried out in positive and negative ionisation mode, and the ionisation mode which produced the pair of transitions with the highest peak intensity was the one selected for each pesticide. Also, the transition with the highest peak intensity was used for quantification and the ratio between both peak areas was used for both the identification and the retention time of the pesticide. The compilation of these data by analyte is presented in Table S1 (supplementary material).
To control the analytical reliability and assure the recovery efficiency and the accuracy of the analytical results of the ASE method, linear range, linearity, method detection limit (MDL), method quantification limit (MQL), extraction recovery and intra-day precision were determined by matrix and by target pesticide. Uncontaminated sediment samples were used for the optimisation and validation of the method. Nine samples of those sediments were spiked with the pesticide mixture work solution with concentrations ranging from 7.5 to 150 ng g−1 dry weight (dw) for all target pesticides. Samples were carefully mixed in a mortar to promote contact and to bind off the pesticides with the sediment matrix, being then subject to the extraction procedure. The linearity of the method was assessed by the calculation of quadratic linear regression (R2) and residual analysis, as well as by the estimation of MDL and MQL, calculated as requested by the Portuguese guideline RELACRE 13 (2000) which defines the MDL and MQL as 3.3 × S(y/x)/b and 10 × S(y/x)/b, respectively, where S(y/x) is the residual standard deviation of the calibration curve and b is the slope of the calibration curve.
For the chromatographic method validation, seven calibration standard solutions were prepared from the pesticide mixture work solution using ultra-pure water, with concentrations ranging from 25 to 200 ng L−1. The overall performance of the method was evaluated by calculating the intermediate precision and recovery of spiked samples. During the validation process, quality control was performed by the analysis of laboratory and sample blanks, as well as by the analysis of independently prepared standard solutions at regular intervals during each run of the analysis.
Risk characterisation of pesticides
The risk characterisation for a specific pesticide was determined as the risk quotient (RQ), the ratio of the maximum measured environmental concentration (MEC) value and the EQS value for that pesticide. Two EQS values are possible for each pesticide, the maximum allowable concentration (MAC)-EQS and the annual average (AA)-EQS, which are the values established for the protection of organisms from lethal and sub-lethal effects, respectively. The concentration addition method for pesticide mixture toxicity determination was applied by the calculation of the RQmix of the pesticides with a similar mode of action (Chèvre et al. 2006). In this approach, the RQmix can be expressed as the sum of the ratios of the measured environmental concentration and the EQS for each pesticide according to
The RQ or RQmix ≥ 1 demonstrates a high potential adverse effect due to pesticide exposure concentration, while the 0.1 < RQ or RQmix < 1 indicates a medium risk and RQ or RQmix ≤ 0.1 a low risk.
Results
Validation of methods
Analytical figures of merit concerning the validation of the ASE method applied to sediment samples by target pesticide are shown in Table S2 (supplementary material), and Table S3 (supplementary material) lists the signal-to-noise (S/N) ratio values for the sediment-spiked extract injected at 7.5 ng g−1 dw. Chromatograms of a real sample and a spiked sample are presented in Figs. S1-A and S1-B (supplementary material), respectively. A linear range starting at 7.5 ng g−1 dw was validated for all pesticides except MCPA, whose linear range started at 15 ng g−1 dw. The estimated MDL and MQL were between 0.09 and 3.72 ng g−1 dw and between 0.27 and 11.28 ng g−1 dw, respectively, for all pesticides. The recovery of the pesticides was accessed through the analysis of five spiked samples at the concentration of 45 ng g−1 dw. The recovery rate values of the method ranged from 75 to 125% for all pesticides except carbofuran, desethyl-atrazine, desethyl-terbuthylazine and simazine, which showed higher recoveries. A precision under 25% was obtained for all pesticides.
Concerning the validation of the chromatographic method, all pesticides presented R2 > 0.995, residuals under ± 25%, MDL between 0.9 and 8.2 ng L−1 and MQL between 2.7 and 24.9 ng L−1. Recoveries between 80 and 115% were obtained for all pesticides, showing that the method presented a good recovery, with a precision under 27% for all pesticides. The compilation of these results is presented in Table S4 (supplementary material), and Table S5 (supplementary material) lists the S/N ratio values for the water standard solution injected at 25 ng L−1. Chromatogram examples of a real sample and a spiked sample are shown in Figs. S2-A and S2-B (supplementary material), respectively.
Pesticide occurrence
The in situ parameters (oxygen, temperature, pH and salinity) measured in the surface water samples, as well as organic matter content in intertidal sediments, are shown in Table 2. Regarding organic matter content in sediments, the results indicated no seasonal differences, since only station 6 showed a value of 1 order of magnitude higher in July when compared to the March sampling period. However, a spatial gradient was evident as, in both sampling periods, two different zones in the sampling area presented high organic matter content (> 5.0%): stations 4, 6 and 7 (an inner zone of the Comporta channel and the first two stations of the Alcácer do Sal channel) and stations 9, 10 and 11 (upstream stations in the Alcácer do Sal channel).
Among the 35 determined pesticides and degradation products, only herbicides (alachlor, bentazon, metobromuron, metribuzin and triclopyr) and one degradation product (desisopropyl-atrazine) of the legacy herbicide atrazine were detected in estuarine surface water in March 2017 (17.7%), and one other herbicide (isoproturon) and one insecticide (dimethoate) also appeared in July 2017 (23.5%), showing seasonal differences (Table 3 by raw data: Tables S6 and S7, supplementary material). Concentrations ranged from the MQLs to 8.87 μg L−1, the highest concentration determined in this study, for bentazon in July. The results of the frequency of occurrence are also presented in Table 3, with the highest values for alachlor (100% both in March and in July) and metribuzin (100% in March and 91% in July), followed by bentazon (82% both in March and in July) and metobromuron (64% in March and 82% in July). All the 11 sampling stations presented quantified residues of pesticides or degradation products, between 3–5 and 4–7 different compounds in each sampling station in March and July, respectively. A spatial gradient was observed, with generally higher residue values and a higher number of compounds in the stations around the Comporta village (stations 3, 4 and 6). Nevertheless, in July, station 5 (trench used for irrigation) and stations 10 and 11 (upstream stations, near the town of Alcácer do Sal) also presented both higher concentration values and a higher number of compounds quantified per sampling station, unlike what had happened in March.
Among the analysed pesticides, only the herbicides alachlor and metribuzin and the insecticide chlorpyrifos were measured in sediments above their respective MQL, both in March and in July, while bentazon was only measured in July and triclopyr was only measured in March. Desisopropyl-atrazine (atrazine degradation product) was measured above its respective MQL in both sampling periods (Table 3), with alachlor, chlorpyrifos and triclopyr presenting a log Kow value ≥ 3.
Sado estuary environmental condition
The aquatic MAC-EQS and AA-EQS values for the pesticides measured above their respective MQLs in the Sado estuary were obtained in available regulatory and scientific literature (Table 4). Nevertheless, aquatic EQS values are still lacking for certain active substances as metobromuron, as well as for degradation products (desisopropyl-atrazine).
Based on the maximum MEC here determined for each pesticide and its correspondent EQS value, reported in Table 4, the risk for the aquatic organisms of the River Sado estuary due to exposure concentrations to individual pesticides was considered low to medium (Table 5). The gathered results showed that no RQ ≥ 1 was obtained, being the highest RQ calculated for long-term effects of metribuzin (RQ = 0.85).
Regarding the interactive mixture toxicity for the water compartment, the maximum number of co-occurring pesticides determined was 6 for station 5 in July, with bentazon (3.22 μg L−1), isoproturon (0.058 μg L−1), metobromuron (0.027 μg L−1) and metribuzin (1.16 μg L−1), which present the same mode of action (inhibition of photosynthesis), and alachlor (0.161 μg L−1) and triclopyr (0.030 μg L−1), with different modes of action, inhibition of very long–chain fatty acid and mimic of plant hormones (auxins), respectively. The two latter independently acting pesticides presented no individual risk for aquatic organisms (see Table 5) and therefore were not considered for the combined toxicity effect determination.
The short-term RQ values for the co-occurring pesticides measured in the River Sado estuary surface water, which exert a similar mode of action, are as follows: 0.012 for bentazon, 0.058 for isoproturon and 0.58 for metribuzin, while the long-term RQ values are 0.0006 for bentazon, 0.19 for isoproturon and 0.61 for metribuzin. Since no individual aquatic EQS was found for metobromuron, the potential toxicity of this pesticide in the mixture was not considered. Overall, regarding the water quality criteria for the pesticide mixture present in the Sado estuary, no RQmix ≥ 1 was obtained (short-term RQmix = 0.65 and long-term RQmix = 0.81), and thereby, no potential adverse effects of the use of agricultural pesticides on aquatic organisms in the Lower Sado are expected.
Concerning sediments, individual EQSs were not found, even for pesticides with log Kow ≥ 3 as alachlor, chlorpyrifos and triclopyr. In the River Sado estuary, a maximum of four pesticides co-occurred in sediment samples, in stations 1 and 10, in July, with only two pesticides, bentazon (0.031 μg g−1 dw) and metribuzin (0.038 μg g−1 dw) presenting the same mode of action, in station 10. In this latter station, chlorpyrifos (0.032 μg g−1 dw) and desisopropyl-atrazine (0.103 μg g−1 dw) were also measured. In station 1, all the measured pesticides, alachlor (0.030 μg g−1 dw), chlorpyrifos (0.039 μg g−1 dw) and metribuzin (0.043 μg g−1 dw), present different modes of action (see Table 3), and desisopropyl-atrazine (0.058 μg g−1 dw) was also measured. However, since no individual EQSs were found for pesticides in sediments, the potential toxicity of pesticide mixtures in sediments was not determined.
Discussion
A new method was developed and validated for the analysis of 35 pesticides and three triazine pesticide degradation products in estuarine water and sediment. Regarding the use of the ASE method for the extraction of pesticide residues in sediment samples, the method presented better recoveries, between 77 and 125% for most pesticides, than those provided in other studies which used different approaches, such as QuEChERS or pressurised liquid extraction methods (e.g. Dagnac et al. 2005; Masiá et al. 2015). Even though those publications report slightly lower MQL values (0.15–15 ng g−1 dw) than the ones estimated in the present study for some pesticides, this is mainly due to the way MDLs and MQLs were determined. In the present study, the limits were estimated in accordance with the RELACRE guideline, which requires the use of the residual standard deviation of the calibration curve. However, the S/N ratio, a ratio which usually provides much lower MDL and MQL values than the ones obtained from estimations with the residual standard deviation of the calibration curve, is much more frequently seen in publications. We obtained a S/N ratio > 100 for the lowest-concentration spiked samples of the majority of the pesticides (Table S3, supplementary material). This demonstrates that if we had estimated MQLs based on the S/N ratio, we could have presented remarkably lower limits than those reported by the above-mentioned authors.
Concerning chromatographic method validation, the method permitted lower MDLs and better recoveries than those found in the literature (Chiron et al. 1994; Nogueira et al. 2004; Potter et al. 2007), even though the estuarine water matrix is more complex and poses more challenges than freshwater or water for human consumption. Also, as mentioned above, the MDLs and MQLs determined in the present study were estimated according to the RELACRE guideline. Since our study presented a S/N ratio > 1000 for most pesticides in the lowest standard solution injected during the linearity tests (Table S5, supplementary material), it can be assumed that the MDLs and MQLs would have been at least 10 to 100 times lower if they had been estimated experimentally by injecting increasingly less concentrated standard solutions of those pesticides.
In general, the validated methods show good linearity since R2 > 0.995 for all pesticides in both water and sediment methods, despite the differences in their chemical properties. Since the matrices are complex, the method also showed good precision and recoveries for both matrices, with all pesticides exhibiting an intermediate precision under 27%, and recoveries between 75 and 120%. The developed chromatographic method is also fast, as it only takes 12 min per sample and allows a high throughput for estuarine water samples. As for sediment samples, the method takes longer due to the additional sediment drying and ASE steps needed.
The present study showed that, from the 35 pesticides and triazine degradation products determined in the River Sado estuary in 2017 belonging to the fungicide, herbicide, insecticide and nematocide pesticide groups, only herbicides (20%) and insecticides (5.7%) were measured above their respective MQLs in surface water and sediments. A degradation product of legacy atrazine, desisopropyl-atrazine, was found in estuarine surface water and sediment samples in both sampling periods (March and July), suggesting higher persistence than the parent compound atrazine or the other two degradation products determined, desethyl-atrazine and desethyl-terbuthylazine. For instance, it is known that atrazine degradation products were more persistent than atrazine in pore water (Panshin et al. 2000), and that atrazine is rapidly incorporated into organic matter and clay colloids of sediments, becoming strongly absorbed and not extractable (Papilloud et al. 1996). Even though alachlor and isoproturon are EU-banned pesticides, they were found in the water compartment, which highlights the need to include legacy pesticides in monitoring programmes. In addition, dimethoate, metobromuron, metribuzin and triclopyr were also found, even if they are not authorised for rice crops, thus stressing the need to select a wide range of pesticides in monitoring programmes. The number of quantified compounds in water was higher in July, in line with the pesticide application period in the agricultural area located upstream of the estuary. Also, five herbicides (alachlor, bentazon, metobromuron, metribuzin and triclopyr) were measured before the beginning of the production season in the Lower Sado (in March), as well as after (in July), suggesting long-term aquatic exposure. From those, alachlor, linked to maize and vine production in Portugal, is listed as a priority substance. Alachlor (100%) and metribuzin (≥ 91%) were the most frequent pesticides, whereas bentazon (8.87 μg L−1) and metribuzin (1.61 μg L−1) were those found at higher concentrations. Alachlor has also been found in other Portuguese coastal systems, with lower maximum concentrations in the water compartment: 0.10 μg L−1 in the Mondego estuary in 2010–2011 (Cruzeiro et al. 2016a) and 0.012 μg L−1 in the Ria Formosa Lagoon in 2012–2013 (Cruzeiro et al. 2015). Nevertheless, the highest maximum value (6.1 μg L−1) was reported for the Albemarle-Pamlico estuarine system (USA) in 2000 (Powell et al. 2017). Regarding bentazon, which is highly soluble in water (500 mg L−1), it was found dissolved in the water phase in both sampling periods (max. 8.87 μg L−1), representing a low risk to the aquatic organisms of the Sado estuary. A maximum concentration of 3.4 μg L−1 has also been found in the Mondego estuary in 2014 and 2016 (Rodrigues et al. 2018). Dimethoate is a broad-spectrum organophosphate insecticide and acaricide which was found in the study area only in July, and only in estuarine water, with a maximum concentration of 0.18 μg L−1, thus representing a medium risk in long-term exposure to the aquatic organisms of the Sado estuary. Comparable maximum concentrations of 0.20 μg L−1 were found in 2012–2013 along the Ria Formosa Lagoon (Cruzeiro et al. 2015). Isoproturon is an herbicide only found in July in the study area, and only in estuarine water, with a maximum concentration of 0.11 μg L−1. The non-renewal of the approval of isoproturon as an active substance in accordance with Regulation 1107/2009 was performed only in 2016, and it may still be in use by Lower Sado farmers. In the Sado estuary, this herbicide poses a medium risk to aquatic organisms. A similar maximum value was found during 2010–2012 in Sjaelland, Denmark (McKnight et al. 2015). Surprisingly, no studies were found reporting worldwide metobromuron concentrations. In the Sado estuary, this herbicide was found only in the water compartment in a maximum concentration of 0.034 μg L−1. The EFSA peer review for the metobromuron risk assessment indicates a no-observed-effect concentration (NOEC) value of 500 μg L−1 for fish (EFSA 2014), and thereby no adverse effects were expected in the Sado estuary. Concerning metribuzin, this herbicide was found in the water of the San Francisco Bay (USA) by Klosterhaus et al. (2013) and in the Ria Formosa Lagoon by Cruzeiro et al. (2015) in very low concentrations (0.0002 μg L−1 and 0.0014 μg L−1, respectively). The concentrations mentioned were lower than the maximum concentration found in the Sado estuary (1.6 μg L−1), where it represents a medium risk to estuarine organisms. Finally, the herbicide triclopyr was found in the Sado estuary in the water (max. 0.079 μg L−1) and in both sampling periods, presenting a low risk to aquatic organisms, and a similar maximum concentration (0.068 μg L−1) was found during 2010–2012 in the Strymonas river basin (Northern Greece) by Papadakis et al. (2018).
Sediments were less contaminated, with a lower number of quantified compounds, lower frequencies (< 80%) and lower concentrations in the study area. This conclusion was also attained by Ccanccapa et al. (2016) in the Ebro river basin, in Spain. Bentazon was measured in sediments in the present study, showing temporal and spatial variations, since it was only measured in July (max. 0.037 μg g−1), and in the sampling stations with higher organic matter content (> 7%). This herbicide was also found in sediments of the Llobregat river basin (Spain) in 2005–2006, but in a lower concentration (0.009 μg g−1) (Ricart et al. 2010). The high soil adsorption and non-mobility properties (Pesticides Properties DataBase (PPDB), https://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm), as well as the moderate water solubility (1.05 mg L−1 at 20 °C, PPDB) of chlorpyrifos indicate its preferential partitioning into the organic matter rather than in water, resulting in a strong binding with sediment. Also, because chlorpyrifos is an unauthorised pesticide in Portugal (DGAV 2016), this broad-spectrum organophosphate insecticide was only measured in sediment samples of the Sado estuary, with a maximum concentration of 0.072 μg g−1. Similar maximum concentrations (0.036 μg g−1 and 0.044 μg g−1) were determined in the sediment samples collected from the Ebro river basin (Spain) in 2011 (Ccanccapa et al. 2016) and from the Orcutt Creek (Santa Maria estuary, California, USA) in 2008 (Smalling et al. 2013), respectively. Metribuzin was also found in the sediments of the Sado estuary, in a maximum concentration of 0.046 μg g−1. Triclopyr was found in a single measurement in the sediments (0.025 μg g−1) of the Sado estuary.
Maximum concentrations of 0.069 μg L−1 and 0.10 μg g−1 were measured in water and sediment for desisopropyl-atrazine, respectively. In sediments, in July, possibly due to higher temperatures that intensify biological transformation, as well as higher solar radiation that increases both direct and indirect photodegradation (Vieno et al. 2005; Musolff et al. 2009), a maximum value of 1 order of magnitude higher was determined when compared with the March sampling period. A concentration of 0.25 μg L−1 was measured during 2006–2007 in the water of the Bay of Vilaine (France) (Caquet et al. 2013). The toxicity of degradation products is not often taken into account, and no EQS or NOEC values have yet been set. Nevertheless, Ralston-Hooper et al. (2009) found an LC50,96 h (95% confidence interval), a 50% lethality, where a concentration of 7200 (6200–8400) μg L−1 was determined after exposing Hyalella azteca, an amphipod crustacean, to desisopropyl-atrazine. Desisopropyl-atrazine also significantly inhibited the metamorphosis of the larval prawn Penaeus monodon within the following concentration range: 3.5–917 μg L−1 (Mercurio et al. 2018). The desisopropyl-atrazine concentrations found in the present study were far from the toxicity values reported in the literature, indicating a low likelihood of effects on aquatic organisms.
Conclusions
A fast and almost solvent-free environmentally friendly method with a good performance for both estuarine water and sediment samples which enables achieving the MQLs required by the strict EU legislation for pesticide monitoring is proposed. The research carried out under this study established both seasonal and spatial occurrences of pesticides and degradation products on a temperate estuarine system (River Sado estuary). In surface water, alachlor, metribuzin and bentazon were the most frequent in March, while metobromuron also joined this group in July. Metribuzin and bentazon were at the highest concentrations. From the 35 studied pesticides, four (azoxystrobin, bentazon, imidacloprid and MCPA) are authorised for use in rice crops, the main crop of Lower Sado, being bentazon the only pesticide determined above quantification limits, which indicates compliance with pesticide use requirements since no risk to aquatic organisms was determined from bentazon use. Two pesticides already banned under EU regulations were quantified in surface water (alachlor and isoproturon), highlighting the need to include legacy pesticides in monitoring programmes. In general, the compounds measured in sediments were similar to the ones measured in the water, with the exception of the insecticide dimethoate and the herbicides isoproturon and metobromuron, which were measured only in water, and the insecticide chlorpyrifos, which appeared only in sediments. Sediments were less contaminated, with a lower number of quantified compounds, lower frequencies and lower concentrations in the study area. No potential high adverse effects were expected on the aquatic organisms of the River Sado estuary due to the use of agricultural pesticides in the Lower Sado, even when the potential combination effect of pesticide mixtures was considered. Nevertheless, individually, alachlor, dimethoate and isoproturon were found above acceptable aquatic short- and long-term risk values worldwide.
References
Adams CD, Thurman EM (1991) Formation and transport of deethylatrazine in the soil and vadose zone. J Environ Qual 20:540–547. https://doi.org/10.2134/jeq1991.00472425002000030007x
Bettencourt AM, Bricker SB, Ferreira JG, Franco A, Marques JC, Melo J-J, Nobre A, Ramos L, Reis CS, Salas F, Silva MC, Simas T, Wolff WJ (2004) Typology and reference conditions for Portuguese transitional and coastal waters. INAG & IMAR, Portugal
Caeiro S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Medeiros G, Painho M (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Indic 5:151–169. https://doi.org/10.1016/j.ecolind.2005.02.001
Caquet T, Roucaute M, Mazzella N, Delmas F, Madigou C, Farcy E, Burgeot T, Allenou J-P, Gabellec R (2013) Risk assessment of herbicides and booster biocides along estuarine continuums in the Bay of Vilaine area (Brittany, France). Environ Sci Pollut Res 20:651–666. https://doi.org/10.1007/s11356-012-1171-y
Ccanccapa A, Masia A, Navarro-Ortega A, Pico Y, Barceló D (2016) Pesticides in the Ebro River basin: occurrence and risk assessment. Environ Pollut 211:414–424. https://doi.org/10.1016/j.envpol.2015.12.059
Chèvre N, Loepfe C, Singer H, Stamm C, Fenner K, Escher BI (2006) Including mixtures in the determination of water quality criteria for herbicides in surface water. Environ Sci Technol 40:426–435
Chiron S, Dupas S, Scribe P, Barceló D (1994) Application of on-line solid-phase extraction followed by liquid chromatography-thermospray mass spectrometry to the determination of pesticides in environmental waters. J Chromatogr A 665:295–305
Cruzeiro C, Pardal MA, Rocha E, Rocha MJ (2015) Occurrence and seasonal loads of pesticides in surface water and suspended particulate matter from a wetland of worldwide interest-the Ria Formosa Lagoon, Portugal. Environ Monit Assess 187:669. https://doi.org/10.1007/s10661-015-4824-8
Cruzeiro C, Rocha E, Pardal MA, Rocha MJ (2016a) Environmental assessment of pesticides in the Mondego River Estuary (Portugal). Mar Pollut Bull 103:240–246. https://doi.org/10.1016/j.marpolbul.2015.12.013
Cruzeiro C, Rocha E, Pardal MA, Rocha MJ (2016b) Seasonal-spatial survey of pesticides in the most significant estuary of the Iberian Peninsula—the Tagus River estuary. J Clean Prod 126:419–427. https://doi.org/10.1016/j.jclepro.2016.03.005
Cuevas N, Martins M, Costa PM (2018) Risk assessment of pesticides in estuaries: a review addressing the persistence of an old problem in complex environments. Ecotoxicology 27:1008–1018. https://doi.org/10.1007/s10646-018-1910-z
Dagnac T, Bristeau S, Jeannot R, Mouvet C, Baran N (2005) Determination of chloroacetanilides, triazines and phenylureas and some of their metabolites in soils by pressurised liquid extraction, GC-MS/MS, LC-MS and LC-MS/MS. J Chromatogr A 1067:225–233. https://doi.org/10.1016/j.chroma.2004.11.058
DGAV (2016) Guia dos produtos fitofarmacêuticos - lista dos produtos com venda autorizada. Ministério da agricultura, florestas e desenvolvimento rural, Direção-geral de alimentação e veterinária (DGAV), Lisboa, Portugal. Available on: http://www.dgv.min-agricultura.pt/portal/page/portal/DGV/genericos?generico=4046540&cboui=4046540. Accessed 7 May 2019
Díez S, Lacorte S, Viana P, Barceló D, Bayona JM (2005) Survey of organotin compounds in rivers and coastal environments in Portugal 1999-2000. Environ Pollut 136:525–536. https://doi.org/10.1016/j.envpol.2004.12.011
Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities L327
Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy. Official Journal of the European Union L348.
Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Official Journal of the European Union L226.
Duodu GO, Goonetilleke A, Ayoko GA (2017) Factors influencing organochlorine pesticides distribution in the Brisbane River Estuarine sediment, Australia. Mar Pollut Bull 123:349–356. https://doi.org/10.1016/j.marpolbul.2017.09.022
EFSA (2014) Conclusion on the peer review of the pesticide risk assessment of the active substance metobromuron. European Food Safety Authority, Parma, Italy. EFSA J 12:3541. https://doi.org/10.2903/j.efsa.2014.3541
EFSA (2015) Scientific opinion on the effect assessment for pesticides on sediment organisms in edge-of-field surface water. European Food Safety Authority, Parma, Italy. EFSA J 13(7):4176
European Commission (2011) Technical guidance for deriving environmental quality standards under the Water Framework Directive. Guidance Document No 27. https://doi.org/10.2779/43816
European Union (2012) SCHER, SCCS, SCENIHR, opinion on the toxicity and assessment of chemical mixtures. https://doi.org/10.2772/21444
Goolsby DA, Thurman EM, Pomes ML, Meyer MT, Battaglin WA (1997) Herbicides and their metabolites in rainfall: origin, transport, and deposition patterns across the Midwestern and Northeastern United States, 1990-1991. Environ Sci Technol 31:1325–1333. https://doi.org/10.1021/es960847o
Jablonowski ND, Hamacher G, Martinazzo R, Langen U, Köppchen S, Hofmann D, Burauel P (2010) Metabolism and persistence of atrazine in several field soils with different atrazine application histories. J Agric Food Chem 58:12869–12877. https://doi.org/10.1021/jf103577j
Johnson I, Atkinson C, Hope S-J, Sorokin N (2007) Proposed EQS for Water Framework Directive Annex VIII substances: dimethoate. Science Report HOEP670085/SR17, UK Environment Agency
Klosterhaus S, Yee D, Sedlak M, Wong A, Sutton R (2013) Contaminants of emerging concern in San Francisco Bay: a summary of occurrence data and identification of data gaps. RMP Contribution 698. San Francisco Estuary Institute, Richmond 121 pp
Masiá A, Vásquez K, Campo J, Picó Y (2015) Assessment of two extraction methods to determine pesticides in soils, sediments and sludges. Application to the Túria River Basin. J Chromatogr A 1378:19–31. https://doi.org/10.1016/j.chroma.2014.11.079
McKnight US, Rasmussen JJ, Kronvang B, Binning PJ, Bjerg PL (2015) Sources, occurrence and predicted aquatic impact of legacy and contemporary pesticides in streams. Environ Pollut 200:64–76. https://doi.org/10.1016/j.envpol.2015.02.015
Mercurio P, Eaglesham G, Parks S, Kenway M, Beltran V, Flores F, Mueller JF, Negri AP (2018) Contribution of transformation products towards the total herbicide toxicity to tropical marine organisms. Sci Rep 8:4808. https://doi.org/10.1038/s41598-018-23153-4
Musolff A, Leschik S, Möder M, Strauch G, Reinstorf F, Schirmer M (2009) Temporal and spatial patterns of micropollutants in urban receiving waters. Environ Pollut 157:3069–3077. https://doi.org/10.1016/j.envpol.2009.05.037
Nogueira JMF, Sandra T, Sandra P (2004) Multiresidue screening of neutral pesticides in water samples by high performance liquid chromatography-electrospray mass spectrometry. Anal Chim Acta 505:209–215. https://doi.org/10.1016/j.aca.2003.10.065
Nunes M, Vernisseau A, Marchand P, Le Bizec B, Ramos F, Pardal MA (2014) Occurrence of PCDD/Fs and dioxin-like PCBs in superficial sediment of Portuguese estuaries. Environ Sci Pollut Res 21:9396–9407. https://doi.org/10.1007/s11356-014-2891-y
Panshin SY, Carter DS, Bayless ER (2000) Analysis of atrazine and four degradation products in the pore water of the vadose zone, central Indiana. Environ Sci Technol 34:2131–2137. https://doi.org/10.1021/es990772z
Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Makris KC, Papadopoulou-Mourkidou E (2015) A pesticide monitoring survey in rivers and lakes of Northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf 116:1–9. https://doi.org/10.1016/j.ecoenv.2015.02.033
Papadakis EN, Tsaboula A, Vryzas Z, Kotopoulou A, Kintzikoglou K, Papadopoulou-Mourkidou E (2018) Pesticides in the rivers and streams of two river basins in Northern Greece. Sci Total Environ 624:732–743. https://doi.org/10.1016/j.scitotenv.2017.12.074
Papilloud S, Haerdi W, Chiron S, Barceló D (1996) Supercritical fluid extraction of atrazine and polar metabolites from sediments followed by confirmation with LC-MS. Environ Sci Technol 30:1822–1826. https://doi.org/10.1021/es9503342
Porsbring T, Backhaus T, Johansson P, Kuylenstierna M, Blanck H (2010) Mixture toxicity from photosystem II inhibitors on microalgal community succession is predictable by concentration addition. Environ Toxicol Chem 29:2806–2813. https://doi.org/10.1002/etc.346
Potter TL, Mohamed MA, Ali H (2007) Solid-phase extraction combined with high-performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry analysis of pesticides in water: method performance and application in a reconnaissance survey of residues in drinking water in Greater Cairo, Egypt. J Agric Food Chem 55:204–210. https://doi.org/10.1021/jf062512o
Powell KW, Cope WG, LePrevost CE, Augspurger T, McCarthy AM, Shea D (2017) A retrospective analysis of agricultural herbicides in surface water reveals risk plausibility for declines in submerged aquatic vegetation. Toxics 5:21. https://doi.org/10.3390/toxics5030021
Ralston-Hooper K, Hardy J, Hahn L, Ochoa-Acuña H, Lee LS, Mollenhauer R, Sepúlveda MS (2009) Acute and chronic toxicity of atrazine and its metabolites deethylatrazine and deisopropylatrazine on aquatic organisms. Ecotoxicology 18:899–905. https://doi.org/10.1007/s10646-009-0351-0
RELACRE 13 (2000) Validação de Métodos Internos de Ensaio em Análise Química. RELACRE, Lisboa
Ricart M, Guasch H, Barceló D, Brix R, Conceição MH, Geiszinger A, López de Alda MJ, López-Doval JC, Muñoz I, Postigo C, Romaní AM, Villagrasa M, Sabater S (2010) Primary and complex stressors in polluted Mediterranean rivers: pesticide effects on biological communities. J Hydrol 383:52–61. https://doi.org/10.1016/j.jhydrol.2009.08.014
Rodrigues ET, Pardal MA, Salgueiro-González N, Muniategui-Lorenzo S, Alpendurada MF (2016) A single-step pesticide extraction and clean-up multi-residue analytical method by selective pressurized liquid extraction followed by on-line solid phase extraction and ultra-high-performance liquid chromatography-tandem mass spectrometry for complex matrices. J Chromatogr A 1452:10–17. https://doi.org/10.1016/j.chroma.2016.05.036
Rodrigues ET, Alpendurada MF, Ramos F, Pardal MA (2018) Environmental and human health risk indicators for agricultural pesticides in estuaries. Ecotoxicol Environ Saf 150:224–231. https://doi.org/10.1016/j.ecoenv.2017.12.047
Saraiva S, Pina P, Martins F, Santos M, Braunschweig F, Neves R (2007) Modelling the influence of nutrient loads on Portuguese estuaries. Hydrobiologia 587:5–18. https://doi.org/10.1007/s10750-007-0675-9
SEPA (2015) Supporting guidance (WAT-SG-53): environmental quality standards and standards for discharges to surface waters. Scottish Environment Protection Agency v6.0, 34
Serafim A, Company R, Lopes B, Pereira C, Cravo A, Fonseca VF, França S, Bebianno MJ, Cabral HN (2013) Evaluation of sediment toxicity in different Portuguese estuaries: ecological impact of metals and polycyclic aromatic hydrocarbons. Estuar Coast Shelf Sci 130:30–41. https://doi.org/10.1016/j.ecss.2013.04.018
Silva E, Cerejeira MJ (2015) Concentration addition-based approach for aquatic risk assessment of realistic pesticide mixtures in Portuguese river basins. Environ Sci Pollut Res 22:6756–6765. https://doi.org/10.1007/s11356-014-3857-9
Silva E, Daam MA, Cerejeira MJ (2015) Aquatic risk assessment of priority and other river basin specific pesticides in surface waters of Mediterranean river basins. Chemosphere 135:394–402. https://doi.org/10.1016/j.chemosphere.2015.05.013
Smalling KL, Kuivila KM, Orlando JL, Phillips BM, Anderson BS, Siegler K, Hunt JW, Hamilton M (2013) Environmental fate of fungicides and other current-use pesticides in a central California estuary. Mar Pollut Bull 73:144–153. https://doi.org/10.1016/j.marpolbul.2013.05.028
Stara A, Zuskova E, Kouba A, Velisek J (2016) Effects of terbuthylazine-desethyl, a terbuthylazine degradation product, on red swamp crayfish (Procambarus clarkii). Sci Total Environ 566–567:733–740. https://doi.org/10.1016/j.scitotenv.2016.05.113
Tasca AL, Puccini M, Fletcher A (2018) Terbuthylazine and desethylterbuthylazine: recent occurrence, mobility and removal techniques. Chemosphere 202:94–104. https://doi.org/10.1016/j.chemosphere.2018.03.091
Vasconcelos RP, Reis-Santos P, Fonseca V, Maia A, Ruano M, França S, Vinagre C, Costa MJ, Cabral H (2007) Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: a multi-metric index. Sci Total Environ 374:199–215. https://doi.org/10.1016/j.scitotenv.2006.12.048
Velisek J, Koutnik D, Zuskova E, Stara A (2016) Effects of the terbuthylazine metabolite terbuthylazine-desethyl on common carp embryos and larvae. Sci Total Environ 539:214–220. https://doi.org/10.1016/j.scitotenv.2015.08.152
Verbruggen EMJ, van den Brink PJ (2010) Review on mixture toxicity of pesticides to aquatic organisms. RIVM Report 601400001/2010
Vieno NM, Tuhkanen T, Kronberg L (2005) Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ Sci Technol 39:8220–8226. https://doi.org/10.1021/es051124k
Funding
The Portuguese Foundation for Science and Technology (FCT) supported the present study through a fellowship attributed to Elsa Teresa Rodrigues (SFRH/BPD/116152/2016), which was funded by the Human Potential Operating Programme of the European Social Fund and by the Portuguese budget through the Ministry of Education and Science. This study was developed within the FishFree Project (PTDC/AAG-TEC/4966/2014), supported by FCT through national funds (3599-PPCDT), and the co-funding of the European Regional Development Fund (POCI-01-0145-FEDER-016875). It was also supported within the PT2020 Partnership Agreement and COMPETE 2020 by the Centre for Functional Ecology Strategic Project (UID/BIA/04004/2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 15595 kb)
Rights and permissions
About this article
Cite this article
Rodrigues, E.T., Alpendurada, M.F., Guimarães, A. et al. The environmental condition of an estuarine ecosystem disturbed by pesticides. Environ Sci Pollut Res 26, 24075–24087 (2019). https://doi.org/10.1007/s11356-019-05751-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05751-5