Abstract
Three macrophyte species, Typha augustifolia (T. augustifolia), Phragmites australis (P. australis), and Acorus calamus L. (A. calamus L.), have been grown in hydroponic cultivation systems fed with synthetic wastewater. The experiment was designed as 3 × 2 factorial, with three species and two ratios of NH4 +/NO3 − so as to investigate the nitrogen transformation and nitrogen removal capacity of each species. The nitrogen removal mechanism was further disclosed by comparing biomass production, nitrogen mass balance, and root exudates of the three plant species under different NH4 +/NO3 − ratios. The results indicated there exists a linear relationship, with positive significance (r = 0.946, p < 0.05), between plant biomass and total nitrogen (TN) removal efficiency; in other words, biomass could best reflect plant ability to remove nitrogen. It is also found that NH4 +/NO3 − ratio could influence plant biomass and root exudates significantly. Additionally, the hydrogen donor and source of energy in denitrification happened in this research were mainly organic acids and soluble sugars, accounting for approximately 50 % of the composition in root exudates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Given the widespread extent of water quality degradation associated with nutrient enrichment, eutrophication has and continues to pose a serious threat to water environment (Anderson et al. 2002). A variety of strategies and treatment methods have been taken and developed to minimize the effect (Lurling and van Oosterhout 2013; Sanchez-Carrillo and Alvarez-Cobelas 2001). Among them, constructed wetlands (CWs), as an emerging ecological engineering technology, have shown their attractions and been widely employed due to high nitrogen removal efficiency and low operation cost (Liu et al. 2009; Vymazal 2005). The nitrogen pollutants usually treated by CWs, different from the organic nitrogen present in amino acids and urea, are mainly in the form of ionic inorganic nitrogen, including ammonium (NH4 +), nitrate (NO3 −), and nitrite (NO2 −) (Lee et al. 2009). Plant uptake and microbial denitrification are considered to be the two main mechanisms of nitrogen removal in wetlands (Lin et al. 2002; Tanner et al. 2002), while the interactions between plants, water, and microorganisms are little known.
In most cases, macrophytes with comparable size and growth form show significant differences in pollutant removal capacity. For example, Coleman et al. showed that under the same daily 0.22 g m−2 loading rate of total Kjeldahl nitrogen (TKN), Typha latifolia L. significantly outperformed Juncus effusus L. and Scirpus validus L. in growth and its ability to improve effluent quality (Coleman et al. 2001). Similarly, Akratos found that cattails removed TKN more efficiently than common reeds under daily organic loads ranging from 0.46 to 1.6 g m−2 (Akratos and Tsihrintzis 2007). Nitrogen absorption rates by plants in wetlands have been reported to be in the range of 4–90 % (Tanner 2001; Jan Vymazal 2007; Borin and Salvato 2012). This high variability is caused by the growth characteristics of different plant species and their age, climatic conditions, type of applied wastewater, and other environmental conditions (Borin and Salvato 2012).
In terms of NH4 +/NO3 − ratio which varies from different sources of wastewater, different plant species may have their specific tolerances and preferences to it. Some studies have demonstrated that the NH4 +/NO3 − ratio in the nitrogen supply can affect the rate of plant growth and biomass allocation, and most species grow better and accumulate more nitrogen when supplied with a mixture of NO3 − and NH4 + (Brueck and Guo 2006; Guo et al. 2002; Ali et al. 2001).
The intensity of microbial nitrification and denitrification is also affected by plants, which release organic compounds and O2 from their roots (Landi et al. 2006; Yin et al. 2013). In the process of nitrogen removal in CWs, root exudates initiate and modulate the interactions between the roots and soil microbes (Kumar et al. 2013). However, few studies have systematically investigated the behavior of the major components of macrophyte exudates.
This study focused on nitrogen degradation and transformation in a hydroponic cultivation system with three macrophytes, Typha augustifolia (T. augustifolia), Phragmites australis (P. australis), and Acorus calamus L. (A. calamus L.). The predominant influencing factors on nitrogen removal were investigated through the comparison of nitrogen removal under different NH4 +/NO3 − ratios. Meanwhile, the experiments were also designed to explore how plant exudates (organic acids and soluble sugars) affect denitrification. The results have implications for the screening of wetland plants and provide insight into the mechanisms of nitrogen removal in wetland systems.
2 Materials and Methods
2.1 Plant Materials
The three macrophyte species, T. augustifolia, P. australis, and A. calamus L., were collected from the Dengbeiqiao wetland, a simulated surface flow CWs located in Yunnan, China (26.01 N, 100.09 E). The wetland was built for purification of nitrogen-polluted wastewater. After soil was washed off from their roots rinsing in distilled water, the plants were divided into homogenous groups and cultivated for 14 days in Hoagland culture solution (Hoagland and Arnon 1950) in a greenhouse.
2.2 Experimental Setup
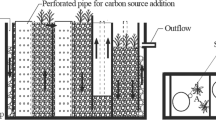
A hydroponic cultivation system (10 L, 25 cm Ø, 28 cm H, glass vessels) with 6 L of nutrient solution was used. An XAD-4 resin-packed column (50 mL, 2.6 cm Ø, 16 cm H) was installed on top of the system with a Teflon stopper (Fig. 1), and culture medium was passed through the apparatus at a rate of 2 mL min−1 during the experiment. The glass vessel exteriors were covered with aluminum foil to avoid photodegradation. Six to ten similar-sized plants of each species were selected and distributed at random between the treatments, with a coverage area of 200 cm2 (approximately 40 % coverage). On the day of planting, the fresh mass of each plant species was measured; the average fresh weight was 46.7 ± 2.1, 71.9 ± 3.3, and 62.5 ± 1.9 g (mean ± SD) for T. augustifolia, P. australis, and A. calamus L., respectively.
The experiment was designed as 3 × 2 factorial, with three species and two ratios of NH4 +/NO3 − (1.85 ± 0.05/9.44 ± 0.24 mg L−1, approximately equal to 1:5; and 7.4 ± 0.11/4.0 ± 0.09 mg L−1, approximately equal to 2:1). The nutrient solution was prepared using (NH4)2SO4 and KNO3; other nutrients including Ca2+, Mg2+, Fe2+, and Na+ in the solution were referenced to Hoagland nutrient medium (Hoagland and Arnon 1950); the solution does not contain any organic matter; daily total nitrogen (TN) loading rate was 0.3 g m−2; solution pH was 7.0; each treatment had three replicates. In addition, a control experiment without plants was carried out. Considering evapotranspiration and sampling from the vessels, lost water was supplemented with distilled water every day. The greenhouse was programmed with a day/night cycle of 25:22 °C, 12:12 h light, and 70 % relative air humidity. The photon flux density at the base of the plants was approximately 400 μmol m−2 s−1.
2.3 Sampling
Sampling of the culture solution in the vessels was conducted every 3 days during 1 month experiment. Culture solution from each vessel was collected and filtered through a 0.45-μm pore size mixed cellulose ester membrance filter (Sangon Biotech, China) and analyzed for NH4 + and NO3 − using a flow injection analysis instrument (QC8500, Hach, USA). At the end of the experiment, the resin column was eluted with 200 mL of distilled water, followed by another elution of 200 mL of methanol. After the elution, the residual methanol was removed by evaporating the column under dry vacuum on a rotary evaporator at 40 °C, and the dried exudates were dissolved in 10 mL of distilled water for high-performance liquid chromatography (HPLC) (Table 2, Waters, USA) and total organic carbon (TOC) analysis (multi 3100, Analytik Jena, Germany). All fractions were stored in a freezer at −20 °C. The total fresh plant mass, maximum root length, and maximum leaf length of individual plants were measured at days 0 and 30 to calculate fresh mass production and change in maximum root and leaf length.
2.4 Exudate Analysis
The following organic acids were targeted for analysis: oxalic acid, tartaric acid, malic acid, malonic acid, citric acid, acetic acid, succinic acid, and propionic acid (GR, Aladdin, China). The analyzed sugars included glucose, sucrose, fructose, xylose, galactose, and arabinose (GR, Aladdin, China). A detailed description of the HPLC procedures and the analytical materials is thoroughly described in Table 1.
2.5 Statistical Analysis
Statistical analyses were performed with SPSS software (version 22 for Windows). One-way ANOVA and post hoc Tukey’s HSD tests were used to compare the plant growth parameters in the different species at the 5 % significance level. Pearson correlation analyses were used to evaluate the relationships between the biomass production and nitrogen removal efficiency, nitrogen mass balance and nitrogen removal efficiency, and exudate concentration and nitrogen mass balance.
3 Results
3.1 Plant Growth
We selected two NH4 +/ NO3 − ratios for the experiment, and TN was approximately 11 ± 0.34 mg L−1. The NH4 +/NO3 − ratio of 1:5 simulated agricultural wastewater, which derived more NO3 − from fertilizer. The NH4 +/NO3 − ratio of 2:1 simulated wastewater from a sewage treatment system, which derived more NH4 + from domestic wastewater. The species showed different growth characteristics: biomass production was P. australis < A. calamus L. < T. augustifolia under both two NH4 +/ NO3 − ratio conditions (Table 2). The individual physiological difference of plants significantly affected the growth location of fresh mass; in contrast, P. australis, T. augustifolia, and A. calamus L. developed many more roots and leaves. In terms of maximum root length, T. augustifolia was 1.5–2 times longer than A. calamus L. and P. australis, while T. augustifolia had significantly higher maximum leaf length than P. australis and A. calamus L. Comparing the results of the two NH4 +/NO3 − ratios, plants had 2.5–10.7 % greater fresh mass production under the NH4 +/NO3 − ratio of 2:1 than that under the ratio of 1:5.
3.2 Removal Performance
NH4 + in the solution was almost completely removed by all three plant species during the 30-day experiment under both NH4 +/NO3 − ratios but at different rates (Fig. 2). T. augustifolia and A. calamus L. removed nearly 100 % of NH4 + during days 15–21 for both NH4 +/ NO3 − ratios, in contrast to P. australis, which reached that level after 27 days. These indicated that the influence of plant species was inferior to that of NH4 +/NO3 − ratio. Low dissolved oxygen was in the experimental system (oxygen filling the experimental device was not mandatory), which was not conducive to aerobic nitrification of ammonium; NH4 + removed mainly through plant absorption without considering ammonia volatilization losses; T. augustifolia and A. calamus L. had faster absorption rate of NH4 + compared to P. australis. However, degradation of NO3 − was mainly influenced by NH4 +/NO3 − ratio. Under the 1:5 ratio, P. australis and A. calamus L. had similar degradation rates; by day 30, the NO3 − concentrations were 1.96 and 1.35 mg L−1, respectively, and removal efficiencies were 81–86 %. In contrast, the removal efficiency was only 44–54 % with the 2:1 ratio. At the same time, NO3 − was completely removed by T. augustifolia during the 30-day experiment under both ratios. Above all, the three species responded differently to inorganic nitrogen composition: T. augustifolia had the highest nitrogen removal efficiency under both NH4 +/NO3 − ratios, and P. australis had the lowest NH4 + removal efficiency under both NH4 +/NO3 − ratios. Further, considering different NH4 +/ NO3 − ratios, A. calamus L. and P. australis had greater NO3 − removal efficiency with a NH4 +/NO3 − ratio of 1:5 than under the NH4 +/NO3 − ratio of 2:1. As the result, for the configuration of plant, T. augustifolia should be preferred, and in lower NH4 +/NO3 − ratio, A. calamus L. and P. australis had greater NO3 − removal efficiency.
3.3 Plant Uptake of Nitrogen
Plant nitrogen accumulation was the result of multiplication by the dry biomass production and plant nitrogen mass concentration. Plant nitrogen mass concentration will not change obviously in a short time (Krywult et al. 2013), so the nitrogen accumulation can be determined by the biomass production, the more biomass growth, the more nitrogen accumulation. The mean nitrogen mass concentrations were significantly different between the plant species, with T. augustifolia < A. calamus L. < P. australis. Over 30 days, plant nitrogen accumulation was P. australis < A. calamus L. < T. augustifolia. This order is mainly affected by the plant’s dry mass production: T. augustifolia, even though its nitrogen mass concentration was the least, had the highest dry mass production. All species had a greater plant nitrogen accumulation under the NH4 +/NO3 − ratio of 2:1 than that under the NH4 +/NO3 − ratio of 1:5 (Table 3). Under the 2:1 ratio, plants had much greater dry mass production, indicating that in the higher NH4 +/NO3 − ratio, these plants grew better, possibly because of preferred N forms: some plants are better adapted to NH4 + than to NO3 − supply (Rewald et al. 2016).
3.4 Mass Balance of Nitrogen
Although the plants used in the experiments were cleaned carefully prior to setup in the hydroponic cultures, the root systems were not axenic; therefore, microorganisms may have been contributing to metabolism.
In general, large percentages (from 56.9 to 75.3 %) of plant nitrogen uptake were found in six different treatments (Fig. 3). These figures were similar if compared with experiments at similar scale and same plant species reported in the literature, different species made some difference (Table 4). The highest plant uptake (75.3 %) was observed for T. augustifolia under the NH4 +/NO3 − ratio of 2:1; further, the plant uptake for T. augustifolia was also the highest (69.9 %) with the NH4 +/NO3 − ratio of 1:5. However, the metabolized portion negatively correlated with the NH4 +/NO3 − ratio, and the metabolized percentage was lower under NH4 +/NO3 − ratio of 2:1 than that under NH4 +/NO3 − ratio of 1:5. The differences in the plants’ contributions were essentially attributed to their different biomass productions. TN removal efficiency and the plant removal contribution percentage were significantly correlated with the biomass production of plants (r = 0.937 and r = 0.949, respectively, p < 0.01). NO3 − removal efficiency was significantly correlated with microbial metabolism (r = 0.873, p < 0.01).
The percentage contribution of nitrogen removal in the form of plant uptake, microbially metabolized and remaining in the culture solution at the end of the 30-day experiment (the left figure is the percentage under the NH4 +/NO3 − ratio of 1:5; the right figure is the percentage under the NH4 +/NO3 − ratio of 2:1)
3.5 Root Exudates
Biotic interactions are crucial for the understanding of ecosystem functioning. These interactions are often governed through chemical signals, especially between plants and microorganisms at the root level. Given this context, the quality of root exudates is a key element in the establishment and regulation of such interactions.
Figure 4 shows the concentrations of the root secretion TOC, the total organic acids, and the total soluble sugars in the culture solution dealt with six different treatments in 30-day experiment. Organic acids and soluble sugars were converted to reflect carbon concentration in the form of total carbon (TC). Among the three plant species, T. augustifolia had the highest exudate concentrations, including TOC, organic acids, and soluble sugars; the reason for this may be that T. augustifolia had the highest biomass production of the plant species. TC was about half of TOC, which demonstrated that root exudates were mainly composed of organic acids and soluble sugar.
Concentrations of TOC, total organic acids, and total soluble sugars in the culture solutions dealing with three plant species and two NH4 +/NO3 − ratios. The six treatments are as follows: T. (a) and T. (b) are T. augustifolia cultured under ratios of 1:5 and 2:1, respectively; P. (a) and P. (b) are P. australis cultured under ratios of 1:5 and 2:1, respectively; and A. (a) and A. (b) are A. calamus L. cultured under ratios of 1:5 and 2:1, respectively. Note that TC is organic acids and soluble sugars converted to carbon concentration
The exudate composition differed between the six treatment systems (Fig. 5). In every system, five types of organic acid - tartaric acid, malonic acid, malic acid, succinic acid, and citric acid - were detected; succinic acid was always detected in high concentrations. Glucose and arabinose were detected in every system, but sucrose and galactose were only present in some systems, and arabinose appeared in a higher concentration compared to other soluble sugars. This indicates that the different NH4 +/NO3 − ratios affected the root’s secretions. The concentration of organic acids was ten times higher than that of soluble sugar.
Concentrations of organic acids and soluble sugars in the culture solutions dealing with three plant species and two NH4 +/NO3 − ratios. The horizontal ordinate is the same as in Fig. 4
4 Discussion
During the study, all three plant species grew well and conduced nitrogen degradation. However, biomass, root length, and leaf length of the three species turned out differently during the experiment. These differences are possibly associated with adjustments to their physiology (i.e., net assimilation) and morphology (Ma et al. 2010), as many species have various mechanisms for aquatic/drought adaptation, including the enhancement of root systems, adjustments to growth rate, modifications to plant structure, and more efficient water utilization. According to Li et al. (2014) and Wang et al. (2013), environmental conditions during the test may also have influenced their adaptability and growth. At the end of the study, the mean nitrogen mass concentration of T. augustifolia was the lowest compared with P. australis and A. calamus L., but the former had the highest biomass production, which likely plays a decisive role in its highest rank in the final TN removal. The linear relationship between dry biomass and TN removal efficiency was positively significant (r = 0.946, p < 0.05), which meant that the dry biomass best reflects the ability of plant species to remove nitrogen from wastewater; these results are similar to those reported by Zhu et al. (2011). Generally, plants that can store large amounts of nitrogen in their tissue are usually the best choice for nitrogen removal, making total biomass an important factor in nitrogen removal from wastewater (Chang et al. 2010). Thus, plant biomass is not only the important factor for the bioenergy production after plant harvest but also constitute an important index for choosing macrophytes to remediate environmental pollution (Zhen et al. 2016; Kumar et al. 2016).
Different sources of wastewater have distinct NH4 +/NO3 − ratios. The inorganic nitrogen composition in wastewater can range from 0.4 to 99.6 % for NH4 + or NO3 −. Plant species may differ in their tolerance to and preference for NH4 + or NO3 − (Sundaravadivel and Vigneswaran 2001; Cea-Barcia et al. 2014). Some species, such as peas (Pisum sativum L.) and tomatoes (Lycopersicon esculentum Miller), prefer NO3 − (Lasa et al. 2001; J. Li et al. 2007), while tea (Camellia sinensis L.) prefers NH4 + as its nitrogen source (Ruan et al. 2007). In this study, T. augustifolia removed more nitrogen than the other two species because of its greater biomass. Meanwhile, there was difference in nitrogen mass balance for species between the two NH4 +/NO3 − ratios. For all three plant species, the higher NH4 +/NO3 − ratio (2:1) was better for N removal than the lower NH4 +/NO3 − ratio (1:5). Plants generally prefer NH4 + rather than NO3 − in habitats with NH4 + and NO3 − mixes. In general, the NH4 + absorbed from water by macrophytes can be used directly in the synthesis of amino acids, while NO3 − must be metabolized; therefore, acquisition and assimilation of NH4 + is considered less carbon-costly than that of NO3 − (Rewald et al. 2016; Bloom et al. 1992). Hence, most plants absorb NH4 + preferentially. Results from the current study correspond to findings from some previous studies, which showed that different NH4 +/NO3 − ratios could significantly impact plant biomass, thus greatly affecting their nitrogen removal ability (Rewald et al. 2016).
The nitrogen balance did not account for organic nitrogen or the volatilization of nitrogen; the metabolized percentage in the nitrogen mass balance was microbially metabolized. The NO3 − removal efficiency significantly correlated with the metabolized percentage (r = 0.873, p < 0.05). It was found that the metabolized percentage was higher under the NH4 +/NO3 − ratio of 1:5 than the NH4 +/NO3 − ratio of 2:1. These results suggest that denitrification may be the main contribution of microbes, and more NO3 − may be conducive to microbial metabolization.
Combining the above analyses, we conclude that there was denitrification in our research. Plants secreted some organic matter that contributed to microbe denitrification. It has been found that root exudates, mainly low molecular weight organic matter, are able to act as sources of energy and as hydrogen donors (Zhai et al. 2013; Salvato et al. 2012). In the research, detected organic acids and soluble sugars were converted to TC. This TC was about half of the TOC of exudates; additionally, TC was significantly correlated with TN removal (r = 0.820, p < 0.05), at the same time, TOC was not significantly correlated with TN removal (r = 0.741). These findings demonstrate that organic acids and soluble sugars, as low molecular weight organic matter, are contributors to microbial denitrification, but there is some organic matter in the TOC that are non-beneficial to denitrification microbes. Among different plant species, higher TOCs were accompanied by higher biomass. Additionally, the same plants under a NH4 +/NO3 − ratio of 1:5 had more TOC than those under a ratio of 2:1, even if plants in the latter solution had more biomass production, which indicates that plant secretion is affected by not only biomass but also solution composition.
In the analysis of organic acids, different organic acids and soluble sugars expressed different concentrations under different treatments. Succinic acid, malic acid, and tartaric acid were the main organic acids, while arabinose and glucose were the main soluble sugars. This phenomenon may be due to differences in the plant species, differences in growth conditions, and sampling. Moreover, we found that soluble sugar detection was trace contrasted to organic acids; more research on organic acids is needed to further understanding of these acids.
5 Conclusions
This study investigated nitrogen transformations in three macrophyte species cultivated in hydroponic systems representing the presence of wastewater with two NH4 +/NO3 − ratios. Our results suggest that the linear relationship between dry biomass and TN removal efficiency was positively significant (r = 0.946, p < 0.05), indicating that dry biomass could best reflect the ability of plant species to uptake nitrogen out of wastewater, and the choice of larger mass macrophyte species should be priority selection. Different NH4 +/NO3 − ratios could significantly impact plant biomass, thus greatly affecting their nitrogen removal. NO3 − removal efficiency was significantly correlated with microbial metabolism (r = 0.873, p < 0.01), meaning that denitrification may be the main role of microbes; higher levels of NO3 − may be conducive to microbial metabolization. Organic acids, as a low molecular weight organic matter, were mainly root secretions that contributed to microbial denitrification. Above all, plant absorption occupies large proportion in the process of wetland nitrogen removal, and choice of plants needs combination with water quality, and microbial denitrification also should be considered.
References
Akratos, C. S., & Tsihrintzis, V. A. (2007). Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecological Engineering, 29(2), 173–191.
Ali, A., Tucker, T. C., Thompson, T. L., & Salim, M. (2001). Effects of salinity and mixed ammonium and nitrate nutrition on the growth and nitrogen utilization of barley. Journal of Agronomy and Crop Science-Zeitschrift Fur Acker Und Pflanzenbau, 186(4), 223–228.
Anderson, D. M., Glibert, P. M., & Burkholder, J. M. (2002). Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries, 25(4B), 704–726.
Bloom, A. J., Sukrapanna, S. S., & Warner, R. L. (1992). Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiology, 99(4), 1294–1301.
Borin, M., & Salvato, M. (2012). Effects of five macrophytes on nitrogen remediation and mass balance in wetland mesocosms. Ecological Engineering, 46, 34–42.
Borin, M., & Tocchetto, D. (2007). Five year water and nitrogen balance for a constructed surface flow wetland treating agricultural drainage waters. Science of the Total Environment, 380(1–3), 38–47.
Brueck, H., & Guo, S. (2006). Influence of N form on growth and photosynthesis of Phaseolus vulgaris L. plants. Journal of Plant Nutrition and Soil Science-Zeitschrift Fur Pflanzenernahrung Und Bodenkunde, 169(6), 849–856.
Cea-Barcia, G., Buitron, G., Moreno, G., & Kumar, G. (2014). A cost-effective strategy for the bio-prospecting of mixed microalgae with high carbohydrate content: diversity fluctuations in different growth media. Bioresource Technology, 163, 370–373.
Chang, J., Liu, D., Cao, H., Chang, S. X., Wang, X., Huang, C., et al. (2010). NO3 −/NH4 + ratios affect the growth and N removal ability of Acorus calamus and Iris pseudacorus in a hydroponic system. Aquatic Botany, 93(4), 216–220.
Chung, A. K. C., Wu, Y., Tam, N. F. Y., & Wong, M. H. (2008). Nitrogen and phosphate mass balance in a sub-surface flow constructed wetland for treating municipal wastewater. Ecological Engineering, 32(1), 81–89.
Coleman, J., Hench, K., Garbutt, K., Sexstone, A., Bissonnette, G., & Skousen, J. (2001). Treatment of domestic wastewater by three plant species in constructed wetlands. Water, Air, and Soil Pollution, 128(3–4), 283–295.
Guo, S., Bruck, H., & Sattelmacher, B. (2002). Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants—nitrogen form and water uptake. Plant and Soil, 239(2), 267–275.
Hoagland, D. R., & Arnon, D. I. (1950). The water culture method for growing plants without soil. Circular California Agricultural Experiment Station, 347, 1–32.
Krywult, M., Smykla, J., & Wincenciak, A. (2013). The presence of nitrates and the impact of ultraviolet radiation as factors that determine nitrate reductase activity and nitrogen concentrations in Deschampsia antarctica Desv. around penguin rookeries on King George Island, Maritime Antarctica. Water Air Soil Pollut, 224(5), 1563.
Kumar, D., Shivay, Y. S., Dhar, S., Kumar, C., & Prasad, R. (2013). Rhizospheric flora and the influence of agronomic practices on them: a review. Proceedings of the National Academy of Sciences India Section B-Biological Sciences, 83(1), 1–14.
Kumar, G., Zhen, G. Y., Kobayashi, T., Sivagurunathan, P., Kim, S. H., & Xu, K. Q. (2016). Impact of pH control and heat pre-treatment of seed inoculum in dark H-2 fermentation: a feasibility report using mixed microalgae biomass as feedstock. International Journal of Hydrogen Energy, 41(7), 4382–4392.
Landi, L., Valori, F., Ascher, J., Renella, G., Falchini, L., & Nannipieri, P. (2006). Root exudate effects on the bacterial communities, CO2 evolution, nitrogen transformations and ATP content of rhizosphere and bulk soils. Soil Biology & Biochemistry, 38(3), 509–516.
Lasa, B., Frechilla, S., Lamsfus, C., & Aparicio-Tejo, P. M. (2001). The sensitivity to ammonium nutrition is related to nitrogen accumulation. Scientia Horticulturae, 91(1–2), 143–152.
Lee, C. G., Fletcher, T. D., & Sun, G. Z. (2009). Nitrogen removal in constructed wetland systems. Engineering in Life Sciences, 9(1), 11–22.
Li, J., Zhou, J. M., & Duan, Z. Q. (2007). Effects of elevated CO2 concentration on growth and water usage of tomato seedlings under different ammonium/nitrate ratios. Journal of Environmental Sciences-China, 19(9), 1100–1107.
Li, M., Sheng, G. P., Wu, Y. J., Yu, Z. L., Banuelos, G. S., & Yu, H. Q. (2014). Enhancement of nitrogen and phosphorus removal from eutrophic water by economic plant annual ryegrass (Lolium multiflorum) with ion implantation. Environmental Science and Pollution Research, 21(16), 9617–9625.
Lin, Y. F., Jing, S. R., Wang, T. W., & Lee, D. Y. (2002). Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environmental Pollution, 119(3), 413–420.
Liu, D., Ge, Y., Chang, J., Peng, C., Gu, B., Chan, G. Y. S., et al. (2009). Constructed wetlands in China: recent developments and future challenges. Frontiers in Ecology and the Environment, 7(5), 261–268.
Lurling, M., & van Oosterhout, F. (2013). Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Research, 47(17), 6527–6537.
Ma, X., Ma, F., Li, C., Mi, Y., Bai, T., & Shu, H. (2010). Biomass accumulation, allocation, and water-use efficiency in 10 Malus rootstocks under two watering regimes. Agroforestry Systems, 80(2), 283–294.
Rewald, B., Kunze, M. E., & Godbold, D. L. (2016). NH4: NO3 nutrition influence on biomass productivity and root respiration of poplar and willow clones. Global Change Biology Bioenergy, 8(1), 51–58.
Ruan, J., Gerendas, J., Hardter, R., & Sattelmacher, B. (2007). Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Annals of Botany, 99(2), 301–310.
Salvato, M., Borin, M., Doni, S., Macci, C., Ceccanti, B., Marinari, S., et al. (2012). Wetland plants, micro-organisms and enzymatic activities interrelations in treating N polluted water. Ecological Engineering, 47, 36–43.
Sanchez-Carrillo, S., & Alvarez-Cobelas, M. (2001). Nutrient dynamics and eutrophication patterns in a semi-arid wetland: the effects of fluctuating hydrology. Water, Air, and Soil Pollution, 131(1–4), 97–118.
Sundaravadivel, M., & Vigneswaran, S. (2001). Constructed wetlands for wastewater treatment. Critical Reviews in Environmental Science and Technology, 31(4), 351–409.
Tanner, C. C. (2001). Growth and nutrient dynamics of soft-stem bulrush in constructed wetlands treating nutrient-rich wastewaters. Wetlands Ecology and Management, 9(1), 49–73.
Tanner, C. C., Kadlec, R. H., Gibbs, M. M., Sukias, J. P. S., & Nguyen, M. L. (2002). Nitrogen processing gradients in subsurface-flow treatment wetlands—influence of wastewater characteristics. Ecological Engineering, 18(4), 499–520.
Vymazal, J. (2005). Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecological Engineering, 25(5), 478–490.
Vymazal, J. (2007). Removal of nutrients in various types of constructed wetlands. Science of the Total Environment, 380(1–3), 48–65.
Wang, Z., Zhang, Z., Zhang, Y., Zhang, J., Yan, S., & Guo, J. (2013). Nitrogen removal from Lake Caohai, a typical ultra-eutrophic lake in China with large scale confined growth of Eichhornia crassipes. Chemosphere, 92(2), 177–183.
Yin, H. J., Li, Y. F., Xiao, J., Xu, Z. F., Cheng, X. Y., & Liu, Q. (2013). Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Global Change Biology, 19(7), 2158–2167.
Zhai, X., Piwpuan, N., Arias, C. A., Headley, T., & Brix, H. (2013). Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecological Engineering, 61, 555–563.
Zhen, G. Y., Lu, X. Q., Kobayashi, T., Kumar, G., & Xu, K. Q. (2016). Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: kinetic modeling and synergistic impact evaluation. Chemical Engineering Journal, 299, 332–341.
Zhu, L., Li, Z., & Ketola, T. (2011). Biomass accumulations and nutrient uptake of plants cultivated on artificial floating beds in China’s rural area. Ecological Engineering, 37(10), 1460–1466.
Acknowledgments
The authors are grateful for the financial support of the Major Science and Technology Program for Water Pollution Control and Treatment of China (No. 2012ZX07105-003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, H., Xu, K., He, X. et al. Removal of Nitrogen by Three Plant Species in Hydroponic Culture: Plant Uptake and Microbial Degradation. Water Air Soil Pollut 227, 324 (2016). https://doi.org/10.1007/s11270-016-3036-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3036-3