Abstract
A field trial was carried out to examine the influence of residual acidified biochar (a 3:100 (w/w) mixture of citric acid and citrus wood biochar) on soil properties, growth, water status, photosynthetic efficiency, metal accumulation, nutrition status, yield, and irrigation use efficiency (IUE) of maize grown under salty soil and metal-contaminated irrigation water. The acidified biochar (ABC) was applied to faba bean in 2016/2017 in saline soil (electrical conductivity (ECe) 7.6 dS m−1) with three levels 0, 5, and 10 t ha−1 with 4 replications. The results summarized that after a year of utilization, acidified biochar still significantly affected the growth and yield by improved soil properties and decreased maize uptake of sodium by transient sodium (Na+) binding because of its high adsorption capacity. Growth, physiology, and maize yields were influenced positively by ABC application, under metal-contaminated irrigation water. It was summarized that the utilization of ABC had a significant residual (P ≤ 0.05) effect on reducing nickle (Ni), lead (Pb), cadmium (Cd), and chromium (Cr) accumulation in maize under heavy metal–contaminated irrigation water. However, more detailed open-field experiments should be carried out to assess the long-term residual impacts of ABC for sustaining maize production under biotic stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is the third largest planted crop worldwide after wheat and rice (Malcovska et al. 2014) and becomes the most important essential cereal and feed crop in Egypt and other nations (FAO 2017), performing an increasingly considerable role in supporting food security and stabilizing of socio-economic status. Global maize production has expanded by nearly 50% in the course of recent decades. Maize production under semi-arid and arid zones in many African countries including Egypt is severely obstructed by several abiotic stresses such as drought, salinity, heavy metal ions, and low soil fertility (Abdel Latef and Tran 2016). Because of the shortage of freshwater, growers in some parts in developing countries like Egypt are forcing to utilize partially blending freshwater with raw sewage water to irrigate and manage their crops (Abdel-Azim and Allam 2005). This water occasionally contains heavy metals (i.e., Cd, Pb, Ni, Cr, Hg, etc.) over as far as possible causing serious problems for soil and various environmental habitats (i.e., plants, biota, and human) via translocation into the plants and getting into food chain due to their prolonged poisonous impacts (Vodyanitskii 2016; Etesami 2017). Recently restricted water resources having acceptable quality has resulted in the reuse of water with less quality such as agricultural drainage water in these areas (Ali et al. 2017a, b). Over the past few years, there has been a prominent increase in research on the use of naturally derived and/or organic materials to be integrated into sustainable agriculture. Some of these environmentally friendly bio-stimulants may increase yield and resistance to different biotic and abiotic stresses. The most encouraging choices incorporate biochar (Akhtar et al. 2015; Calvo et al. 2014; Semida et al. 2019), which can efficiently improve soil fertility, stimulate plant growth, and elevate plant resilience to unfavorable conditions. Various investigations had been documented that adding biochar develops soil physical properties such as water-holding capacity, bulk density, and permeability; chemical properties (i.e., nutrient availability, cation exchange capacity (CEC) and soil pH); and soil biota (i.e., microbial population, and microbial activities), thus ultimately enriching crop yield, and it is very important for the improvement of degraded topsoil (Abd El-Mageed et al. 2020; Glaser et al. 2002; Lehmann et al. 2006; Yuan and Xu 2011). The availability of toxic elements to plant growth (i.e., Al, Cu, Fe, Ni, Co, Pb, and Mn) can be reduced by biochar addition, maybe due to surface adsorption and precipitation (Kloss et al. 2014; Masto et al. 2013). Utilizing soil alterations like biochar for diminishing the availability of toxic metals and its uptake by maize had been archived by Ali et al. (2017a, b) and Al-Wabel et al. (2015). They reported that biochar fundamentally decreased extractable soil heavy metal content, indicating metal immobilization, increasing shoot dry biomass of maize. In addition, the utilization of biochar significantly reduced the contents of Fe, Mn, Zn, Cu, and Cd in maize. Numerous studies report the ameliorative effects of applied biochar on crops grown in polluted soils by several heavy metal elements such as Cd and Pb (Ali et al. 2017a, b; Al-Wabel et al. 2015). Soil of Egypt is mostly categorized by slight alkaline to alkaline which is mostly because of its high temperature, little rainfall, and low relative humidity, and the evaporation rate is very high leading to corrupted soil (Abd-Alla et al. 2014; Abd El-Mageed et al. 2018, 2019; Semida et al. 2014). Soil alkalinity is deliberated as the greatest problem in semi-arid and arid zones like Egypt and characterized by high pH values (7.5–8.7) (Clark 1996). Alkaline soils are characterized by reduced availability of macro and micronutrients (N, P, K, Cu, Fe, Zn, and Mn); also, alkali stress generally includes a grouping of stresses, osmotic, ion-induced damage, and increased soil pH (Chen et al. 2011; Lynch and St Clair 2004). Additionally, it is widely reported that supplying biochar to soils has resulted in increases in pH (Chan et al. 2007; Uchimiya et al. 2011; Bell and Worrall 2011). For this reason, we added citric acid to citrus wood biochar (3:100 (w/w)) to decrease the pH of biochar in our previous experiment. However, to the best of our knowledge, no studies have been carried out to study the response of maize to residual acidified biochar under saline soil and metal-contaminated irrigation water in open-field condition. Therefore, the main objective of the current study is to determine how residual acidified biochar influences on the soil properties, growth, morpho-physiological responses, photosynthetic efficiency, and accumulation of toxic metals in maize plants irrigated by polluted water by hazardous limits of heavy metal ions.
Materials and methods
Experimental site, setup, and growth conditions
A field trial was achieved in a private farm positioned at Fayoum, Egypt (29° 02′ N and 30° 23′ E) in the summer season of 2017 and the experimental layout was a randomized block design with 4 replicates. Every experimental plot area was 9 m2; 15 m length × 0.6 m width; and about 0.25 m between plants within rows. A year before the current experiment (2016/2017), the selected soil was treated with acidified biochar at a rate of 0 t ha–1 (ABC0) as a control, 5 t ha–1 (ABC5), and 10 t ha–1 (ABC10) (a 3:100 (w/w) mixture of citric acid (CA) and citrus wood biochar (BCH), respectively) for different experiments. The pH of citrus wood biochar was 8.82 and after acidification process (addition of citric acid), the pH changed to 7.6. The main characteristics of acidified biochar, soil biota, and soil physicochemical as influenced by acidified biochar applications were assessed according to the procedures of Page et al. (1982) and Klute (1986) and are listed in Tables 1, 2, and 3.
In the summer of 2017, the residual impacts of the previously applied (2016/2017) acidified biochar (RABC) were studied using healthy and uniform grains of Zea maize (hybrid 360) which were sown on 15 April and terminated on 5 August in 2017. Gains were sown 5 cm away from the drip line at a depth of 4 cm and drip irrigated with one line and one dripper per plant giving 4.0 L h−1. Chemical fertilization was practiced at the recommended rate for corn production in this area: 160 kg N ha−1, 75 kg P ha−1, and 100 kg K ha−1. The cultural practices (i.e., pest and disease management) were the same as local commercial crop production.
Measurements of growth and yield characteristics
90 days after planting (DAT), 5 plants were carefully removed from every experimental plot (n = 20). The flag leaf area (FLA) was estimated using the following formula: FLA = length of mean flag leaf × width of mean flag leaf × 0.75
The lengths of shoots were estimated by meter scale, and the numbers of leaves per plant were counted. The weight of 1000 grains (g) was taken from every plot and then these grains were weighted using an electronic balance. For each treatment, grains were cleaned and weighted and grain yield (kg ha−1) was calculated. The shelling ratio (SR) was determined by utilizing the following formula.
Shoots were weighed to record their fresh weights and then sited in an oven at 70 °C until the constant weight and the dry matter were recorded. Irrigation use efficiency was calculated (as kg yield per m3 water) for different treatments after harvest utilizing the equation of Jensen (1983).
Determinations of relative water content and membrane stability index
Fully expanded fresh leaves (n = 20) were used for the determination of relative water content (RWC%) and membrane stability index (MSI%). After excluding the midrib, leaves were processed according to the following methods. The RWC% was determined according to Hayat et al. (2007). Fully expanded leaf discs (10 discs) with a 2-cm diameter were weighed (fresh mass) and directly floated on distilled water in Petri dishes for 8 h, in a dark room; the turgid mass was then weighted. Discs’ dry mass was weighted after dehydration at 70 °C until the constant weight. The following equation was used to calculate the values of RWC:
The MSI% of the cell was assessed by Premchandra et al. (1990) methods. The leaf sample (0.2 g) was placed in a test tube containing 10 mL of distilled water. Tubes were heated at 40 °C in a water bath for 30 min, and the electrical conductivity (EC1) of the solution was registered using a conductivity meter. A second sample was boiled at 100 °C for 10 min, and the conductivity was measured (EC2). The MSI was calculated using the formula:
where EC1 is the EC of the solution at 40 °C and EC2 is the EC of the solution at100 °C.
Chlorophyll fluorescence measurements
Chlorophyll fluorescence was measured on two different sunny days using a portable fluorometer (Handy PEA, Hansatech Instruments Ltd., Kings Lynn, UK). One leaf (the same age) was chosen per plant from five plants from each plot. A total of 20 measurements per treatment were made. Fluorescence measurements (Fv/Fm) were calculated according to Maxwell and Johnson (2000). The performance index of photosynthesis based on equal absorption (PIABS) was calculated as reported by Clark et al. (2000). Stomatal conductance (gs) was measured (n = 12) with a portable photosynthetic system (CIRAS-2, PP Systems, Hitchin, UK). Also, the leaf chlorophyll content (SPAD value) (n = 20) was determined with SPAD502 (KONICAMINOLTA. Inc., Tokyo).
Nutrients and heavy metal determinations
Maize leaves were dried and grounded to powdered form then the contents of macronutrients (nitrogen (N), phosphorus (P), potassium (K), and calcium (Ca)) and heavy metals (lead (Pb), nickel (Ni), cadmium (Cd), Chromium (Cr), and copper (Cu)) were determined by using an atomic absorption spectrophotometer (Perkin-Elmer, Model 3300) as depicted by Chapman and Pratt (1961) procedure.
Statistical analysis
Data collected were exposed to ANOVA and carried out by Genstat statistical package (VSN International Ltd, Oxford, UK).
Results and discussion
Effect of residual acidified biochar on soil properties
As listed in Table 3, physical properties (i.e., total porosity, useful pores, water-holding pores, field capacity), chemical properties (i.e., nitrogen content (N%), phosphorus (P mg kg–1), and potassium (K mg kg–1 soil), and soil biota (number of bacterial cells/g soil) enhanced with the increasing concentrations of RABC (Table 3). In contrast, ECe, pH, and bulk density showed significant decreases with increasing rates of RABC. Therefore, RABC has the potential to mitigate salinity-induced reductions in mineral uptake and may be a novel technique to alleviate the deleterious effects of salinization in semi-arid and arid zones and contaminated soils (Abd El-Mageed et al. 2020; Kim et al. 2016; Thomas et al. 2013). The addition of 10 t ha−1 of acidified biochar yielded the most positive impacts on soil biota and soil physicochemical properties. The RABC increased favorable soil characteristics, namely total porosity, water-holding pores, field capacity, available water content, organic matter %, N content, K+ content, and cell bacterial number in the topsoil. The analysis of soil properties after a year revealed that the application of 10 t ha−1 of acidified biochar increased organic matter by 52.38% compared with control (0 t ha−1), whereas N, P, and K soil contents were increased by 13.33, 76.86, and 75.74%, respectively. The correlation between the beneficial outcomes of RSBC on soil quality and plant nutritional status indicates that improving soil quality factors will improve plant health. This is logical because RSBC appeared to improve the formation of micro-aggregates over time, which improves soil porosity (Brodowski et al. 2006; Cheng et al. 2006). There was also a significant reduction in bulk density, ECe, and pH. These effects are further supported by decreases in bulk densities of treated soils; the low bulk density of biochar (BCH) (~ 0.79 g m−3) and its highly stable organic carbon have the possibility to decrease soil bulk density and increase its total porosity (Gwenzi et al. 2014). However, soil bulk density was reduced by 91.19%. Furthermore, total porosity (TP), water-holding pores (WHP), useful pores (UP), field capacity (FC), and available water (AW) were increased by 85.27, 61.50, 55.45, 76, and 78.99%. Acidified biochar is fundamental to improve the biological activity where the bacterial cell number was increased from 2.0 × 106 to 6.5 × 106 cell g−1 soil. This increment might be because of the higher content of carbon as a source of biological energy for soil microorganisms and increases of available water. Our findings are in accordance with those of Asai et al. (2009), Antonio et al. (2013), and Laird et al. (2010). They reported that some physical soil properties such as porosity, water-holding capacity, bulk density, and soil structure; and particle size distribution can likewise improve with the utilization of biochar. The results proved that supplying acidified biochar to salty soil results in more micropores (i.e., useful pores and increased water-holding capacity) which increased capillary potential. Similarly, the increased porosity and decreased bulk density of soil are similar to the results of Brewer et al. (2014) and Sun et al. (2012). They found that the highly porous structure of biochar creates interstitial space within the BCH soil matrix. These findings also show that in addition to improving soil quality of saline soils, RABC can be applied to decrease the uptake of Na+ (Akhtar et al. 2015) and Cd (Rizwan et al. 2018).
Effect of residual acidified biochar on growth characteristics
Data in Table 4 show that residual acidified biochar has a dynamic role in enhancing growth attributes of maize grown under salty soil and irrigated by contaminated water. As an effect of RABC, growth attributes of maize increased considerably. Plant height ranged between 182 (0 t ha−1 acidified biochar) and 212 cm (10 t ha−1 acidified biochar) whereas the number of leaves/plant increased from 11.2 to 14.4 cm. However, RABC may cause an increment in other growth characteristics of plants such as flag leaf area, stem diameter, shoot fresh, and dry weight which increased from 135.2 to 180.5 dm2, 2.2 to 3.1 cm, 472.3 to 682.59 g, and 401.5 to 579.47 g, respectively. Plant height, stem diameter, leaf area plant−1, and shoot dry plant−1 (g) were significantly (P ≤ 0.05) decreased under control treatment (0 t ha−1 ABC). However, RABC influence on growth was more important under a biotic stress (saline soil and irrigated by contaminated water). Results indicated that dry matter of plant was adversely influenced by salt stress (untreated soil). Moreover, the dry weight of the whole plant as well as that of individual plants was higher in RABC10 than RABC5 or RABC0. These findings are in agreement with those of Wolka and Melaku (2015), Haider et al. (2015), and Rady et al. (2018). Residual acidified biochar improved maize plant growth because of the improvement of soil-plant water relations (improved relative water content and membrane stability index) and stimulated photosynthesis by increasing the electron transport rate of photosystem II (Fig. 1, Haider et al. 2015). The other mechanisms by which residual acidified biochar–enhanced maize growth may include increased soil moisture content, modified soil properties (i.e., organic matter content; NPK content and availability; total porosity, water-holding pores; useful pores), and increased the activity of soil biota (number of bacterial cells/g soil, Table 3). Therefore, RABC may overcome the injurious effects caused by a biotic stress and enhancement maize growth.
Maize water status
Responses of MSI and RWC of plants for RABC are proposed in Fig. 1. Statistical analysis carried out on MSI and RWC showed a significant difference (P ≤ 0.05) among RABC levels. The values of membrane stability index (MSI) and relative water content (RWC) were improved with increasing of RABC. The height values of MSI and RWC (71.8 and 80.5%) were obtained under RABC10 compared with that under RABC0 treatment (65.8 and 54.3%). As compared with RABC0 (control, 0 t ha−1) treatment, plants treated by either 5 or 10 t RABC t ha−1 revealed a significant increase (P ≤ 0.05) in dehydration resilience as far as improved MSI and RWC% (Fig. 1). Relative water content may be mirroring the metabolic movement in tissues of plants and it is considered a measure of water relations of plant (Sinclair and Ludlow 1986). Results are in accordance with those of Akhtar et al. (2014) and Haider et al. (2015). They concluded that the utilization of higher rates of biochar increased RWC and MSI of maize and tomato under abiotic stresses. According to Abd El-Mageed et al. (2018); Agami et al. (2018); and Kabir et al. (2004), crops having greater biomass can maintain higher water content in leaf, leading to a higher tolerance to a biotic stress.
Physiological responses
Data related to stomatal conductance (gs) and chlorophyll content (SPAD value) of maize are illustrated in Fig. 1. Results revealed that gs and SPAD responses differ significantly (P ≤ 0.05) due to residual acidified biochar. As expected, plants grown under RACB10 had the highest gs and SPAD values (253 and 54) in contrast to untreated soil (116.5 and 26.3), respectively. Compared with those of the RACB0, the readings of gs and SPAD in leaves of maize increased by 26% and 43% for RACB5 and by 117 and 104% for RACB10, respectively. SPAD and gs significantly increased in RABC treatments under abiotic stresses, which may be attributed to the enhanced N and K uptake (Table 3) (Van Hoorn et al. 2001). These effects are supported by the findings from Akhtar et al. (2014) and Seehausen et al. (2017). They reported that stomatal conductance was improved significantly under the utilization of biochar.
Chlorophyll fluorescence
The impacts of RABC on Fv/Fm and performance index (PI) of maize grown under contaminated irrigation are listed in Fig. 1. Chlorophyll efficiency significantly increased gradually with the gradual increase in RABC. However, RABC has been exposed to alleviation of the deleterious effects of salinity and/or contaminated irrigation water stress on chlorophyll fluorescence of maize. The highest Fv/Fm and PI values were recorded under RABC10 (0.82 and 4.1) compared with that under RABC0 treatment (0.72 and 1.8). Higher chlorophyll fluorescence produced higher grain and biomass yields of maize (Table 7). Numerous investigations suggested that the analysis of chlorophyll fluorescence could be utilized as a dependable method to evaluate the fluctuations in the function of PSII under antagonistic conditions (Abd El-Mageed et al. 2018; Cakmak 2005). Our results demonstrated decreases in Fv/Fm and PI (Fig. 1) under untreated soil, which were potentially because of the decrease in leaf photosynthetic pigments and RWC required for photosynthesis. These responses are well confirmed by earlier findings in other investigations of Seehausen et al. (2017). They reported that chlorophyll fluorescence (Fv/Fm) was increased, with increasing biochar application. Conversely, Akhtar et al. (2015) stated that no significant influence of biochar was observed on the photochemical efficiency of photosystem II (Fv/Fm) in wheat and maize crops.
Nutrients status of maize
Residual acidified biochar significantly improved leaf N, P, K+, and Ca2+ contents in relation to the respective control (Table 5). In contrast, leaf Na+ concentration and Na+/K+ ratio were significantly decreased by RABC. Leaf Na+ concentration and Na+/K+ ratio concentration decreased at RABC10 compared with that at RABC0. Comparable with the control (non-residual acidified biochar), RABC reduced Na+ concentration while increased K+ concentration thus lowered Na+/K+ ratio in the maize leaves. N concentration increased by 11.4 and 14.3%, P by 63.5 and 76.2%, K by 22.6 and 38.2%, and Ca by 32.0 and 37.6% which was calculated at RABC5 and RABC10, compared with their corresponding control. Results are parallel with those obtained by Major et al. (2010). They reported that biochar addition caused an increase in K, Mg, and Ca uptake in maize. Furthermore, soil treated with acidified biochar decreased Na and Na+/K+ concentration by 8.0 and 15.9% for Na and by 24.9 and 39.2% for Na+/K+ when contrasted with untreated soil, respectively. The results are in harmony with that of Akhtar et al. (2015). They observed that residual biochar utilization has positive impacts in reducing Na+ uptake in the following wheat crop. The decrease in the pH of the soil (Table 3) probably enhanced the nutrient status of maize plants. Therefore, this decrease in soil pH led to the enhancing mineralization of both organic materials and solubilization of nutrients particularly in the root zone which could have resulted in increased bioavailability of essential nutrients in soil and ultimately their uptake and assimilation in maize plants. These results are in agreement with those obtained by Ramzani et al. (2016) and Paradelo et al. (2016). Table 5 shows significant increases in N and P content in plants grown under 10 t ha−1 RABC by 14.2 and 66.7% compared with non-acidified biochar–amended soil. The increase of N and P maize uptake is most likely because of the induced improvement in soil chemical properties, i.e., the pH value, total N and available P2O5 contents, CEC, and base saturation. The results are in agreement with those of Yamato et al. (2006) and Yeboah et al. (2009) and are contrary to findings by Mau and Utami (2014). In this concern, Mau and Utami (2014) reported that using biochar alone did not enhance maize growth or P uptake.
Concentration of heavy metals
Data related to toxic metal (i.e., Ni, Pb, Cr, Cd, and Cu) concentrations in leaves and grains of maize are listed in Table 6 and revealed that Ni, Pb, Cd, and Cr differ significantly (P ≤ 0.05) due to residual acidified biochar. Table 6 demonstrates that maize grown in soil received 5 or 10 tons of acidified biochar per hectare has lower concentrations of Ni, Pb, Cd, and Cr by 33.2–41.4%, 26.4.9–12.1%, 20–40%, and 8–16% for leaves and by 43.4–52.5%, 25.2–30.3%, 28.1–40.6%, and 25.6–33.6% for grains compared with control (untreated soil). According to our results, the availability of toxic elements (i.e., Ni, Pb, Cd, and Cr) to plant growth can be reduced by residual acidified biochar, most likely because of surface adsorption and precipitation of acidified biochar. The obtained results are in agreement with those obtained by Kloss et al. (2014) and Masto et al. (2013). Reduction of metal contents for crops could be because of the surface functional groups and adsorption sites on residual acidified biochars which could increase CEC and consequently increase soil metal exchange capacity through the creation of complexes with cationic heavy metals. In this regard, Al-Wabel et al. (2015) demonstrate that adding biochar significantly decreased concentrations (i.e., Mn, Zn, Cu, and Cd) of shoot plants in accordance with increasing supply rates. In dry conditions which described by high soil pH, the justification for supplying acidified biochar to environmental matrices is that it can work as a sorbent for metals in solution by establishing a new balance between the concentrations sorbed to surfaces and that in solution, and its greater resistance to degradation should render longevity of the effect. The decrease of maize heavy metal uptake could be referred to two reasons. First, residual acidified biochar was effective in increasing soil CEC and immobilizing heavy metals (i.e., Ni, Pb, Cd Cr, and Cu). These findings are in accordance with those of Jiang et al. (2012) for Cu and Pb and Shen et al. (2012) for Cr. The second one is the indirect effect of residual acidified biochar on characteristics of soil (physical, biological, and chemical) that then impact on heavy metal retention or release. The addition of acidified biochar to soils can increase microbial biomass, organic carbon, nutrient content, and WHP (Table 3) (Sohi et al. 2010; Karami et al. 2011; Lehmann et al. 2011), which may in turn impact of heavy metal retention and release.
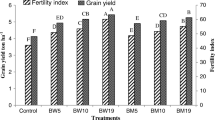
Yield components, yields, and IUE
Data in Table 7 reveal that RABC had significant (P ≤ 0.05) influences on yield components, yields, and irrigation use efficiency (IUE). Comparable with RABC0, the weight of 1000 grain significantly (P ≤ 0.05) increased by 27.3%, shelling ratio by 10.3%, HI by 10.5%, biomass yield by 39.2%, grain yield by 78.3%, and IUE by 83.1%. Such findings are in harmony with Kannan et al. (2017) and Vaccari et al. (2015). Harvest index (HI) was relatively increased from 0.19 to 0.21 (Table 7). In this concern, Major et al. (2010) and Wang et al. (2012) noticed that higher utilization of biochar produced significantly (P ≤ 0.05) higher HI values. Applications of acidified biochar at 5 and 10 t ha–1 significantly increased grain yield by 41 and 78% and increased biomass yield by 18.8 and 39.2% in contrast to the control, respectively. Likewise, the IUE of maize was increased by 42.3 and 84% compared with the control. The findings are parallel with those of Kimetu et al. (2008), Oguntunde et al. (2004), and Uzoma et al. (2011). The increases in the weight of 1000 grain, shelling ratio, HI, biomass yield, grain yield, and IUE could be attributed to the impacts of RABC. Residual acidified biochar improved physical soil properties (Table 3) (i.e., total porosity, water-holding pores, field capacity, and available water content); chemical properties (organic matter content, ECe, pH, NPK content); and soil biota (number of bacterial cells in the soil). Moreover, RABC enhanced growth attributes (Table 4), plant nutritional status (Table 5), maize water relations, physiological responses, and photochemical efficiency (Fig. 1) and reduced accumulation of Ni, Pb, Cd, Cr, and Cu (Table 6). All of these factors are mainly responsible for improved crop productivity (Rondon et al. 2007; Thies and Rilling 2009; Yamato et al. 2006).
Conclusions
A year after the addition, the constructive outcome of acidified biochar was still persisting. The present examination revealed that the utilization of acidified biochar had positive residual effects which could overcome the destructive of salinity and/or heavy metal stress by releasing mineral nutrients (particularly N, P, K+, and Ca++) and increasing RWC and MSI acting as osmotic and metabolic regulators or substrates and in part as cell component stabilizers. The results concluded that acidified biochar is useful for improving the soil properties, growth (i.e., plant height, number, and leaf area/plant and plant dry weight), nutrient status, IUE, and yield and reducing the Ni, Pb, Cd, and Cr uptake in maize under heavy metal–contaminated irrigation water. This gives additional insight into the application of acidified biochar under abiotic stresses.
References
Abd El-Mageed TA, El-Samnoudi IM, Ibrahim AM, Abd El Tawwab AR (2018) Compost and mulching modulates morphological, physiological responses and water use efficiency in sorghum (bicolor L. Moench) under low moisture regime. AgricWater Manage 431–439
Abd El-Mageed TA, El-Sherif AMA, Abd El-Mageed SA, Abdou NM (2019) A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric Water Manag 226(105831):1–9
Abd El-Mageed TA, Rady MM, Taha RS, Abd El Azeam S, Simpsond CR, Semida WM (2020) Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci Hortic 261(108930):1–10
Abd-Alla MH, El-Enany AE, Nafady NA, Khalaf DM, Morsy FM (2014) Synergistic interaction of Rhizobium leguminosarum bv. Viciae and arbuscular mycorrhizal fungi as a plant growth promoting bio fertilizers for faba bean (Vicia faba L.) in alkaline soil. Micro Res 169:49–58
Abdel Latef AA, Tran LSP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci 7:243
Abdel-Azim R, Allam M (2005) Agricultural drainage water reuse in Egypt: strategic issues and mitigation measures. Non-conventional water use: WASAMED project Bari: CIHEAM / EU DG Research
Agami RA, Saad AM, Abd El-Mageed TA, Abousekken MS, Mohamed H (2018) Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric Water Manag 210:261–270
Akhtar SS, Li G, Andersen MN, Liu F (2014) Biochar enhances yield and quality of tomato under reduced irrigation. Agric Water Manag 138:37–44
Akhtar SS, Andersen MN, Liu F (2015) Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric Water Manag 158:61–68
Ali A, Guo D, Zhang Y, Sun X, Jiang S, Guo Z, Huang H, Liang W, Li R, Zhang Z (2017a) Using bamboo biochar with compost for the stabilization and phytotoxicity reduction of heavy metals in mine-contaminated soils of China. Sci Rep 7:2690
Ali HN, Saeed BN, Abdol Rahim H (2017b) Management practices using of agricultural drainage water with drip irrigation for crop production and lands sustainability in arid and semi-arid areas. 13th ICID International Drainage Workshop, Ahwaz, Iran, March. www.idwi3.org
Al-Wabel MI, Usman ARA, El-Naggar AH, Aly AA, Ibrahim HM, Elmaghraby S, Al-Omran A (2015) Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J Biol Sci 22:503–511
Antonio JA, Salazar P, Barron V, Torrent J, Maria D, Campillo C, Gallardoand A, Villar R (2013) Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron Sustain Dev 33:475–484
Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y, Inoue Y, Shiraiwa T, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos. 1. Soil physical properties, leaf SPAD and grain yield. Field Crop Res 111:81–84
Bell MJ, Worrall F (2011) Charcoal addition to soils in NE England: a carbon sink with 978 environmental co-benefits? Sci Total Environ 409:1704–1714
Brewer CE, Chuang VJ, Masiello CA, Gonnermann H, Gao X, Dugan B, Driver LE, Panzacchi P, Zygourakis K, Davies CA (2014) New approaches to measuring biochar density and porosity. Biomass Bioenergy 66:176–185
Brodowski S, John B, Flessa H, Amelung W (2006) Aggregate-occluded black carbon in soil. Eur J Soil Sci 57:539–546
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41
Chan K, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of greenwaste biochar as a soil amendment. Aust J Soil Res 45:629–634
Chapman HD, Pratt PF (1961) Methods of analysis for soil, plants and water. University of California, Division of Agricultural Science, Berkeley, pp 56–63
Chen L, Yin H, Xu J, Liu X (2011) Enhanced antioxidative responses of a salt-resistant wheat cultivar facilitate its adaptation to salt stress. Afr J Biotech 10(74):16887–16896
Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37:1477–1488
Clark JS (1996) The pH values of soils suspended in dilute salt solutions. Soil Sci Soc Am Proc 30:11–14
Clark AJ, Landolt W, Bucher JB, Strasser RJ (2000) Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ Pollut 109:501–507
Etesami H (2017) Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicol Environ Saf 147:175–191
FAO (2017) Crop Statistics, FAOSTAT. Food and Agriculture Organization of the United Nations (FAO), Rome (Italy)
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal - a review. Biol Fertil Soils 35:219–230
Gwenzi W, Chaukura N, Mukome FND, Machado S, Nyamasoka B (2014) Biochar production and applications in sub-Saharan Africa: opportunities, constraints, risks and uncertainties. J Environ Manag 150:250–261
Haider G, Koyro HW, Azam F, Steffens D, Müller C, Kammann C (2015) Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 395:141–157
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Jensen ME (1983) Design and operation of farm irrigation systems. ASAE, Michigan, p 827
Jiang J, Xu R, Jiang T, Li Z (2012) Immobilization of Cu (II), Pb (II) and Cd (II) by the 1149 addition of rice straw derived biochar to a simulated polluted Ultisol. J Hazard Mater 229-230:145–150
Kabir ME, Karim MA, Azad MAK (2004) Effect of potassium on salinity tolerance of mung bean (Vigana radiata L. Wilczek). J Biol Sci 4:103–110
Kannan V, Srinivasan G, Babu R, Thiyageshwari S, Sivakumar T (2017) Effect of biochar, mulch and Ppfm spray on leaf relative water content, leaf Proline, chlorophyll stability index and yield of cotton under moisture stress condition. Int J Curr Microbiol Appl Sci 6:604–611
Karami N, Clemente R, Moreno-Jimenez E, Lepp NW, Beesley L (2011) Efficiency of greenwaste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J Hazard Mater 191:41–48
Kim HS, Kim KR, Yang JE, Ok YS, Owens G, Nehls T, Wessolek G, Kim KH (2016) Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 142:153–159
Kimetu JM, Lehmann J, Ngoze SO, Mugendi DN, Kinyangi JM, Riha S, Verchot Recha JW, Pell AN (2008) Reversibility of soil productivity decline with matter of differing quality along a degradation gradient. Ecosystems 11:726–739
Kloss S, Zehetner F, Wimmer B, Buecker J, Rempt F, Soja G (2014) Biochar application to temperate soils: effects on soil fertility and crop growth under greenhouse conditions. J Plant Nutr Soil Sci 177:3–15
Klute A (1986) Methods of soil analysis. Part-I: Physical and mineralogical methods, 2nd edn. American Society of Agronomy, Madison
Laird DA, Fleming P, Davis DD, Horton R, Wang B, Karlen DL (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–449
Lehmann J, Gaunt J, Rondon M (2006) Biochar sequestration in terrestrial ecosystems - a review. Mitig Adapt Strateg Glob Chang 11:403–427
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota - a review. Soil Biol Biochem 43:1812–1836
Lynch JP, St Clair SB (2004) Mineral stress: the missing link in understanding how global climate change will affect plants in real world soils. Field Crops Res 90:101–115
Major J, Rondon M, Molina D, Susan JR, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128
Malcovska SM, Ducaiova Z, Backor M (2014) Impact of silicon on maize seedlings exposed to short-term UV-B irradiation. Biologia 69:1349–1355
Masto RE, Ansari A, George J, Selvi VA, Ram LC (2013) Co-application of biochar and lignite fly ash on soil nutrients and biological parameters at different crop growth stages of Zea mays. Ecol Eng 58:314–322
Mau AEE, Utami SR (2014) Effects of biochar amendment and arbuscular mycorrhizal fungi inoculation on availability of soil phosphorus and growth of maize. J Degrad Min Lands Manag 1:69–74
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51(345):659–668
Oguntunde PG, Fosu M, Ajayi AE, Van De Giesen N (2004) Effects of production on maize yield, chemical properties and texture of soil. Biol Fertil Soils 39:295–299
Page AI, Miller RH, Keeny DR (1982) Methods of soil analysis. Part II. Chemical and Microbiological Methods, 2nd ed. Am Soc Agron Madison WI USA, pp 225–246
Paradelo R, Vazquez-Nion D, Silva B, Gonzalez A, Barral MT (2016) Acidification of mixtures of granite powder and compost for reuse in plant production. Comp Sci Uti 24:1e10
Premchandra GS, Saneoka H, Ogata S (1990) Cell membrane stability, an indicator of drought tolerance as affected by applied nitrogen in soybean. J Agric Sci Camb 115:63–66
Rady MM, Abd El-Mageed TA, Abdelhamid MT, Abd El-Azeam MMM (2018) Integrative potassium humate and biochar application reduces salinity effects and contaminants and improves growth and yield of eggplant grown under saline conditions. Int J Empir Educ Res 1:37–56
Ramzani PMA, Iqbal M, Kausar S, Ali S, Rizwan M, Virk ZA (2016) Effect of different amendments on rice (Oryza sativa L.) growth, yield, nutrient uptake and grain quality in Ni-contaminated soil. Environ Sci Pollut Res 23:18585–18595
Rizwan M, Ali S, Abbas T, Adrees M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Qayyum MF, Nawaz R (2018) Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J Environ Manag 206:676–683
Rondon MA, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43:699–708
Seehausen M, Gale N, Dranga S, Hudson V, Liu N, Michener J, Thurston E, Williams C, Smith S, Thomas S (2017) Is there a positive synergistic effect of biochar and compost soil amendments on plant growth and physiological performance? Agronomy 7:13
Semida WM, Abd El-Mageed TA, Howladar SM (2014) A novel organo-mineral fertilizer can alleviate negative effects of salinity stress for eggplant production on reclaimed saline calcareous soil. Acta Hortic 1034:493–499
Semida WM, Beheiry HR, Setamou M, Simpson CR, Abd El-Mageed TA, Rady MM, Nelson SD (2019) Biochar implications for sustainable agriculture and environment: a review. S Afr J Bot 127:333–347
Shen YS, Wang SL, Tzou YM, Yan YY, Kuan WH (2012) Removal of hexavalent Cr by coconut coir and derived chars—the effect of surface functionality. Bioresour Technol 104:165–172
Sinclair TR, Ludlow MM (1986) Influence of soil water supply on the plant water balance of four tropical grain legumes. Aust J Plant Physiol 13:329–341
Sohi S, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82
Sun H, Hockaday WC, Masiello CA, Zygourakis K (2012) Multiple controls on the chemical and physical structure of biochars. Ind Eng Chem Res 51:3587–3597
Thies JE, Rilling MC (2009) Characteristics of biochar: biological properties. In: Biochar for Environmental Management
Thomas SC, Frye S, Gale N, Garmon M, Launchbury R, Machado N, Melamed S, Murray J, Petroff A, Winsborough C (2013) Biochar mitigates negative effects of salt additions on two herbaceous plant species. J Environ Manag 129:62–68
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM (2011) Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem 59:2501–2510
Uzoma KC, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011) Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag 27:205–212
Vaccari FP, Maienza A, Miglietta F, Baronti S, Di Lonardo S, Giagnoni L, Lagomarsino A, Pozzi A, Pusceddu E, Ranieri R, Valboa G, Genesio L (2015) Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric Ecosyst Environ 207:163–170
Van Hoorn JW, Katerji N, Hamdy A, Mastrorilli M (2001) Effect of salinity on yield and nitrogen uptake of four grain legumes and on biological nitrogen contribution from the soil. Agric Water Manag 51:87–98
Vodyanitskii YN (2016) Standards for the contents of heavy metals in soils of some states. Ann Agrar Sci 14:257–263
Wang T, Arbestain C, Hedley M, Bishop M (2012) Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org Geochem 51:45–54
Wolka K, Melaku B (2015) Exploring selected plant nutrient in compost prepared from food waste and cattle manure and its effect on soil properties and maize yield at Wondo Genet, Ethiopia. Environ Syst Res 4:9
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495
Yeboah E, Ofori P, Quansah GW, Dugan E, Sohi SP (2009) Improving soil productivity through biochar amendments to soils. Afr J Environ Sci Technol 3:34–41
Yuan JH, Xu RK (2011) The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag 27:110–115
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abd El-Mageed, T.A., Abdurrahman, H.A. & Abd El-Mageed, S.A. Residual acidified biochar modulates growth, physiological responses, and water relations of maize (Zea mays) under heavy metal–contaminated irrigation water. Environ Sci Pollut Res 27, 22956–22966 (2020). https://doi.org/10.1007/s11356-020-08847-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08847-5