Abstract
To investigate the effects of antibiotics on nitrogen removal and uptake by wetland plants, four typical macrophyte species, Cyperus alternifolius L., Typha angustifolia L., Lythrum salicaria L., and Acorus calamus L., were grown in hydroponic cultivation systems and fed wastewater polluted with 10 μg L−1 Ofloxacin (OFL) and Tetracycline (TET). Biomass production, nitrogen mass concentration, chlorophyll content, root exudates, and nitrogen removal efficiency of hydroponic cultivation were investigated. The results indicated that in all hydroponic systems, NH4+–N was entirely removed from the hydroponic substrate within 1 day and plant nitrogen accumulation was the main role of the removed NO3−. OFL and TET stimulated the accumulation of biomass and nitrogen of A. calamus but significantly inhibited the NO3−–N removal ability of L. salicaria (98.6 to 76.2%) and T. augustifolia (84.3 to 40.2%). This indicates that A. calamus may be a good choice for nitrogen uptake in wetlands contaminated with antibiotics. OFL and TET improved the concentrations of total organic carbon (TOC), total nitrogen (TN), organic acid, and soluble sugars in root exudates, especially for oxalic acid. Considering the significant correlation between TOC of root exudates and nitrogen removal efficiency, the TOC of root exudates may be an important index for choosing macrophytes to maintain nitrogen removal ability in wetlands contaminated with antibiotics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication caused by nitrogen runoff from agricultural systems is a serious worldwide environmental problem (Vymazal 2010) that produces an abundance of micro-polluted surface water, with relatively low concentrations of nitrogen and carbon pollutants (typically < 10 mg L−1) (Huang et al. 2012; Wu et al. 2017). As an efficient and low-cost treatment facility, wetlands have been constructed to remove various environmental pollutants, especially nitrogen (Liu et al. 2009; Huang et al. 2012; Wu et al. 2017). Uptake of nitrogen by plants is one of the main mechanisms of nitrogen removal in wetlands (Lin et al. 2002), and macrophytes show large differences in the capacity for pollutant removal depending on the species, size, age, climatic conditions, type of wastewater, and other environmental conditions (Tanner 2001; Vymazal 2007; Borin and Salvato 2012). A previous study has shown that plants can remove 4–90% of total nitrogen (TN) in wetlands (Wu et al. 2017). Wang et al. reported that the TN removed by 22 plant species, such as Typha latifolia L. and Cyperus alternifolius L., contributed 9.66% to total nutrient removal in a free-water surface wetland (2010). In addition, 65% total kay type nitrogen (TKN) was removed by T. latifolia L. in a sub-surface horizontal flow constructed wetland (Chung et al. 2008).
Antibiotics are emerging environmental pollutants in aquatic environments that have spread widely throughout surface waters in the world (Liu et al. 2013a, b; Wang et al. 2014; Huang et al. 2015; Song et al. 2017; Wang et al. 2017; He et al. 2018). The effects of antibiotics on plant growth have been well-studied in regard to the physiological toxicity of antibiotics on cell walls, proteins, nucleic acids, metabolic pathways, and bacterial membrane structures (Naamala et al. 2016). Wetland plants have varied tolerance levels to antibiotics. Chen et al. studied the response and tolerance of 10 clonal wetland plants grown in soil spiked with 10 mg kg−1 of sulfadiazine and found that Panicum paludosum and Cyperus malaccensis var. brevifolius showed the least reduction of growth parameters, such as plant shoots, total biomass, root number, and chlorophyll content, whereas the growth of both Alocasia macrorrhiza and Saururus chinensis was severely inhibited (2016). Root-mediated exudates, major sources of organic carbon released by plant roots, are common plant responses to biotic and abiotic stressors such as antibiotic contaminants (Weir et al. 2006; Badri and Vivanco 2009). The toxic effect of plants grown under different concentrations of various antibiotics also varies. Results from another study illustrated that under hydroponic conditions, high ciprofloxacin, oxytetracycline, and sulfamethazine concentrations (> 10 μg L−1) had a toxic effect on root activity and leaf chlorophyll of Phragmites australis, while hormesis occurred at low concentrations of the antibiotics (0.1–1 μg L−1) (Liu et al. 2013b). Although an abundance of researches have focused on the toxicity of antibiotics on plant growth, studies on the effects of antibiotics on nitrogen accumulation of wetland plants are rare. This could inevitably impact the nitrogen removal in constructed wetlands.
This study focused on the removal and plant accumulation of nitrogen in a hydroponic cultivation system with four common wetland plants, Cyperus alternifolius L., Typha angustifolia L., Lythrum salicaria L., and Acorus calamus L.(Deng et al. 2004; Leto et al. 2013). We also investigated how plant exudates (organic acids and soluble sugars) respond to antibiotics, which, in turn, affects plant nitrogen accumulation. Hydroponic conditions prevent the potential adsorption of antibiotics into soil or substrate particles, which would result in lower concentrations of exposure and a changed phytotoxicity (Hillis et al. 2011). Further, because multiple coexisting antibiotics have been detected in surface water, the solution included a mixture of the antibiotics Ofloxacin (OFL) and Tetracycline (TET). In this study, OFL and TET were selected as the test antibiotics because of their wide usage in livestock production and high detection frequency in aquatic environments (Liu et al. 2016; Zhao et al. 2016). Our results have implications for selecting wetland plants with efficient nitrogen removal for constructed wetlands contaminated with antibiotics.
Materials and methods
Plant materials
The four wetland plant species used in this study, C. alternifolius (Cy), T. angustifolia (Ty), L. salicaria (Ly), and A. calamus (Ac), were obtained from a plant cultivation base in Jiangsu Province, China. The plants were rinsed with distilled water, and soil was carefully washed from the roots. The plants were divided into homogenous groups and cultivated for 15 days in Hoagland culture solution (Wu et al. 2016) in a greenhouse.
Experimental setup
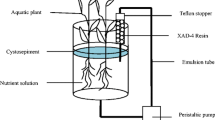
A hydroponic cultivation system (2 L, 13.8 cm Ø, 19.5 cm H, glass vessels) with 1 L of nutrient solution was used. The exteriors of the glass vessels were covered with aluminum foil to avoid photodegradation of antibiotics. Six to 10 similar-sized plants of each species were selected and distributed at random between the treatments. On the day of planting, the fresh mass of each plant species was determined; the average fresh weight was 59.4 ± 5.1 g, 52.5 ± 1.9 g, 69.8 ± 3.4 g, and 55.9 ± 2.9 g (mean ± SD) for Cy, Ac, Ty, and Ly, respectively.
The experiment was designed as 4 × 2 factorial with four species and two concentrations of antibiotics (the concentrations of OFL and TET were 0 μg L−1 in control groups and 10 μg L−1 in test groups). Throughout the study, the experimental systems were irrigated with 1 L modified Hoagland’s nutrient solution, and the pH was adjusted to 7.0. The nutrient solutions were replaced every 6 days. The modified Hoagland’s nutrient solution was prepared with tap water, KH2PO4, (NH4)2SO4, and KNO3, as well as 2.94 ± 0.13 mg L−1 NH4+–N, 8.14 ± 0.29 mg L−1 NO3−–N, 11.08 ± 0.59 mg L−1 TN, and 0.78 ± 0.12 mg L−1 PO43−–P to simulate the nutrient composition of micro-polluted water. Each treatment had four replicates. Plants were kept under greenhouse conditions (25 °C and 12:12 h light to dark) for 24 days.
Sampling
Water samples were collected every 6 days and analyzed for NH4+ and NO3− using a flow injection analysis instrument (QC8500, Hach, USA). The total fresh plant mass was measured every 6 days, and maximum root length and maximum leaf length were measured at days 0 and 24. Exudate analysis, chlorophyll content, and nitrogen elemental analysis were conducted at the end of the experiment. After the experiment, the plants were oven-dried at 60 °C to a constant weight and homogenized to a fine powder. Nitrogen elemental analysis was conducted to obtain nitrogen mass concentration. In this study, nitrogen accumulation was calculated by multiplying the dry biomass production (Table 2) with the plant nitrogen concentration.
Chlorophyll concentrations (Chl a, Chl b, and total Chl) of plants were measured according to Huang et al. (2004). First, cutting plant leaves (0.1 g/three parallel individuals in each container) into 0.5 cm segments and incubating them in 80% (v/v) aqueous acetone for 48 h in the dark. Second, measuring the absorbance of the solutions at 663 and 645 nm, and the chlorophyll contents (mg ml-1) were calculated using the following equations:
Exudate analysis
As Wu et al. reported for the exudate analysis, at the beginning of the experiment, the resin column was placed in hydroponic cultivation systems to persistently adsorb the organic matter discharged by the roots (see in Fig. 1) (2017). After 24 days, the resin column was eluted with water and methanol, and then the methanol was removed by evaporation to concentrate the organic matter of root exudates. Finally, the exudates were analyzed by high-performance liquid chromatography (HPLC) (Table 1, Waters, USA) and total organic carbon (TOC, Analytik Jena Multi 3100, Germany). All fractions were stored in a freezer at − 20 °C.
Some common organic acids and sugars in root exudates were targeted for analysis: oxalic acid, tartaric acid, malonic acid, citric acid, succinic acid, glucose, sucrose, fructose, xylose, and arabinose. A detailed description of the HPLC procedures and the analytical materials are thoroughly described in Table 1 (Wu et al. 2017).
Statistical analysis
For nitrogen removal, chlorophyll, and plant growth parameters, significant differences among samples were evaluated through a parametric one-way analysis of variance (ANOVA). Significant (p < 0.05) differences were detected by a multiple Tukey comparison test. Pearson correlation analyses were used to evaluate the relationships between the nitrogen mass balance and nitrogen removal efficiency. Statistical analyses were performed using the commercial software SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA).
Results
Plant growth
In control groups, the species showed different growth characteristics. Ly had the greatest fresh biomass production, followed by Ty, Ac, and Cy. The individual physiological differences of plants significantly affected the growth location of the fresh mass. For example, in antibiotic and control groups, Ty and Ac both developed longer leaves than Cy and Ly. During the experiment, antibiotics strongly inhibited the biomass accumulation of Ty and Ly (Fig. 2), with a biomass production of only 50.5% and 42.4%, respectively, compared with control groups. However, the antibiotics had an opposite effect on Cy and Ac. For Ac, after the first cultivation cycle, the biomass of antibiotic groups exceeded that of control groups, but for Cy, the biomass of antibiotic groups was always greater than the control. In terms of fresh biomass production, Cy and Ac in antibiotic groups had 1.3 and 1.7 times greater biomass than that in control groups, respectively (Table 2).
Effects of antibiotics on chlorophyll content
Plant total chlorophyll, chlorophyll a, chlorophyll b, and the Chl a/Chl b ratio are shown in Fig. 3. At the end of the treatment period, in the control group, the total Chl content of Cy, Ac, Ty, and Ly were 28.2, 43.7, 14.2, and 40.6 mg mL−1, respectively. Ac had the greatest total content of chlorophyll, chlorophyll a, and chlorophyll b and the lowest Chl a/Chl b ratio. Antibiotics clearly stimulated plant Chl b and total chlorophyll production, while the Chl a/Chl b ratio of the four plant species was slightly decreased. The influence of antibiotics on Chl a was different in the different plant species. For Cy and Ty, antibiotics definitely increased Chl a, but for Ac and Ly, the effects were opposite. However, although antibiotics can affect the content of total chlorophyll, chlorophyll a, and chlorophyll b, the effects were not significant (p > 0.05).
Nitrogen removal performance
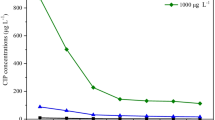
In both control and antibiotic groups, NH4+ in the solution was almost completely removed from hydroponic substrate during the 24-day experiment (98.8–99.1%; Fig. 4). The removal process was fast; in the last 6 days of the hydroponic cycle, NH4+ concentrations dropped from 2.94 to 0.103–0.028 mg L−1 in 1 day (Fig. 5), indicating that plant species and antibiotics had no influence on NH4+ removal. A high dissolved oxygen content was detected in the experimental systems (3.85–6.25 mg L−1), which was conducive to aerobic nitrification of ammonium. Considering the mild pH (6.92–7.11) of the culture medium, ammonia volatilization losses cannot happen. Therefore, NH4+ was thought to be removed mainly through nitrification and plant uptake, and the appearance of NO2− in the culture solutions also supported this inference.
Average (± SD) removal rates of NH4+, NO3−,, and TN in the culture solutions of the four plant species (n = 4) and two antibiotic concentrations during the 24-day experiment (-c and -ACs represent control groups and antibiotic groups, respectively. a and b indicates there is a significant difference between these two treatments (p < 0.05))
In control groups, systems planted with Ty and Ly had higher removal rates of NO3− compared to systems planted with Cy and Ac, and this may have been caused by their own physiological characteristics. Antibiotics clearly influenced the NO3- removal from water in four systems except the one planted with Cy. Under the antibiotic treatment, the NO3- removal rates were 40.2% and 76.2% for systems planted with Ty and Ly, respectively, which is also significantly lower than control groups (83.4% and 99.3%, respectively). In contrast, the NO3− removal efficiency of systems planted with Ac was stimulated by antibiotics, increasing from 56.6 to 60.2%. The concentration changes of NO3− after 6 days of cultivation was also similar (Fig. 5), and the influence of antibiotics worked from the beginning to the end. Due to the major proportion of NO3− in TN, the trend of TN was similar with NO3−. Above all, systems planted with any of the four different plant species all had high NH4+ removal efficiencies that were not affected by 10 μg L−1 OFL and TET. However, the NO3− removal rates were significantly inhibited by antibiotics in Ty and Ly systems, whereas NO3− removal rates were slightly stimulated for Ac systems.
Plant uptake of nitrogen
Figure 7 shows that the nitrogen mass concentration of four plant species changed from 2.0 to 3.8%, and the nitrogen mass concentration (percent by dry weight) was in the following order Cy < Ac < Ty < Ly. Antibiotics slightly increased the nitrogen concentration of the four plant species, except in Ly, in which the nitrogen concentration dropped from 3.77 to 3.44 in treatments with OFL and TET. Over the 24-day experimental period, in antibiotic groups, plant nitrogen accumulation was Ac > Ly > Ty > Cy (Fig. 6). Similar to NO3− removal from water, for nitrogen accumulation, antibiotics caused a significant inhibitory effect on Ty and Ly and a promoting effect on Ac, which has the highest nitrogen accumulation in the antibiotic treatments.
Mass balance of nitrogen
Although plant uptake and the remaining culture solution were two important parts of nitrogen fates in hydroponic systems, there were other processes involved, for example, microbial metabolism and plant root adsorption. In culture solutions, plant root surfaces are negatively charged, for which some nutrients can be absorbed to root surface (Liu et al. 2016), but these nutrient ions still do not cross cell membrane, therefore plant root adsorption cannot be classified as plant accumulation. These unknown processes contributed 0.6–36.0% of the TN fate in all systems. In general, different percentages (from 23.5–81.3%) of plant nitrogen uptake were found in control groups (Fig. 7). The highest plant uptake percentage (81.3%) was observed for the Ly system in control groups, followed by the Ty system of control groups. The percentage of plant uptake in the Ac and Cy system was relatively low (29.4% and 26.8%). However, in the antibiotics group, the plant uptake percentage of Ly and Ty groups decreased significantly (81.3 to 51.0% and 67.1 to 20.5%, respectively). For the other two systems, the presence of antibiotics promoted plant uptake, especially for Ac (29.4 to 49.8%). These results were in accordance with those described in “Nitrogen removal performance” section and “Plant uptake of nitrogen” section, indicating the different response of the four plants to antibiotic treatment.
Root exudates
The release of organic compounds from roots is important for nutrient mineralization (Sun et al. 2016). The concentrations of organic acids and soluble sugars in root exudates were 41–98% of TOC, and this demonstrated that root exudates were mainly composed of organic acids and soluble sugars (Fig. 8). In control groups, the exudate TOC concentrations in the four plant species were extremely different; Ly had the highest concentration (0.624 mg g−1 day−1; Fig. 8), which may be because Ly had the highest biomass production. For TN in root exudates, there were no significant differences found among the four plant species. For all plants, antibiotics improved the TOC concentration of root exudates, especially for Ly and Ac. Among them, the TOC concentration of Ly increased 1.67 times, which showed the most significant effect. Moreover, antibiotics also slightly increased the TN concentration of root exudates.
Concentrations of TOC, organic acids, and soluble sugars (expressed as OASS for short, and presented by the sum of their carbon concentration) and TN of root exudates in the culture solutions from the four plant species and two treatments (-c and -ACs represent control groups and antibiotic groups, respectively)
The exudate composition differed between the eight treatment systems (Table 3). In each system, oxalic acid, succinic acid, and arabinose were detected. Malonic acid, citric acid, and glucose were only present in some systems, and tartaric acid, sucrose, fructose, and xylose could not be detected in any system. Among these organic matters, oxalic acid was always detected in high concentrations. Antibiotics significantly stimulated the concentrations of all detected organic matter. For example, the oxalic acid concentration of Cy increased from 0.313 to 1.221 mg g−1 d−1, which is approximately four times the former.
Discussion
Four wetland plant species varied in their tolerance to antibiotics, which might be related to their different capacity for antibiotic uptake and detoxification, but this has yet to be verified (Chen et al. 2016). The OFL and TET antibiotics clearly inhibited the biomass production of Ly and Ty, which is in agreement with previous studies (Mikes and Trapp 2010; Ahmed et al. 2015). For example, Michelini et al. reported that the biomass of barley plants treated with 11,500 μg L−1 sulfadimethoxine and sulfamethazine showed a slight decrease compared to controls (2013). A negative effect on lettuce growth and reduced fresh weight was observed with 10 and 100 mg kg−1 sulfathiazole (Caban et al. 2018). In contrast, the biomass of Cy and Ac increased dramatically under OFL and TET stress, which may be a hormesis effect. Similarly, Michelini et al. stated that 10 mg kg−1 sulfadiazine enhanced the plant growth of willow and maize (2012), and Ma et al. also reported that oxytetracycline at < 5 mg kg−1 significantly increased the shoot biomass of Sedum plumbizincicola and alfalfa (2016).
Plant biomass production could be attributed to its nutrients assimilated from its environment, especially for nitrogen uptake. In this study, the NH4+–N was almost entirely removed in one day. Although most plants absorb NH4+–N preferentially over NO3−–N (Bloom et al. 1992; Rewald et al. 2016), considering the fast removal rate and the aerobic condition in systems, nitrification, and plant uptake were thought to be involved in the removal of NH4+–N. On the other hand, NO3−–N, the main component of TN in this research, was removed over the duration of the whole 6-day hydroponic cycle. The removal efficiency of NO3−–N in the hydroponic substrate was significantly correlated with the nitrogen accumulation and nitrogen uptake percentage of plants (r = 0.958, p < 0.05; r = 0.871, p < 0.05, respectively), indicating that plant uptake might be the main contribution of NO3−–N removal.
Differences in nitrogen uptake could reflect the different tolerances of plants toward antibiotics. Under antibiotic stress, NO3−–N assimilation of Ly and Ty was clearly lower than in the control. Unlike Ly and Ty, the nitrogen uptake of Ac and Cy was stimulated by the addition of OFL and TET, which could a hormesis effect. Researchers have demonstrated that the root uptake of NO3−–N, an active transport process, is actually the balance between two concomitant opposite fluxes, influx, and efflux, likely mediated by different carrier proteins (McClure et al. 1990; Miller and Smith 1996). Antibiotics were found to induce root electrolyte release, such as K+, possibly due to an impairment of membrane permeability. This cellular leakage can lead to a lowering in root functions, such as water transport and ion supply, thereby potentially compromising plant productivity (Huang et al. 2005; Michelini et al. 2013), which may help to explain the antibiotic’s inhibition of plant NO3−–N uptake.
When exposed to a range of biotic and abiotic stressors at the root zone, roots will discharge a different blend of chemicals to protect against negative influences and encourage positive interactions (Badri and Vivanco 2009). In this study, the TOC of root exudates was significantly positively correlated with NO3−–N and TN removal efficiency, respectively (r = 0.786, p < 0.05; r = 0.789, p < 0.05), indicating that the TOC of root exudates was related to nitrogen removal. The quantity and quality of root exudates are influenced by external factors like biotic and abiotic stressors (Badri and Vivanco 2009). The TOC and oxalic acid in root exudates of the four plant species were all stimulated by OFL and TET. Some previous research has reported that plants responded to stress situations, such as antibiotics, by overproduction of reactive oxygen species (ROS), which can set off a chain reaction leading to lipid peroxidation, ATP depletion, nucleic acid damage, and uncontrolled cell death (Cruz-Ortega et al. 2002; Zacchini and De Agazio 2004; Gujarathi et al. 2005). Oxalic acid, an important organic acid in root exudates, is a natural antioxidant that has also been shown to inhibit lipid peroxidation (Kayashima 2002). Additionally, oxalic acid can block the generation of ROS in susceptible plants and reduce oxidative damage generated in response to catechin (Weir et al. 2006).
In this study, the oxalic acid concentration and the percentages in TOC (from 21.4–53.2% to 22.3–78.2%) were significantly improved by OFL and TET. It might be inferred that the increasing discharge of oxalic acid is used for protecting plant roots from ROS caused by antibiotics. Considering the positive relationship between TOC and nitrogen removal efficiency, the protection of roots from ROS damage might finally help to maintain plant nitrogen assimilation to some extent. Thus, the TOC of root exudates is not only a plant physiological index, but it also constitutes an important index for choosing macrophytes to maintain nitrogen removal efficiency in constructed wetlands contaminated with antibiotics.
The described hydroponic system certified that antibiotics have various effects on wetland plants; however, this system also has limits. In real wastewater treatment, there are abundant pollutants and antibiotics, which may interfere with plants and affect nitrogen removal. Meanwhile, the matrix in constructed wetlands may also influence the effect of antibiotics on plants, which needs further research.
Conclusions
This study investigated the nitrogen removal ability of four macrophyte species cultivated in hydroponic systems simulating the presence of wastewater with 10 μg L−1 of the antibiotics OFL and TET. OFL and TET stimulated the biomass and nitrogen accumulation of Ac and Cy but significantly inhibited the NO3−–N uptake in Ly and Ty, indicating that Ac may be a more appropriate wetland species for the removal of nitrogen in antibiotic contaminated wastewater. Considering the significant correlation between the TOC of root exudates and nitrogen removal efficiency, the TOC of root exudates may be an important index for choosing macrophytes to maintain nitrogen removal efficiency in constructed wetlands contaminated with antibiotics.
References
Ahmed MBM, Rajapaksha AU, Lim JE et al (2015) Distribution and accumulative pattern of tetracyclines and sulfonamides in edible vegetables of cucumber, tomato, and lettuce. J Agric Food Chem 63:398–405. https://doi.org/10.1021/jf5034637
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant, Cell Environ 32:666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x
Bloom AJ, Sukrapanna SS, Warner RL (1992) Root Respiration Associated With Ammonium and Nitrate Absorption and Assimilation By Barley. Plant Physiol 99:1294–1301. https://doi.org/10.1104/pp.99.4.1294
Borin M, Salvato M (2012) Effects of five macrophytes on nitrogen remediation and mass balance in wetland mesocosms. Ecol Eng 46:34–42. https://doi.org/10.1016/j.ecoleng.2012.04.034
Caban JR, Kuppusamy S, Kim JH et al (2018) Hairy Vetch Incorporated as Green Manure Inhibits Sulfathiazole Uptake by Lettuce in Soil. Water Air Soil Pollut 229:104. https://doi.org/10.1007/s11270-018-3710-8
Chen J, Xu H, Sun Y et al (2016) Interspecific differences in growth response and tolerance to the antibiotic sulfadiazine in ten clonal wetland plants in South China. Sci Total Environ 543:197–205. https://doi.org/10.1016/j.scitotenv.2015.11.015
Chung AKC, Wu Y, Tam NFY, Wong MH (2008) Nitrogen and phosphate mass balance in a sub-surface flow constructed wetland for treating municipal wastewater. Ecol Eng 32:81–89. https://doi.org/10.1016/j.ecoleng.2007.09.007
Cruz-Ortega R, Ayala-Cordero G, Anaya AL (2002) Allelochemical stress produced by the aqueous leachate of Callicarpa acuminata: Effects on roots of bean, maize, and tomato. Physiol Plant 116:20–27. https://doi.org/10.1034/j.1399-3054.2002.1160103.x
Deng H, Ye ZH, Wong MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Pollut 132:29–40. https://doi.org/10.1016/j.envpol.2004.03.030
Gujarathi NP, Haney BJ, Park HJ et al (2005) Hairy roots of Helianthus annuus: A model system to study phytoremediation of tetracycline and oxytetracycline. Biotechnol Prog 21:775–780. https://doi.org/10.1021/bp0496225
He S, Dong D, Zhang X et al (2018) Occurrence and ecological risk assessment of 22 emerging contaminants in the Jilin Songhua River (Northeast China). Environ Sci Pollut Res 25:24003–24012. https://doi.org/10.1007/s11356-018-2459-3
Hillis DG, Fletcher J, Solomon KR, Sibley PK (2011) Effects of ten antibiotics on seed germination and root elongation in three plant species. Arch Environ Contam Toxicol 60:220–232. https://doi.org/10.1007/s00244-010-9624-0
Huang L, Gao X, Liu M et al (2012) Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol Eng 46:98–106. https://doi.org/10.1016/j.ecoleng.2012.06.004
Huang X, Lakso AN, Eissenstat DM (2005) Interactive effects of soil temperature and moisture on Concord grape root respiration. J Exp Bot 56:2651–2660. https://doi.org/10.1093/jxb/eri258
Huang X, Liu C, Li K et al (2015) Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res 70:109–117. https://doi.org/10.1016/j.watres.2014.11.048
Huang XD, El-Alawi Y, Penrose DM et al (2004) A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ Pollut 130:465–476. https://doi.org/10.1016/j.envpol.2003.09.031
Kayashima T (2002) Oxalic acid is available as a natural antioxidant in some systems. Biochim Biophys Acta - Gen Subj 1573:1–3. https://doi.org/10.1016/S0304-4165(02)00338-0
Leto C, Tuttolomondo T, La Bella S et al (2013) Effects of plant species in a horizontal subsurface flow constructed wetland - phytoremediation of treated urban wastewater with Cyperus alternifolius L. and Typha latifolia L. in the West of Sicily (Italy). Ecol Eng 61:282–291. https://doi.org/10.1016/j.ecoleng.2013.09.014
Lin YF, Jing SR, Wang TW, Lee DY (2002) Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environ Pollut 119:413–420. https://doi.org/10.1016/S0269-7491(01)00299-8
Liu D, Ge Y, Chang J et al (2009) Constructed wetlands in China: Recent developments and future challenges. Front Ecol Environ 7:261–268. https://doi.org/10.1890/070110
Liu L, Liu C, Zheng J et al (2013a) Chemosphere Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands. Chemosphere 91:1088–1093. https://doi.org/10.1016/j.chemosphere.2013.01.007
Liu L, Liu YH, Liu CX et al (2013b) Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol Eng 53:138–143. https://doi.org/10.1016/j.ecoleng.2012.12.033
Liu X, Zhang H, Li L et al (2016) Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries. Mar Pollut Bull 109:597–602. https://doi.org/10.1016/j.marpolbul.2016.05.033
Ma T, Zhou L, Chen L et al (2016) Oxytetracycline Toxicity and Its Effect on Phytoremediation by Sedum plumbizincicola and Medicago sativa in Metal-Contaminated Soil. J Agric Food Chem 64:8045–8053. https://doi.org/10.1021/acs.jafc.6b02140
McClure PR, Kochian LV, Spanswick RM, Shaff JE (1990) Evidence for cotransport of nitrate and protons in maize roots: I. Effects of nitrate on the membrane potential. Plant Physiol 93:281–289. https://doi.org/10.1104/pp.93.1.281
Michelini L, La Rocca N, Rascio N, Ghisi R (2013) Structural and functional alterations induced by two sulfonamide antibiotics on barley plants. Plant Physiol Biochem 67:55–62. https://doi.org/10.1016/j.plaphy.2013.02.027
Michelini L, Reichel R, Werner W et al (2012) Sulfadiazine uptake and effects on salix fragilis l. and zea mays l. plants. Water Air Soil Pollut 223:5243–5257. https://doi.org/10.1007/s11270-012-1275-5
Mikes O, Trapp S (2010) Acute toxicity of the dissociating veterinary antibiotics trimethoprim to willow trees at varying pH. Bull Environ Contam Toxicol 85:556–561. https://doi.org/10.1007/s00128-010-0150-6
Miller AJ, Smith SJ (1996) Nitrate transport and compartmentation in cereal root cells. J Exp Bot 47:843–854. https://doi.org/10.1093/jxb/47.7.843
Naamala J, Jaiswal SK, Dakora FD (2016) Antibiotics Resistance in Rhizobium: Type, Process, Mechanism and Benefit for Agriculture. Curr Microbiol 72:804–816. https://doi.org/10.1007/s00284-016-1005-0
Rewald B, Kunze ME, Godbold DL (2016) NH4: NO3nutrition influence on biomass productivity and root respiration of poplar and willow clones. GCB Bioenergy 8:51–58. https://doi.org/10.1111/gcbb.12224
Song C, Zhang C, Kamira B et al (2017) Occurrence and human dietary assessment of fluoroquinolones antibiotics in cultured fish around tai lake, China. Environ Sci Pollut Res 36:2899–2905. https://doi.org/10.1002/etc.3876
Sun L, Lu Y, Kronzucker HJ, Shi W (2016) Quantification and enzyme targets of fatty acid amides from duckweed root exudates involved in the stimulation of denitrification. J Plant Physiol 198:81–88. https://doi.org/10.1016/j.jplph.2016.04.010
Tanner CC (2001) Growth and nutrient dynamics of soft-stem bulrush in constructed wetlands treating nutrient- rich wastewaters. Wetl Ecol Manag 9:49–73
Vymazal J (2010) Constructed Wetlands in the Czech Republic: 20 Years of Experience (Water and Nutrient Management in Natural and Constructed Wetlands). Springer, Dordrecht
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65. https://doi.org/10.1016/j.scitotenv.2006.09.014
Wang L, Gan H, Wang F et al (2010) Characteristic analysis of plants for the removal of nutrients from a constructed wetland using reclaimed water. Clean - Soil, Air, Water 38:35–43. https://doi.org/10.1002/clen.200900162
Wang W, Wang H, Zhang W et al (2017) Occurrence, distribution, and risk assessment of antibiotics in the Songhua River in China. Environ Sci Pollut Res 24:19282–19292. https://doi.org/10.1007/s11356-017-9471-x
Wang Z, Liu C, Liao J et al (2014) Nitrogen removal and N 2 O emission in subsurface vertical fl ow constructed wetland treating swine wastewater : Effect of shunt ratio. Ecol Eng 73:446–453. https://doi.org/10.1016/j.ecoleng.2014.09.109
Weir TL, Bais HP, Stull VJ et al (2006) Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeus to a phytotoxin produced by Centaurea maculosa. Planta 223:785–795. https://doi.org/10.1007/s00425-005-0192-x
Wu H, Wang X, He X et al (2017) Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci Total Environ 598:697–703. https://doi.org/10.1016/j.scitotenv.2017.04.150
Wu H, Xu K, He X, Wang X (2016) Removal of Nitrogen by Three Plant Species in Hydroponic Culture: Plant Uptake and Microbial Degradation. Water Air Soil Pollut 227:324. https://doi.org/10.1007/s11270-016-3036-3
Zacchini M, De Agazio M (2004) Spread of oxidative damage and antioxidative response through cell layers of tobacco callus after UV-C treatment. Plant Physiol Biochem 42:445–450. https://doi.org/10.1016/j.plaphy.2004.03.007
Zhao S, Liu X, Cheng D et al (2016) Temporal–spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River Delta, China. Sci Total Environ 569–570:1350–1358. https://doi.org/10.1016/j.scitotenv.2016.06.216
Funding
The authors received financial support from the Major Science and Technology Program for Water Pollution Control and Treatment of China (NO. 2017ZX07203-005) (NO. 2012ZX07105-003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tong, X., Wang, X., He, X. et al. Effects of antibiotics on nitrogen uptake of four wetland plant species grown under hydroponic culture . Environ Sci Pollut Res 26, 10621–10630 (2019). https://doi.org/10.1007/s11356-019-04184-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04184-4