Abstract
Cadmium (Cd) toxicity in agricultural crops is a widespread problem. Little is known about biochar and arbuscular mycorrhizal fungi (AMF) effect on Cd concentration in maize plant either applied separately or in combination. Current study was performed to demonstrate effects of biochar and Rhizophagus clarus on plant growth, photosynthesis activity, nutrients (P, Ca, Mg, Fe, Cu, and Mn), and Cd concentration in maize grown in Cd-spiked soil. The alkaline soil was spiked by Cd factor at three levels: 0 (Cd 0), 5 (Cd 5), and 10 (Cd 10) mg/kg; biochar factor at two levels: 0 and 1%; and mycorrhizal inoculum factor at two levels: MF0 and MF1 (R. clraus). Plants were harvested after 70 days of seed germination, and various morphological and physiological parameters, as well as elemental concentration and root colonization, were recorded. Addition of biochar increased plant biomass by 21% (Cd 5) and 93% (Cd 10), MF1 enhanced by 53% (Cd 0) and 69% (Cd 10), while biochar + MF1 enhanced dry plant biomass by 70% (Cd 0) and 94% (Cd 10). Results showed maximum increase of 94% (Cd 10) in plant biomass was observed in Cd-spiked soil. Root colonization decreased proportionally by increasing Cd concentration and at Cd 10, colonization was 36.7% and 31.7% for MF1 and biochar + MF1 treatments, respectively. Besides that, addition of biochar enhanced root attributes (root length, volume, and surface area) by 34–58% compared to control in Cd 10. The MF1 increased these attributes by 11–78% while biochar + MF1 enhanced by 32–61% in Cd-spiked soil. However, biochar + MF1 neutralized Cd stress in maize plant for gaseous attributes (assimilation rate, transpiration rate, intercellular CO2, and stomatal conductance). The MF1 enhanced Cd concentration in plant as it was 3.32 mg/kg in Cd 5 and 6.73 mg/kg in Cd 10 treatments while addition of biochar phytostabilized Cd and reduced its concentration in plants by 2.0 mg/kg in Cd 5 and 4.27 mg/kg in Cd 10. The biochar + MF1 had 2.9 mg/kg and 4.8 mg/kg Cd concentration in Cd 5 and Cd 10 plants, respectively. Phosphorus concentration was augmented in shoots (up to 26%) and roots (up to 20%) of maize plant in biochar-amended soil than control plants. In biochar + MF1, concentration of P was 1.01% and 0.73% in Cd 5 and Cd 10, respectively. It is concluded that biochar + MF1 treatment enhances plant biomass while addition of sole biochar reduced Cd uptake, slightly indifferent to earlier treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution exacerbated a worldwide problem and heavy metal toxicity attained primary concern (Torregroza-Espinosa et al. 2018). Increasing contamination of soil and water by cadmium (Cd) appeared as a major threat to the ecosystem, food security, and environmental sustainability (Rizwan et al. 2017, 2018). Cadmium is a most common heavy metal and has a non-biodegradable property which allows them to accumulate in soil and enter into food chain (Reimann et al. 2019). Previous studies have revealed that Cd is highly toxic to plants and inhibits plant growth and even plant death may occur (Rizwan et al. 2016a, 2012). Cadmium toxicity has been shown to reduce photosynthesis and mineral nutrition in plants (Rizwan et al. 2016b). Several bioremediation approaches have been suggested to reduce soluble heavy metals in soil (Abhilash et al. 2012; Teng et al. 2015). Among rhizospheric microbes, arbuscular mycorrhizal fungi (AMF) assist plant roots in enhancing access to water, and nutrients. It also plays a key role in phytostabilization where polyphosphate complexes are precipitated in plant roots and fungal mycelium by retaining heavy metal. Moreover, AMF forms specific structures known as radical mycelium, which improves plant adaptation to environmental stress (Wu et al. 2016). Besides that, AMF also alters physicochemical properties of rhizospheric soil and microbial community shaping rhizosphere which reduces metal phytoavailability (Ogar et al. 2015).
In recent years, biochar has gained growing attention as a soil amendment in immobilizing heavy metals such as lead (Pb), Cd, copper (Cu), chromium (Cr), zinc (Zn), and nickle (Ni) in soil (Zhang et al. 2018). Moreover, biochar improves crop productivity by enhancing nutrient concentration in soil (Xu et al. 2015). Large specific surface area, high surface charge density, porous structure, and pH of biochar might immobilize detrimental compounds in soil (Abbas et al. 2017). However, presence of surface functional groups (e.g., hydroxyl, carboxyl, phenol) on biochar contribute to reduction of heavy metal ions. Diversified surface structure of biochar makes it a suitable sorbent by significantly binding heavy metals to functional groups, complexation (Vithanage et al. 2015). Biochar addition to soil also benefits AMF, likely by modifying soil properties which assist in mycorrhizal spore germination, hyphal branching, and further growth (Hammer et al. 2014). Combination of biochar and AMF in polluted soil can alter nutrient cycling paradigm, and soil microbial community structuring, therefore, influences heavy metal speciation which immobilizes them in soil (Hammer et al. 2014).
Maize (Zea mays L.) is a major staple food and supposed as noticeable source of Cd intake by human (Anjum et al. 2016). Maize is also a favorable AMF colonizer (Cao et al. 2017) and it is frequently used in phytomanagement of polluted soils such as Cd-contaminated soils. Maize plant tolerates Cd stress and produces high biomass (Rizwan et al. 2017). Liu et al. (2014) studied Cd phytostabilization in maize plant inoculated with Rhizophagus constrictum, R. intraradices, and R. mosseae separately where Cd uptake was reduced. Biochar application to soil also phytostabilized Cd in maize plant (Zhao et al. 2016). Biochar addition to soils could significantly enhance heavy metal adsorption and immobilization capacity (Rizwan et al. 2016c). However, only one study (Liu et al. 2018) has been reported to our knowledge where R. intraradices in presence of biochar was evaluated for Cd concentration in maize plant. Results of the study by Liu et al. (2018) demonstrated about 79% increase in maize plant biomass, and 50–76% decrease in Cd in different parts of maize plants. It was hypothesized that biochar and R. clarus alone or in combination might alleviate Cd toxicity in maize by improving plant morphological, physiological characteristics and alter Cd concentration in plants. Thus, present study was designed to explore morpho-physiological growth of maize to three Cd toxicity concentrations: 0 (Cd 0), 5 (Cd 5), and 10 (Cd 10) mg/kg with two biochar levels: 0% and 1% and R. clarus inoculation at two levels: as MF0 and MF1. Moreover, as R. clarus has a peculiar role in P uptake, key attention was given to study plant and soil P.
Materials and methods
Biochar preparation and soil collection
The feedstock of common reed (Phragmites australis) was collected from vicinity of the research area of Cukurova University, Turkey. This feedstock is a promising energy plant due to its high production potential. The selected feedstock provides 4.4–6.9 kg of biomass per meter square per year and has the ability to survive in harsh winters (Garrido et al. 2017). Before making biochar, feedstock was ground to small size and sieved. The obtained material was passed through a 50-mesh screen away large lumps. Particle size was reduced to 0.7–0.8 mm, and it was dried at 110 °C for 24 h. The feedstock was charred at 550 °C for 2 h using a heating rate of 10 °C min−1 in a closed container under oxygen-limited conditions in a muffle furnace (RD50, REF-SAN, Turkey) (Sánchez et al. 2009). The residence time of preparing biochar was 2 h. Biochar was milled to pass through a 2-mm sieve. Finally, the biochar was analyzed for proximate (moisture content, ash, volatile matrix, and fixed carbon) (ASTM 2007), ultimate, and nutrients and the results have been given in Table 1.
The pH values of biochar were obtained in triplicate by mixing 1.0 g biochar in 20 mL deionized water with a little modification and an increased shaking time of up to 1.5 h. It was modified to ensure sufficient equilibration between solution and biochar surface. The EC was then determined using an Orion model 115A plus conductivity meter (Thermo Fisher Scientific, Waltham, MA) (Rajkovich et al. 2012). Total P, K, Ca, Mg, Fe, Mn, and B were determined after dry combustion by heating biochar up to 500 °C for 2 h and then being retained at 500 °C for 8 h. HNO3 (5 mL) was added to each vessel and digested at 120 °C until dry. Tubes were removed from block and allowed to cool before adding 1.0 mL HNO3 and 4.0 mL H2O2. Samples were placed back into a preheated block and processed at 120 °C until dryness, dissolved in 1.43 mL HNO3, volume was then raised with 18.57 mL deionized water and filtered (Enders and Lehmann 2012). The nutrient concentration in digested plant samples was analyzed by inductively coupled plasma optical emission spectrometry (Optima 8000 ICP–OES), PerkinElmer Inc., USA. Samples were analyzed using an environmental scanning electron microscope (ESEM) model Quanta FEI 650 (FEI, Netherlands). The surface of sample was coated with a thin, electric conductive gold film prior to observation (Fig. 1).
The surface horizon (0–15 cm) of Kiziltapir (Lithic Rpodoxeralf Xeralf Alfisol) soil series by USDA classification located at research farms of Cukurova University was collected, air-dried, passed through aperture sieve (2 mm mesh), and analyzed for its physiochemical properties (Table 2). The soil was sterilized at 121 °C (2 h) for removing soil microbes before using in the experiment.
Experimental design
An experiment was conducted in greenhouse of Cukurova University, Turkey. Exactly 3 kg of soil was added in each pot (21 cm diameter × 18 cm height). Three concentrations of Cd containing 0, 5, and 10 mg kg−1 Cd as CdSO4 were spiked in soil 1 week before adding the soil to pots. The Cd and biochar levels were selected based on the previous studies (Abbas et al. 2017; Ali et al. 2019). The Cd was spiked in soil by dissolving salt in 50 mL distilled water for each pot and then thoroughly mixed in soil. Spiking was done instead of using indigenously contaminated soil to evaluate mechanism of plant growth under different levels of Cd. After this, each concentration of Cd was divided into four sets containing control, biochar, mycorrhizal fungus (MF1), and biochar + MF1. The soil application of biochar was done just after Cd spiking in soil. For biochar treatment, 1% biochar (w/w) was added to pots, and half of pots containing biochar were inoculated with Rhizophagus clarus. The R. clarus (BEG248) was obtained from The International Bank for the Glomeromycota and further multiplied and propagated using sorghum (Sorghum bicolor) as host plant in greenhouse of Cukurova University (Ortas et al. 2017). Briefly, mycorrhizal inoculum of R. clarus (BEG142) was obtained from The International Bank of the Glomeromycota. Sorghum plants were used as trap culture for mycorrhizal spore propogation. The growth medium consisted of a mixture of soil, san, and peat at a ratio 3:1:1 by volume. The inoculum potential was estimated based on number of spores in 100 g of inoculum. Infected roots, hyphae, spores, and substrates were collected. Each mycorrhizal-inoculated pot was filled with 50 g (equivalent to ~ 700 spores) inoculum where as non-mycorrhizal treatments received equal amount of autoclaved inoculum with filtrate (< 20 mm) to obtain native microbial population. This study was designed with three factors such as Cd factor at three levels: 0 (Cd 0), 5 (Cd 5), and 10 (Cd 10) mg/kg; biochar factor at two levels: 0 and 1%; and mycorrhizal inoculum (R. clarus) factor at two levels: MF0 and MF1 forming 36 experimental units in total.
Each pot was provided with basal dose of ammonium nitrate (34% N), potassium dihydrogen phosphate (34% K2O and 52% P2O5), and muriate of potash (MOP, 60% K2O) at recommended rates of 160 kg N ha−1, 80 kg P2O5 ha−1, and 60 kg K2O ha−1 equivalents (NARC 2017). The study was conducted in a completely randomized design with factorial arrangement with three replications and plants were harvested after 70 days of growth. Environmental conditions of greenhouse were 25 ± 3 °C, 80 ± 3% relative humidity, and 16:8 h day/night cycle. Maize seeds were surface disinfected with 15% bleach for 15 min and washed with dH2O for three times. Five seeds of maize (cv. LG 37.10, Anadolu) were sown in each pot. After thinning of germinated plants, only three of them were allowed to grow further in each pot. The plants were irrigated with deionized water in maintaining 70% of field capacity moisture content in soil. Another split dose (half of recommended dose) of N was given 5 weeks after germination in solution form.
Plant harvesting and sampling
After 70 days of seed germination, aboveground and belowground maize plant biomass was harvested. Plant roots and shoots were gently rinsed with deionized water (Kachenko and Singh 2006). Root system of plant was placed in a transparent plastic tray filled with water to put on a scanner (Epson Perfection V700, Photo Long Beach, CA, USA). Various parameters of roots such as root length, root surface area, and root volume were examined by WinRHIZO Pro 3.10 (Regent Instruments Inc.) (Himmelbauer 2004). Finally, shoot and root biomass were oven-dried at 60 °C in paper bags to achieve a constant weight. All dried plant tissues (above- and belowground) were ground with a Tema mill, RM100 (Retsch Solutions in Miling and Sieving, Haan, Germany) to pass through a 0.5-mm mesh sieve, and samples were stored in sealed containers for further analyses.
Gas exchange measurement
Two weeks after Cd spiking in soil, gaseous exchange was investigated. Transpiration rate, assimilation rate, intercellular, and intracellular CO2 of maize plant leaves of all treatments were evaluated via a portable photosynthesis system (LI-COR Model 6800, Lincoln, NE, USA) with an extra clamp-on leaf cuvette that exposed 3 cm2 of leaf area. Temperature and light were 26 ± 0.2 °C and 1500 μmol m−2 s−1, respectively. The LI-6800-01 CO2 injector (LI-COR Lincoln, NE, USA) with a high-pressure liquefied CO2 cartridge source was used to keep a constant level of CO2 at 400 μmol m−2 s−1. The light was executed using the LI-6800-02P light source (LI-COR). Measurements were made at tips of leaf 7 to leaf 8 at midday.
Tissue nutrient analyses and root colonization
Total N concentration (%) was determined for above- and belowground biomass tissue using an elemental analyzer (Thermo Fisher Scientific FLASH 2000 Series CN Elemental Analyzer, Thermo Fisher Scientific, Waltham, USA). Nutrient concentration phosphorus (P), calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), and manganese (Mn), in digested biomass, were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES), Perkin Elmer, USA. To ensure reliability of equipment, chemicals, and digestion process, two blanks were included in each digestion batch. In determining mycorrhizal root colonization, after 70 days of seed germination, maize plant roots were cut into small pieces of 1 cm and further stained with Trypan Blue with some modifications described by Phillips and Hayman (Koske and Gemma 1989). A randomly selected aliquot of stained root segments suspended in lactoglycerin was spread in a Petri dish marked with a 1-cm grid. Further roots were cut to 1 cm and made three slides having 10 root segments on each to facilitate scanning, and viewed under a stereomicroscope at 10 to 80×. The proportion of length of each root segment which contained arbuscules, or hyphae was estimated to nearest 10%. Data were recorded as frequency distributions from samples containing 90 root segments from each plant. The percentage of root length with mycorrhizal endophytes in sample was then calculated from frequency distribution (Biermann and Linderman 1981). Further counting of colonized roots was performed using method described by Giovannetti and Mosse (1980) under microscope.
Cadmium determination in plant
An amount of ca. 0.5 g of homogenized sample was weighed and processed with a mixture of 2 mL of hydrogen peroxide and 6 mL of nitric acid in microwave digestion system with a set program. The subsequent extracts were redissolved in 10 mL of ultrapure water for succeeding analysis by ICP-OES. An amount of ca. 0.5 g of certified reference material (NIST 1573a tomato leaves) was digested in microwave as mentioned above for maize plant samples. The chemical solutions provided by sample treatment and those used to construct calibration curves were made in water containing 0.5% (v/v) HNO3, injected by autosampler of the Optima 8000 ICP-OES (PerkinElmer Inc., USA) (Wheal et al. 2011).
Soil pH, Cd, and P analysis
Soil pH value was determined in deionized water (1:2.5 soil/water ratio) where pH was observed after 30 min. Soil was digested by microwave-assisted digestion (method 3051A) for Cd analysis with HNO3 and HCl (3:1). Soil available P was determined based on Olsen (1954). For extraction of soil P, 5 g soil was taken with 0.5 M NaHCO3 and soil was shaken for 30 min. Filtrate was further processed and read on spectrophotometer.
Calculations and statistical analyses
Data were analyzed using PROC MIXED of Version 9.0 of the SAS System for Windows (SAS Institute, Inc., Cary, NC, USA) (Robert et al. 1997). Differences among the means were analyzed using three-way ANOVA with a completely randomized factorial design. Before ANOVA, the percentage of colonization was subjected to arcsine transformation to adjust them to the normal distribution. Statistical significance was postulated at p ≤ 0.05; biologically interesting differences with 0.05 < p ≤ 0.10 are also presented. Pearson’s correlation coefficient test was performed to estimate relationships between different factors and observed nutrient concentration.
Results
Biochar characterization
The pH and EC (dS m−1) of biochar were 8.98 and 2.38, respectively. Further details of biochar results are given in Table 1. Moreover, porous surface sturcture of biochar was observed in SEM (Fig. 1). As per International Biochar Initiative (IBI) biochar standards, biochar with ≥ 60% Corg lies in class 1 and biochar used in this study had 68%, whereas IBI suggests minimum 10% quantity which shows stability of biochar. Regarding liming class, it belonged to class 0 where CaCO3 – eq < 1%.
Shoot and root biomass
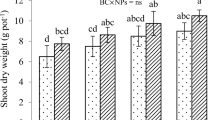
Maximum increase in dry shoot weight was observed in biochar + MF1 treatment in Cd 0 (32%) and Cd 5 (39%) than respective controls, whereas in the Cd 10, 50% increase was observed (Table 3). Besides that, biochar-amended soil increased 52% shoot weight in Cd 10. A similar trend was observed for dry root weight where 29% increase in biochar + MF1 followed by MF1 (27%) in Cd 0. The indifferent trend was followed in Cd 5 concentration, and biochar + MF1 had 54% increase in dry root weight than control while biochar addition enhanced 22% dry root weight. In Cd 10, root attributes were changed tremendously. Biochar + MF1 enhanced 40% dry root weight followed by biochar (31%) and MF1 (20%).
Root characterization and colonization
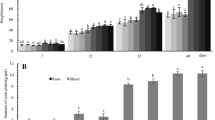
Root colonization was high in Cd 0, and it was maximum as 58% for MF1 treatment. Addition of biochar enhanced colonization by 62% (Fig. 2). Increasing Cd concentration decreased root colonization. In biochar + MF1, 95% root colonization was reduced in Cd 10. Plant root length was enhanced by increasing Cd concentration in comparison to control. In biochar + MF1-inoculated plants, 36% increase was noticed in Cd 0 while 38% enhancement was observed in Cd 5. Only Cd 10 reduced root length by 32% in biochar + MF1-inoculated plants (Table 3). Addition of biochar enhanced 37% root length in Cd 10 while it increased by 44% in MF1-inoculated plants of Cd 5. A similar trend was followed in root surface area where 35%, 37%, and 29% enhancement was observed by biochar + MF1 in Cd 0, Cd 5, and Cd 10, respectively. The MF1 inoculation boosted root surface area by 15%, 42%, and 19% in Cd 0, Cd 5, and Cd 10, respectively. Root volume increased by 33%, 36%, and 25% in biochar + MF1-inoculated plants for Cd 0, Cd 5, and Cd 10. The R. clarus alone contributed 19%, 39%, and 20% enhancement in root volume for Cd 0, Cd 5, and Cd 10, respectively.
Gaseous exchange
In Cd 0, addition of biochar + MF1 significantly enhanced assimilation rate by 27%. In 5 mg Cd kg−1 spiked soil, MF1 and biochar + MF1 enhanced assimilation to 22% and 24%, respectively (Fig. 3a). Increase in Cd concentration decreases assimilation. However, it increased by 10%, 17%, and 15% in biochar + MF1, MF1, and biochar-amended treatments respectively in soil spiked with Cd (10 mg Cd kg−1). Besides that, transpiration rate was maximum for biochar + MF1 by 34% in Cd 0 (Fig. 3b). Biochar + MF1 significantly improved transpiration rate in soil spiked with 5 mg Cd kg−1. Intercellular CO2 in biochar + MF1 (Cd 0) was enhanced by 27% and significantly improved in Cd 5 than control and biochar treatments (Fig. 3c). Stomatal conductance was enhanced by increasing Cd concentration (Fig. 3d).
Nutrient concentration in maize shoot and root
In shoot, biochar enhanced P by 21% (Cd 0) in comparison to control. Generally, P concentration was reduced by increasing Cd concentration. In biochar + MF1 (Cd 10), P uptake was reduced by 85% (Table 4). Similarly, biochar addition enhanced Ca uptake. Maximum Ca uptake of 25% was noticed in biochar-amended soil (Cd 5). Uptake of Mg was enhanced by 4–15% in all treatments, and Mg uptake was proportional to Cd concentration. Similarly, Fe uptake was enhanced by 10–15% in all treatments (Cd 5) while Cd 10 inversely affected Fe uptake. Cu uptake was augmented by Cd concentration, and Cd 10 enhanced Cu uptake by 19% in biochar-amended soil. Whereas, Cd 10 promoted Mn uptake by 6% in MF1-inoculated maize plant, while it was reduced by 6% in MF1-inoculated plant of Cd 5.
In root, biochar enhanced P by 17% (Cd 0) in comparison to control. Generally, P uptake was reduced by increasing Cd concentration. In biochar + MF1 (Cd 10), P uptake was reduced by 60% (Table 5). Biochar + MF1-inoculated plants stimulated P uptake by 15% in Cd 5. Biochar addition to soil enhanced Ca uptake, and biochar-amended soil had 27% increase in Cd 10. Combination of biochar + MF1 stimulated Ca uptake by 32%. Increasing Cd concentration reduced Mg uptake by 16% in biochar-amended soil (Cd 0) followed by 3% (Cd 10). At Cd 5, Fe uptake was enhanced in all treatments by 16–27% while Cd 10 inversely affected Fe uptake. Uptake of Cu was augmented at Cd 5 by 31% increase in MF1-inoculated soil.
Cd concentration in plant
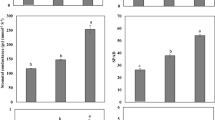
In whole plant, Cd concentration increased by enhancing Cd concentration in soil. Use of MF1 strongly assisted to Cd concentration by enhancing up to 43% in Cd 0. In biochar + MF1, it was enhanced by 15% only (Fig. 4a). Moreover, MF1 enhanced concentration by 20% and 21% in Cd 5 and Cd 10 concentrations respectively compared to control. In assessment to MF1, biochar amendment probably adsorbed Cd and reduced concentration in comparison to rest of treatments.
Soil pH, Cd, and P concentration
Soil pH results showed that addition of biological amendments did not have significant effect on soil pH. Maximum soil pH was observed for biochar + AMF in Cd 0 as 7.57. Maximum increase of 1.7% in soil pH was also observed in biochar + MF1 of Cd 0. Besides that, 28% more soil Cd was observed in Cd 5 (biochar + MF1) than control. Whereas, biochar and MF1 separately reduced soil Cd by 33% and 32% respectively in Cd 5. Similar trend was followed by biochar in Cd 10 by 31% reduction while MF1 reduced it only by 19%. At both levels of Cd, biochar + MF1 increased soil Cd by 8–28%.
During soil P estimation, reduction in soil P was noticed by increasing Cd concentration. Reduction of 36% soil P was noted in the biochar-amended soil at Cd 5 concentration whereas 8% more soil P was residing in biochar-amended soil at Cd 10 concentration than control (Fig. 4d). When MF1 was inoculated in presence of biochar, soil P reduced by 26% in Cd 10.
Pearson’s correlation cofficient test was performed to estimate relationships between different factors contibuted in bioremediation and plant nutrient concentration (S1). Data showed P and Ca in shoot were significantly correlated. Similarly, shoot and root micronutrients were significantly correlated and had positive interaction. Stomatal conductance and assimilation rate of plant were significantly correlated; moreover, soil pH was significantly correlated with assimilation rate.
Discussion
The Cd toxicity in agricultural soils is a major threat to agricultural productivity. Use of soil amendments and inoculants can reduce Cd uptake in plants and phytostabilize it in rhizosphere. In Cd 0, biochar induced more root colonization, whereas in Cd 5 and Cd 10, biochar + MF1 had still additive effect in root colonization. Liu et al. (2018) also observed similar effects in AM fungal treatment and biochar + AM fungal treatment. The mycorrhizal symbiotic relation extracts slow-release nutrients specially P from biohar and make it available to soil. Many studies demonstrated that biochar application positively effects on fungal abundance and root colonization (Mau and Utami 2014; Yamato et al. 2006). In the study, a significant decline in photosynthetic attributes was observed in control treatments at all Cd concentrations (Fig. 3). Studies conducted on maize plant under Cd stress concluded plant biomass reduction, assimilation rate alteration, decline in transpiration rate, and intercellular CO2 modifications (Akhtar et al. 2017). The extent of decline in gaseous exchange parameters varied in accordance with Cd concentration. Plants inoculated with MF1 and biochar + MF1 had more assimilation rate and stomatal conductance followed by biochar-amended soil. The reduction was aligned to increase Cd concentration. Decline in stomatal conductance of plant is one of critical approaches adopted by plant to bound assimilation rate with aim of keeping cellular turgidity (Bertholdi et al. 2018). Stomatal-related activities include stomatal closure and impairment of metabolic processes is associated with decrease in assimilation rate on Cd stress (Wu et al. 2006). It was corroborated that during Cd stress, assimilation rate declines to decrease in chlorophyll content and enzymatic activity responsible for CO2 fixation (Šimonová et al. 2007). It is also attributed to decrease intercellular CO2 subsequently reduced assimilation rate on exposure to Cd stress (Cui and Wang 2006). Results additionally proposed that reduction in gaseous exchange at higher Cd concentration could link with damages in photosynthetic apparatus (Akhtar et al. 2017).
Enhancement in Cd concentration decreased dry plant biomass with exception of MF1-inoculated plants (Table 3). Decrease in plant biomass could be due to drop in photosynthetic activity as leaf photosystems severely affected by Cd (Rizwan et al. 2017). Moreover, plants become stunted, and growth retardation occurs due to denaturing of proteins, where Cd disrupts H-S (hydrogen-sulfur) bond (LIN et al. 2007). Artiushenko et al. (2014) reported that in metal stress, plants develop an effective defense system to alleviate potential adversities. Such defensive system may comprise chelate synthesis, production of an osmolyte, enhanced enzymatic and non-enzymatic antioxidants, suberin lamella formation and cell wall lignification (Lux et al. 2010).
Nutrient absorption through root surface is strongly influenced by root surface area (Zhou et al. 2018). Root hairs increase root surface area. The Cd has an inhibitory effect on root proliferation, but present study showed contrary results (Table 3). Increase in Cd concentration enhanced root surface area linearly. Plants inoculated with MF1 and biochar + MF1 had more root proliferation capability. Root hairs are primary sites for contact between plant root and rhizosphere (Parker et al. 2000). Absorption of nutrients and heavy metals such as Cd, Pb, and Cr are absorbed on these sites (Nedelkoska and Doran 2000). Rate of mineral ion absorption via root cells is dependant on distance between absorbing cells from root tip in plant. Apical zone of root is a most active zone for cation uptake (Boominathan and Doran 2003). It shows proportion of apical surface area and whole root surface area could be a key factor for nutrient enhancement and heavy metal absorption. Maize crop is easily colonized by MF1 due to its property of high mycorrhizal dependency (Cao et al. 2017) and MF1 assist host plants in alleviating Cd toxicity (Liu et al. 2014). Chemophytostabilization practice for heavy metals (e.g., Zn, Cu, Cd, and Pb) enhances MF1 colonization in plant roots (Gucwa-Przepióra et al. 2007). Soil with a high dose of P abates MF1 colonization to plants. It further affects plant growth, and heavy metal bioavailability (Qiao et al. 2015).
Our study showed that concentration of P, Ca, Mg, and Fe in maize shoot was decreased (Table 4), whereas in maize root, only P was decreased by increasing Cd concentration (Table 4). Cadmium alters plasma membrane permeability and related membrane transporters which reduce micronutrient uptake and changes nutrient composition in plants (Sarwar et al. 2010). Consequently, plant observes nutrient deficiency leading to nutrient imbalance (Liu et al. 2017b). Additionally, alteration in nutrient uptake in plants could be due to inhibition in root growth and Cd-induced enzymatic activity (i.e., superoxide dismutase, peroxidase, catalase, polyphenol oxidase) (Chen et al. 2003). In present study, a significant variation in macronutrients and micronutrient concentration was observed in maize (Tables 4 and 5).
Cadmium concentration varied significantly and increased linearly by enhancing concentration of applied Cd. Tanwir et al. (2015) further corroborated results for Cd concentration against a number of maize cultivars. Plant cell wall accumulates Cd as first detoxification strategy to cope with Cd stress (Fernández et al. 2014). This metal sequestration is further aggravated by use of MF1 inoculation, and once metal accumulated in cell wall, their toxic effects are further mitigated by phytochelatins (Fernández-Fuego et al. 2017) that could be due to any type of chemical release by mycorrhizae into soil. Negatively charged cell wall of maize plant has significant potential in Cd+2 binding and retention for a longer time (Polle and Schützendübel 2003). Root is a primary contributor in rhizosphere played a key role in transforming architecture, modifying nutrient mobility, solubility, and their uptake (Keller et al. 2015). Root structuring and their activities further influence Cd concentration in plants. Besides that, addition of biochar significantly reduced the Cd concentration (Zheng et al. 2015). Moreover, porous structure of biochar, its high surface charge density, and large surface area highlight its ability in sorbing inorganic pollutants (Xu et al. 2013). Large surface area of biochar and oxygen-containing functional groups on surface adsorb Cd (Peng et al. 2017). Biochar addition could decrease Cd concentration in maize plant through altering their availability in soil. Alkaline properties of biochar ash influence Cd2+ hydrolysis by transforming Cd into Cd2+ as less mobile form. Addition of biochar into soil alters pH which reduces Cd-soluble form (Bashir et al. 2018). Hydrolysis and dissolution of biochar in soil increase pH and induce precipitation of Cd to Cd3 (PO4)2, resultantly increasing residual Cd in soil (Mehmood et al. 2018). Moreover, effective CEC of phragmites biochar is high which adsorbs cations (Cd, Ni, Fe) on biochar surface and binds them for a longer time (Erdem et al. 2017). Low bioavailability of toxic elements, high water retention, and CEC provided by biochar promote plant growth in polluted substrate (Fellet et al. 2014; Paz-Ferreiro et al. 2014). Because of high aromaticity and high surface area, biochar is considered as a strong and effective sorbent for organic and inorganic pollutants (Tong et al. 2014). Metal sorption occurs primarily due to an electrostatic interaction between positively charged metal ions and negative charge associated with delocalized π-electrons on aromatic structures (Harvey et al. 2011). AM fungal hyphae are much fine in diameter and can re-capture some of adsorbed nutrients and return them to their host plants. By this, a combined management of MF1 and biochar may lead to more closed nutrient cycles and more efficient fertilizer usage (Hammer et al. 2015). Previous studies reported that AMF could immobilize heavy metals in mycorrhizosphere and inhibit their translocation. İt can be possbile becasue of mechanisms (1) mycorrhizal hyphae can serve as a Cd pool to prevent Cd translocation to shoots by adsorbing and binding Cd (Meier et al. 2012), and “dilution effects” linked to an increased plant biomass and a decreased Cd allocation to aboveground tissues (Bai et al. 2008). The amendment of biochar into soil may influence AMF activity. Biochar changes soil physical and chemical conditions, dilution of AMF propagules and altered signalling between plants and AMF as well as sheltering AMF hyphae from fungal grazing. Cations are adsorbed on surface of biochar whereas it also provides access to hyphal branches for nutrient uptake where plant roots cannot get access due to large diameter (Liu et al. 2017a). However, above mechanisms varied with biochar types and growth environment.
Conclusion
By comparison, biochar application was potent in Cd stress alleviation. It increased maize plant growth, and altered gaseous exchange from plant leaves. Biochar as a soil amendment significantly persuaded soil alkalinization which contribute to Cd immobilization. Biochar + MF1 inoculation further showed positive interactive effect on Cd stress tolerance in maize plant, growth enhancement, and concentration of Cd in plant tissues. Alkaline pH of soil lowers available Cd concentration and root colonization in biochar + MF1 treatment which could assist as a tactic to be extensively adopted in alleviating Cd toxicity. These results propose that biochar treatment has most significant impact on reducing Cd concentration in maize plant.
References
Abbas T, Rizwan M, Ali S, Zia-ur-Rehman M, Qayyum MF, Abbas F, Hannan F, Rinklebe J, Ok YS (2017) Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol Environ Saf 140:37–47
Abhilash P, Powell JR, Singh HB, Singh BK (2012) Plant–microbe interactions: novel applications for exploitation in multipurpose remediation technologies. Trends Biotechnol 30:416–420
Akhtar T, Zia-ur-Rehman M, Naeem A, Nawaz R, Ali S, Murtaza G, Maqsood MA, Azhar M, Khalid H, Rizwan M (2017) Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ Sci Pollut Res 24:5521–5529
Ali S, Rizwan M, Noureen S, Anwar S, Ali B, Naveed M, Abd_Allah EF, Alqarawi AA, Ahmad P (2019) Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ Sci Pollut Res 26:11288–11299. https://doi.org/10.1007/s11356-019-04554-y
Anjum SA, Tanveer M, Hussain S, Ullah E, Wang LC, Khan I, Samad RA, Tung SA, Anam M, Shahzad B (2016) Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean Soil Air Water 44:29–36
Artiushenko T, Syshchykov D, Gryshko V, Čiamporová M, Fiala R, Repka V, Martinka M, Pavlovkin J (2014) Metal uptake, antioxidant status and membrane potential in maize roots exposed to cadmium and nickel. Biologia 69:1142–1147
ASTM A (2007) Book of standards volume 15.01: refractories, activated carbon. Advanced ceramics. American Society for Testing Materials, West Conshohocken
Bai J, Lin X, Yin R, Zhang H, Junhua W, Xueming C, Yongming L (2008) The influence of arbuscular mycorrhizal fungi on As and P uptake by maize (Zea mays L.) from As-contaminated soils. Appl Soil Ecol 38:137–145
Bashir S, Zhu J, Fu Q, Hu H (2018) Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 194:579–587
Bertholdi AAD, Costa VE, Rodrigues AL, de Almeida LFR (2018) Water deficit modifies the carbon isotopic composition of lipids, soluble sugars and leaves of Copaifera langsdorffii Desf. (Fabaceae). Acta Bot Bras 32:80–87
Biermann B, Linderman R (1981) Quantifying vesicular-arbuscular mycorrhizae: a proposed method towards standardization. New Phytol 87:63–67
Boominathan R, Doran PM (2003) Cadmium tolerance and antioxidative defenses in hairy roots of the cadmium hyperaccumulator, Thlaspi caerulescens. Biotechnol Bioeng 83:158–167
Cao J, Feng Y, He S, Lin X (2017) Silver nanoparticles deteriorate the mutual interaction between maize (Zea mays L.) and arbuscular mycorrhizal fungi: a soil microcosm study. Appl Soil Ecol 119:307–316
Chen Y, He Y, Luo Y, Yu Y, Lin Q, Wong M (2003) Physiological mechanism of plant roots exposed to cadmium. Chemosphere 50:789–793
Cui Y, Wang Q (2006) Physiological responses of maize to elemental sulphur and cadmium stress. Plant Soil Environ 52:523
Enders A, Lehmann J (2012) Comparison of wet-digestion and dry-ashing methods for total elemental analysis of biochar. Commun Soil Sci Plant Anal 43:1042–1052
Erdem H, Kınay A, Gunal E, Yaban H, Tutuş Y (2017) The effects of biochar application on cadmium uptake of tobacco. Carp J Env Sci 12:447–456
Fellet G, Marmiroli M, Marchiol L (2014) Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci Total Environ 468:598–608
Fernández R, Fernández-Fuego D, Bertrand A, González A (2014) Strategies for Cd accumulation in Dittrichia viscosa (L.) Greuter: role of the cell wall, non-protein thiols and organic acids. Plant Physiol Biochem 78:63–70
Fernández-Fuego D, Bertrand A, González A (2017) Metal accumulation and detoxification mechanisms in mycorrhizal Betula pubescens. Environ Pollut 231:1153–1162
Garrido RA, Reckamp JM, Satrio JA (2017) Effects of pretreatments on yields, selectivity and properties of products from pyrolysis of Phragmites australis (common reeds). Environments 4:96
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gucwa-Przepióra E, Małkowski E, Sas-Nowosielska A, Kucharski R, Krzyżak J, Kita A, Römkens PFAM (2007) Effect of chemophytostabilization practices on arbuscular mycorrhiza colonization of Deschampsia cespitosa ecotype Waryński at different soil depths. Environ Pollut 150:338–346
Hammer EC, Balogh-Brunstad Z, Jakobsen I, Olsson PA, Stipp SL, Rillig MC (2014) A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol Biochem 77:252–260
Hammer EC, Forstreuter M, Rillig MC, Kohler J (2015) Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl Soil Ecol 96:114–121
Harvey OR, Herbert BE, Rhue RD, Kuo L-J (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45:5550–5556
Himmelbauer M (2004) Estimating length, average diameter and surface area of roots using two different image analyses systems. Plant Soil 260:111–120
Kachenko AG, Singh B (2006) Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut 169:101–123
Keller C, Rizwan M, Davidian J-C, Pokrovsky O, Bovet N, Chaurand P, Meunier J-D (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241:847–860
Koske R, Gemma J (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488
Lin A-J, Zhang X-H, Chen M-M, Qing C (2007) Oxidative stress and DNA damages induced by cadmium accumulation. J Environ Sci 19:596–602
Liu LZ, Gong ZQ, Zhang YL, Li PJ (2014) Growth, cadmium uptake and accumulation of maize (Zea mays L.) under the effects of arbuscular mycorrhizal fungi. Ecotoxicology 23:1979–1986
Liu C, Liu F, Ravnskov S, Rubæk GH, Sun Z, Andersen MN (2017a) Impact of wood biochar and its interactions with mycorrhizal fungi, phosphorus fertilization and irrigation strategies on potato growth. J Agron Crop Sci 203:131–145
Liu M, Li Y, Cher YY, Deng SJ, Xiao Y (2017b) Effects of different fertilizers on growth and nutrient uptake of Lolium multiflorum grown in Cd-contaminated soils. Environ Sci Pollut Res 24:23363–23370
Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, Wang Y (2018) Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 194:495–503
Lux A, Martinka M, Vaculík M, White PJ (2010) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Mau A, Utami S (2014) Effects of biochar amendment and arbuscular mycorrhizal fungi inoculation on availability of soil phosphorus and growth of maize. J Degrade Min Lands Manage 1:69–74
Mehmood S, Rizwan M, Bashir S, Ditta A, Aziz O, Yong LZ, Dai Z, Akmal M, Ahmed W, Adeel M (2018) Comparative effects of biochar, slag and ferrous–Mn ore on lead and cadmium immobilization in soil. Bull Environ Contam Toxicol 100:286–292
Meier S, Borie F, Bolan N, Cornejo P (2012) Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit Rev Environ Sci Technol 42:741–775
NARC (2017) How many varieties have been developed by PARC and which are the varieties recommended for general cultivation? Pakistan Agricultural Research Council, Islamabad
Nedelkoska TV, Doran PM (2000) Hyperaccumulation of cadmium by hairy roots of Thlaspi caerulescens. Biotechnol Bioeng 67:607–615
Ogar A, Sobczyk Ł, Turnau K (2015) Effect of combined microbes on plant tolerance to Zn–Pb contaminations. Environ Sci Pollut Res 22:19142–19156
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. United States Department of Agriculture, Washington
Ortas İ, Rafique M, Akpinar C, Kacar YA (2017) Growth media and mycorrhizal species effect on acclimatization and nutrient uptake of banana plantlets. Sci Hortic 217:55–60
Parker JS, Cavell AC, Dolan L, Roberts K, Grierson CS (2000) Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell 12:1961–1974
Paz-Ferreiro J, Lu H, Fu S, Méndez A, Gascó G (2014) Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth 5:65–75
Peng H, Gao P, Chu G, Pan B, Peng J, Xing B (2017) Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ Pollut 229:846–853
Polle A, Schützendübel A (2003) Heavy metal signalling in plants: linking cellular and organismic responses, plant responses to abiotic stress. Springer, Berlin, pp 187–215
Qiao Y, Crowley D, Wang K, Zhang H, Li H (2015) Effects of biochar and arbuscular mycorrhizae on bioavailability of potentially toxic elements in an aged contaminated soil. Environ Pollut 206:636–643
Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, Lehmann J (2012) Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils 48:271–284
Reimann C, Fabian K, Flem B (2019) Cadmium enrichment in topsoil: separating diffuse contamination from biosphere-circulation signals. Sci Total Environ 651:1344–1355
Rizwan M, Meunier J-D, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209:326–334
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016a) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016b) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23:17859–17879
Rizwan M, Ali S, Qayyum MF, Ibrahim M, Zia-ur-Rehman M, Abbas T, Ok YS (2016c) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248
Rizwan M, Ali S, Qayyum MF, Ok YS, Zia-ur-Rehman M, Abbas Z, Hannan F (2017) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health 39:259–277
Rizwan M, Ali S, Abbas T, Adrees M, Rehman MZ, Ibrahim M, Abbas F, Qayyum MF, Nawaz R (2018) Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J Environ Manage 206:676–683
Robert S, Torrie J, Dickey D (1997) Principles and procedures of statistics: a biometrical approach. McGraw-Hill, New York
Sánchez ME, Lindao E, Margaleff D, Martínez O, Morán A (2009) Pyrolysis of agricultural residues from rape and sunflowers: production and characterization of bio-fuels and biochar soil management. J Anal Appl Pyrolysis 85:142–144
Sarwar N, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90:925–937
Šimonová E, Henselová M, Masarovičová E, Kohanová J (2007) Comparison of tolerance of Brassica juncea and Vigna radiata to cadmium. Biol Plant 51:488–492
Tanwir K, Akram MS, Masood S, Chaudhary HJ, Lindberg S, Javed MT (2015) Cadmium-induced rhizospheric pH dynamics modulated nutrient acquisition and physiological attributes of maize (Zea mays L.). Environ Sci Pollut Res 22:9193–9203
Teng Y, Wang XM, Li LN, Li ZG, Luo YM (2015) Rhizobia and their bio-partners as novel drivers for functional remediation in contaminated soils. Front Plant Sci 6:32
Tong H, Hu M, Li F, Liu C, Chen M (2014) Biochar enhances the microbial and chemical transformation of pentachlorophenol in paddy soil. Soil Biol Biochem 70:142–150
Torregroza-Espinosa AC, Martinez-Mera E, Castaneda-Valbuena D, Gonzalez-Marquez LC, Torres-Bejarano F (2018) Contamination level and spatial distribution of heavy metals in water and sediments of El Guajaro reservoir, Colombia. Bull Environ Contam Toxicol 101:61–67
Vithanage M, Rajapaksha AU, Zhang M, Thiele-Bruhn S, Lee SS, Ok YS (2015) Acid-activated biochar increased sulfamethazine retention in soils. Environ Sci Pollut Res 22:2175–2186
Wheal MS, Fowles TO, Palmer LT (2011) A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal Methods 3:2854–2863
Wu F-B, Jing D, Jia G-X, Zheng S-J, Zhang G-P (2006) Genotypic difference in the responses of seedling growth and cd toxicity in rice (Oryza sativa L.). Agric Sci China 5:68–76
Wu S, Zhang X, Chen B, Wu Z, Li T, Hu Y, Sun Y, Wang Y (2016) Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environ Exp Bot 122:10–18
Xu X, Cao X, Zhao L (2013) Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:955–961
Xu C-Y, Hosseini-Bai S, Hao Y, Rachaputi RC, Wang H, Xu Z, Wallace H (2015) Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ Sci Pollut Res 22:6112–6125
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495
Zhang C, Shan BQ, Zhu YY, Tang WZ (2018) Remediation effectiveness of Phyllostachys pubescens biochar in reducing the bioavailability and bioaccumulation of metals in sediments. Environ Pollut 242:1768–1776
Zhao B, Xu R, Ma F, Li Y, Wang L (2016) Effects of biochars derived from chicken manure and rape straw on speciation and phytoavailability of cd to maize in artificially contaminated loess soil. J Environ Manag 184:569–574
Zheng R, Chen Z, Cai C, Tie B, Liu X, Reid BJ, Huang Q, Lei M, Sun G, Baltrėnaitė E (2015) Mitigating heavy metal accumulation into rice (Oryza sativa L.) using biochar amendment—a field experiment in Hunan, China. Environ Sci Pollut Res 22:11097–11108
Zhou XB, Jia ZM, Wang DB (2018) Effects of limited phosphorus supply on growth, root morphology and phosphorus uptake in citrus rootstocks seedlings. Int J Agric Biol 20:431–436
Acknowledgements
This work was supported by TÜBİTAK under the program of Research Fellowship Programme for International Researchers [grant number 21514107–115.02-188888].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Rafique, M., Ortas, I., Rizwan, M. et al. Effects of Rhizophagus clarus and biochar on growth, photosynthesis, nutrients, and cadmium (Cd) concentration of maize (Zea mays) grown in Cd-spiked soil. Environ Sci Pollut Res 26, 20689–20700 (2019). https://doi.org/10.1007/s11356-019-05323-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05323-7