Abstract
This work presents the effect of the ternary oxygenated additive on diesel biodiesel blended fuel to evaluate the engine characteristics. The Calophyllum inophyllum trees being abundant in India can lessen the dependence on petroleum imports to a specific extent. Methyl tertiary butyl ether is used as an oxygenated additive for the ternary blends preparation as 5–20% by volume. Seven blends of neat baseline diesel, biodiesel (Calophyllum inophyllum Methyl Ester), a blend of diesel (50%)-biodiesel (50%), a blend of diesel (50%)-biodiesel-methyl tert-butyl ether (5, 10, 15, and 20%) are prepared which are tested on a single cylinder, constant speed diesel engine. The experimental results were revealed that the replacement of biodiesel by MTBE has shown a slight reduction in brake thermal efficiency with a slight increase in brake-specific fuel consumption. Further, the MTBE addition in ternary blends reduced the unburned hydrocarbon, CO, and NOx by 63.9, 6.4, and 3.37% respectively. In addition, the carbon dioxide emission is almost similar to diesel fuel at a higher addition of MTBE with diesel-biodiesel blend. In the combustion point of view, the addition of 5% MTBE resulted in 3.49 and 5.1% reduction of peak pressure and heat release rate are observed as compared to diesel fuel. Critical analysis in combustion aspects is also carried out and it is witnessed with prolonged ignition delay during MTBE addition with diesel-biodiesel blends.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy, a paramount need, has continuously been viewed as similarly as a list for financial development where better and productive vitality administration has been the key for higher mechanical yield (Lešnik et al. 2013). From that point forward, energy preservation and economy get to be a buzz statement for mechanical parts which resulted in the increase in energy demand which needed a long-term solution (Chauhan et al. 2010). As projected by IEA, remaining global oil resources are sufficient to meet the demands up to 2030. Therefore, many fuel-related techniques were being strategized where the movement of the world toward a sustainable energy era emphasizing the use of renewable energy sources (Jiaqiang et al. 2016). Also, for the expanding worry, something like natural security and furthermore stringent engine emission norms, and future request for fossil fuels, much more efforts are being made worldwide to improve the efficiency of the engine. One of the renewable sources is vegetable oil which can sustain population growth acting as environment-friendly fuel (Viswanathan 2018). Diesel engines have proven their utility in automotive sector due to their efficiency, high torque, durability, and ruggedness as a well-established power-train result in the worldwide business as far as brake particular fuel utilization and lesser pollutant structuring (Vijayakumar et al. 2016). Several engine-related techniques were being followed to restrict nitrogen oxides (NOX), particulate matters (PM), and greenhouse gases which need utmost attention (Kalsi and Subramanian 2017). Also, most of the fuel is being consumed only by engines making energy crisis more severe which arose the researchers to focus on fuel-related techniques like alternate fuel technology (Ong et al. 2014).
Biodiesel and biodiesel with diesel blends
Among the available alternative fuels, biodiesel is one of the most successful renewable, oxygenated, non-toxic, biodegradable with higher flash point and cetane rating contributing emission reduction making it an ideal future fuel with a wide range of feedstock which is an important factor promoting biodiesel production worldwide. The American Society of Testing Materials (ASTM) International defines biodiesel as a blend of long-chain monoalkylic esters from unsaturated fatty acids acquired from renewable assets which can be utilized as a substitute for diesel fuel (Silitonga et al. 2017). In very populated nations like India, the utilization of consumable oils from crops for biodiesel generation is restricted to fulfill the local needs and hence more focus is being done on the non-edible vegetable feedstock for biodiesel extraction (Sezer 2011).
Calophyllum inophyllum, belonging to the Cluisaceae family, is a multi-purpose tree having multiple origins including south coast India, East Africa, and Southeast Asia where the annual rainfall is 1000–5000 mm at 0–200 m altitudes. This tree is slow growing, irregular crown, and low branching in nature which can be grown in tropical regions of the world. It is one of the easily cultivable, more eco-friendly, economic, and widely available feedstocks which can be used as a partial substitute for fossil fuels. The major advantages of Calophyllum inophyllum oil, when compared to other non-edible oils, is that it yields an oil of 4560 kg per hectare when compared with the jatropha having 1560 per hectare (Atabani and da Silva César 2014; Rajamohan and Kasimani 2018a). The wild seeds contain up to 65–75% oil content when compared with jatropha with only 55–60% and its biodiesel meets the US ASTM D6751 and European Union EN 14214 biodiesel standards (Rahman et al. 2013). Also, the physicochemical properties which are mentioned in Table 1 are very consistent which is capable to meet almost all biodiesel standards thereby a suitable feedstock for producing biodiesel in commercial scale. Yet crude oil has a major limitation of rich free fatty acid content in its feedstock which results in engine deposits, piston ring sticking, and poor fuel atomization, increasing fuel spray penetration partly contributing to the thickening of lubricating oil. Hence, the free fatty acids (glycerol) from the feedstock must be removed by transesterification process (Atabani et al. 2013; Rajamohan and Kasimani 2018a, 2018b).
There are considerable research works that have been reported in the literature in the implementation of Calophyllum inophyllum as a biodiesel in compression ignition (CI) engine applications. Arumugam and Ponnusami (2019) reviewed the production methodology and engine output characteristics of the Calophyllum inophyllum biodiesel from feedstocks. They concluded that the yield of biodiesel is 95% and the cultivation in the land is also very high as compared to other feedstocks. Also, the obtained biodiesel properties and engine output characteristics are close to diesel fuel. Ong et al. (2017) utilized a vibration analysis technique to determine the performance of Calophyllum inophyllum in diesel engines. The biodiesel’s performance was mapped out against vibration as an engine parameter to determine optimum blend ratio that can be utilized in engines for which there is the least vibration after isolating the external vibration. Praveen et al. (2017) further evaluated the emission and performance characteristics of 20% Calophyllum inophyllum/diesel by blending with nano-additives of TiO2 and EGR implementation. Oxides of nitrogen (NOx) tradeoff was observed with a decrease in emission upon increment in EGR rate and but increases with the addition of nanoparticles. Also, various additives (Shameer and Ramesh 2017) and higher alcohols (Ramakrishnan et al. 2018; Nanthagopal et al. 2018) are added to the Calophyllum inophyllum biodiesel for improving the engine output characteristics.
Additives with biodiesel
In addition to biodiesel, oxygenated additives are also gaining attention to improve oxidation characteristics of biodiesels. Use of an oxygenated fuel like ethers not only inhibits soot formation but also enhances the soot oxidation rate as soot and gaseous emissions are a concern in diesel engines (Natarajan et al. 2001; Alagu et al. 2018). It would be beneficial if the oxygenate additive has the following properties which are essential for blending:
-
Oxygenates represent a variety of chemical structures including ethers. Different numbers of carbon atoms in the R–O–R linkage are represented.
-
R- is Alkyl group such as methyl, ethyl, propyl, etc.
-
Oxygenates should be completely miscible with different fuels at engine operating temperature.
-
The low-temperature performance of the blend must be adequate for the operating climate.
-
The addition of an oxygenate with diesel or biodiesel should have an impact on its cetane number.
-
The oxygenated fuel must have long-term stability over a period of time.
-
The oxygenate breaking point might have been needed with a chance to be in the extent of working temperatures for diesel segments and the blazing point of the oxygenate was required to be greater than 52 °C to meet diesel fuel fire safety requirements.
Ethers are the best oxygenates for diesel as they can burn more fuel with an expansion in the oxygen centralization of a fuel-air blend, and power output of an engine along with emissions reductions (Ali et al. 2016; Arcoumanis et al. 2008). Analysts, after thorough investigation, report that because of the colossal vehicle usage increment, the request and accessibility of gasoline and diesel were to some degree unequal, and therefore these vitality requests are necessary to be adjusted with a long-term solution. The situation will be more calamitous if this circumstance proceeds with; the gasoline and diesel will be more outrageous and deficient. With expanding the usage and the consumption of non-renewable energy sources (petroleum derivatives), today more concentration and consideration is given to the other fuel innovation. Chauhan et al. (2010) led to investigate the mixing of diesel with methyl esters which have been an incredible differentiating choice for diesel. Distinctive diesel fuel blend mixes of diethyl ether, ethyl-tert-butyl ether (ETBE), mono-ethylene glycol ethyl and butyl ethers, diethylene glycol ethyl ether alongside cottonseed, jatropha, raw Karanja oil, and six other non-edible oils are considered. The outcomes demonstrated an expansion in NOx and a diminishment in hydrocarbons (HC), carbon monoxide (CO), and PM releases stood out from diesel. Biodiesel blended fuel demonstrated fall of brake thermal efficiency (BTE) and slight augmentation in brake-specific fuel consumption (BSFC) stood out from conventional diesel and their work prescribed 10–20% of the blend for whole deal use. Ren et al. (2008) led to inquire about utilizing six unique sorts of diesel-oxygenates which typically di-methoxy methane (DMM), diglyme (DGM), dimethyl carbonate (DMC), diethyl carbonate (DEC), and other ethers tried utilizing a constant speed engine. Their examinations uncovered that the ignition delay diminishes adversely for diglyme and increments for the other five mixes because of cetane number which was higher for diglyme and bring down to the others. Likewise, smoke diminishes without expanding the NOx, CO, and UBHC regardless of the kinds of oxygenated added substances, with the extension of oxygen mass division in the blends. Miyamoto et al. (1998) conducted a study of diesel mixed with four diverse oxygenate fuels typically diglyme, 2-butoxy ethyl alcohol, 2-Ethylhexyl acetate, as well as di-n-butyl ether and demonstrated that the positive change in the fumes outpourings and the brake thermal efficiency depended absolutely on the content of oxygen in the test fuels, paying little attention to the oxygenate to diesel fuel blend extents or type of oxygenate. NOx decreased with the oxygenate addition primarily by milder first-stage combustion and inalienable lower adiabatic flame temperature thus gave applicable data for proper determination of the oxygenate which was to be utilized as an additive to various blend proportions. The objective of the work done by Srihari et al. (2017) was to assess the impact of diethyl ether in biodiesel-diesel mixes on the performance qualities and emission traits in a premixed charge compression ignition-direct injection diesel engine (PCCI-DI). An examination is done on the biodiesel produced from the cottonseed oil. The PCCI-DI engine was worked with primary injection and pilot injection by various proportions of diethyl ether (DEE) with neat diesel with 20% of biodiesel. The emission characteristics showed detectable decreasing in emissions (NOx, BSCO, and BSHC) opposite ternary blends with the main injection. Advantages like diminishment in the quantum of smoke delivered an increase in BTE were likewise seen in cases. There are some beneficial properties of methyl tertiary-butyl ether (MTBE) which seemed to be a better option as an oxygenate in the screening process given below,
-
MTBE is an additive at low percentages as an octane number enhancer, later it was mixed at higher fixation as oxygenate to meet the perfect air prerequisite.
-
Worldwide request of MTBE in 2011 was 12.1 million tonnes at a compound annual growth rate of 4.2% and is moderately exceptionally less expensive.
-
MTBE has a chain-branched type with compact molecular structure, which prompts an upgraded protection from decomposition amid the ignition delay (Roy et al. 2000)
-
Besides, their high oxygen content prompts complete burning, lowering emissions of many air contaminations.
-
The reduced viscosity of MTBE-blended liquid should help advance in-cylinder blending through increased fuel breakup.
-
MTBE a has lower breaking point than diesel causing better evaporation (Yokota et al. 1998).
-
It was additionally expected that MTBE advances premixed combustion because of its low boiling point (Roy 2008).
-
MTBE has high volatility which decidedly influences the rate of rising and also the hike of the premixed combustion stage.
Considering all the above properties of MTBE oxygenate given in the Table 1 compatible for CI engines, few works were carried by the researchers among which Roy (2008) used MTBE as an oxygenate added substance with diesel fuel in extents of 5, 10, and 15% out of a multi-cylinder compression ignition diesel engine and at idle condition, he observed low UBHC. Yet, MTBE was generally utilized as a potentially added substance for a gasoline engine, its impact on diesel fuel on the overall attributes has not been represented in past works. The present examination gives an achievable pathway to meld the favorable aspects of MTBE with the diesel and methyl esters in CI engine. In an investigation of four classes of fuel added substances by Shih (1998), 2-ethylhexyl nitrate, di-tert-butyl peroxide followed by MTBE, Diglyme, and Monoglyme blends were used which affected the fuel spray penetration, fuel-air blending frames, start delay, compound reaction rates, and rate of heat release(HRR) on a three-cylinder Yamaha ME200F diesel engine under various loads and speeds. The interesting outcomes especially by MTBE blends (5 and 10% volume portion) uncovered that HC formation expanded as the convergence of MTBE was more and the smoke was higher for 10% of MTBE and 10% MTBE turned out to be the best fuel added substance for NOx lessening alongside decrease in exhaust gas temperature. Kajitani et al. (1994) probed an agricultural water-cooled single cylinder compression ignition engine and reasoned that the blending of MTBE in diesel fuel can bring down the smoke and NOx discharge levels. In this manner, ignition delay was expanded, and the combustion span was abbreviated. Likewise, this MTBE oxygenate added substance can lessen the concentration of OH radical in the combustion pre-flame zone and postpone the fuel’s spontaneous ignition with the goal that the emissions of the engine can be progressed. Awad et al. (2018) reviewed the effects of various oxygenated fuels for its engine output characteristics on the impact of compression ratio. They suggest that the addition of MTBE to the fuels like diesel and biodiesel results in excess oxygen content which varies the engine output conditions. Also, they suggest that MTBE can be used in the high-power rating engine with variable compression ratio due to its properties. Topgül (2015) studied the impact of MTBE blends varied from 5 to 30% on the volume basis in a spark ignition engine. Experimental results witnessed that the reduction in fuel consumption with improvement in the efficiency. Furthermore, HC and CO emissions are reduced for the higher concentration of MTBE in the blend with the penalty of NOx emissions. Similarly, there are some research works that have been reported on the MTBE as oxygenated additives in SI engines (Cerri et al. 2013; Cataluña et al. 2011).
Objective and novelty of the work
In view of the basic literature overviewed, alternate fuel innovation was picking up prominence because non-edible oil assets are increasing overall consideration, being sparing contrasted with edible oils where Calophyllum inophyllum filling in as a promising feedstock for biodiesel generation highlighting it as a sensible feedstock for making biodiesel in business scale. However, the principle issue related to MTBE is poor cold flow property which prompts the crystallization of fuel particles leading to operation difficulty in icy climatic condition. To defeat this issue, ternary blended diesel fuel normally with the addition of oxygenates are considered where the expansion of oxygenating added substances enhanced the performance and emission outflow characteristics. In most of the earlier literature, MTBE has been used as octane booster additive with gasoline at different concentrations in various types of spark ignition engines and very few works were carried on MTBE as an additive with diesel and thus identifying literature gap. The main target of this experimental study is focused on investigating different characteristic attributes of a CI engine chipping focusing on diesel-CIME and MTBE ternary blended fuel. All the blends are prepared at various blends ratio where the percentage of diesel is kept constant with varying proportions of MTBE and biodiesel. In all the ternary blends, the MTBE fractions are increased from 5 to 20% by reducing the Calophyllum inophyllum methyl ester (CIME) fractions in the blends. Furthermore, the outcomes of these blends operation in the diesel engine are compared with pure diesel and pure biodiesel along with diesel-biodiesel blend. All the fuels are tested in a constant speed diesel engine with varying engine loads.
Materials and methods
The fuel blends have been set up in two stages for the present investigation. At the first stage, the Calophyllum inophyllum seeds oil undergoes transesterification process for biodiesel preparation and the second stage was the preparation of ternary blend blends with diesel, Calophyllum inophyllum biodiesel, and MTBE. The point by point fuel preparation methods in detail is discussed below.
Preparation of CIME
The transesterification process was used for biodiesel extraction from dried seeds of Calophyllum inophyllum as well as to reduce its viscosity. The procedure was conveyed in the three phases as discussed underneath.
Acid-catalyzed esterification process
Ninety-five milliliters of methyl alcohol along with 5 ml of sulfuric acid, typically a methanoic solution in addition to Calophyllum inophyllum seed raw oil at a molar proportion of 16:1. This blend containing the above mixture was then heated to a temperature underneath 600 °C for various time terms (30–90 min with 15 min time gap) at a consistent mixing rate. Based on several iterations, the blend which was heated to a temperature of 600 °C for 45 min term was observed to be an ideal working condition for acid-catalyzed esterification process.
Further esterification procedure proceeded with sodium hydroxide-soluble alkali catalyst to diminish the free unsaturated fatty acids in the Calophyllum inophyllum oil post which in the second stage, the acid-catalyzed Calophyllum inophyllum oil was blended with alkali catalyst. This procedure was done for a few cycles at various molar proportions and time terms. The ideal conditions for the salt transesterification process were gotten for methanol oil molar proportion of 6:1, 1% weight convergence of sodium hydroxide, and a response temperature of 60 °C. This blend must be stirred at a consistent rate for a response time of 30 min. By this procedure, biodiesel was produced and it was assessed the generation cost of Calophyllum inophyllum biodiesel was roughly 3.2 times higher than oil diesel cost. The fatty acid composition Calophyllum inophyllum methyl ester has been presented in Table 2.
Purifying of the resultant oil
The resultant oil obtained from the transesterification was methanol which contained ester of Calophyllum inophyllum where the methanol content available must be removed by purification process where oil with traces of methanol was heated above 75 °C where all the methanol present in the oil gets evaporated and hence the final oil content which was present was Calophyllum inophyllum biodiesel whose yield is 85%.
Preparation of blends
In the present study, all ternary fuel blends are prepared with the help of pure diesel, neat biodiesel (CIME), and MTBE as major fuel components. In total, seven fuels have been prepared at required quantity including pure diesel, transesterified biodiesel, and one binary blend on the basis of equal proportions of diesel and biodiesel on the volume basis (50% of diesel and biodiesel). The autoignition quality of MTBE is very poor and fewer studies also suggested that these kinds of oxygenated blends could be used up to 10% by volume. Therefore, in the present research work, the MTBE has partially replaced the biodiesel (CIME) by 5, 10, 15, and 20% for the preparation of ternary blends. Moreover, very few literatures are available on the effect of ternary blends in diesel engine. During ternary fuel blends preparation, the composition of diesel is kept at a constant level as 50% on a volume basis in the total quantity. The remaining 50% of the fuel blend is varied by varying the concentration of biodiesel and MTBE on the volume basis. In the present study, the MTBE concentration is increased from 5 to 20% in the ternary blends with the reduction of biodiesel concentration. It is also noted that the CIME biodiesel concentration is varied in the total quantity as 45, 40, 35, and 30%. All the prepared fuel compositions are given below. The MTBE percentages are taken as 5, 10, 15, and 20% where the maximum percentage is restricted to 20% because excess oxygenate results in the drop of maximum flame temperature which affects the thermal efficiency. The properties of all ternary diesel-CIME-MTBE have been presented in Table 3.
-
1.
Blend-1: Diesel
-
2.
Blend-2: Biodiesel
-
3.
Blend-3: D50-B50
-
4.
Blend-4: D50-B45-MTBE5
-
5.
Blend-5: D50-B40-MTBE10
-
6.
Blend-6: D50-B35-MTBE15
-
7.
Blend-7: D50-B30-MTBE20
The stability of the CIME-diesel binary blend and four ternary blends of diesel-CIME-MTBE are tested through the standard gravitational technique. Before testing these fuels in diesel engine, all the fuel blends are kept in separate containers for 1-week duration in order to observe the phase separation issue. Notably, the binary blend and four ternary blends are very stable in a container without showing any phase separation difficulty.
Experimental set-up
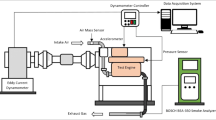
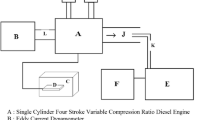
The specialized detail of the diesel engine was exhibited in Table 4 and a simplified outline of the exploratory set up having various additional components is showed in Fig. 1. Load of the engine fluctuated by strategies for the eddy current type dynamometer with the assistance of controller operating in a closed-loop. Intake air flow rate was estimated with the assistance of a standard air tank framework with a manometer. Estimation of engine fuel consumption was determined by noting the time taken for 100 cc through standard burette mechanical assembly. The measurement of exhaust gases typically HC, CO, NOx, and carbon dioxide (CO2) were done by AVL digas 444N gas analyzer. The CO, CO2, and HC emissions are measured based on nondispersive infrared (NDIR) principle and NOx emission are measured through electrochemical method. Table 5 shows the principles of measurement of exhaust gas analyzer. The diesel engine exhaust smoke content is measured using AVL437C smoke meter during engine operation with all fuels. These parameters are then changed over according to outflow standard, i.e., g/kWh. A K-type thermocouple was utilized for deciphering the gas temperature. Also, the in-cylinder pressure was measured by a pressure transducer by KISTLER having an affectability of around 79.5 pC/bar which was mounted on the cylinder head. An AVL 365C Indi Advanced Crank Angle Encoder estimates the respective crank angle and TDC position was recognized by an electro-optical sensor. AVL INDI-MICRA 602-T10602A which was basically a Data Acquisition System of digital type and the computer-based system receives the different signals.
Examinations were led five times and for calculations, the average of all the five values was taken. The present experiments included errors which have been caused due to adjustment, the accuracy of the equipment, natural conditions, and perception blunder and so on. The percentage of uncertainty for various quantities was evaluated based on the square root method which was given in Table 6. The overall uncertainty of the experiment was calculated as follows:
Results and discussions
The prepared blends whose performance and emission characteristics were studied by testing it on a constant speed CI engine at 23° BTDC starting of fuel injection and 200 bar of fuel injection pressure at various loads of 0, 25, 50, 75, and 100% which are discussed below.
Performance characteristics
Performance characteristics include brake thermal efficiency, a measure of fuel efficiency, or brake-specific fuel consumption as well as brake-specific energy consumption were discussed below.
Brake thermal efficiency
Brake thermal efficiency (BTE) variation with brake mean effective pressure (BMEP) for diesel, CIME, D50-B50, and all other ternary blends with increasing MTBE percentages was shown in Fig. 2. For all the fuels tested, the brake thermal efficiency tends to increase with the increment in engine load. The change in BTE at higher loads of the engine was expected to the attainment of higher brake power for the corresponding increase in fuel rate. The maximum brake thermal efficiency was 34.14% for diesel, 27.91% for biodiesel, 33.27% for D50-B50, 33.18% for D50B45MTBE5, 32.46% for D50B40MTBE10, 32.14% for D50B35MTBE15, and 31.83% for D50B40MTBE20 at the maximum load (BMEP). The results showed that for neat CIME and D50-B50, brake thermal efficiency showed fall of 18.24 and 2.54% respectively, whereas for all ternary blends, the brake thermal efficiency was reduced with respect to that of the baseline diesel (not more than 6.7%). This loss was because of the higher density of the blend, higher fuel blend viscosity, and lesser calorific value of both blends (Jaichandar and Annamalai 2013). Overall at higher loads, there are fluctuations in BTE reported for ternary blends and was low when compared with diesel. This was due to the uneven combustion at higher speeds where increase in the fuel viscosity and its density resulted in the poor atomization of the fuel droplets as well as vaporization. Also, the brake thermal efficiency was dependent on the energy content of the fuel (calorific value) and the CV of all blends are comparatively low which was also a reason. It was to be noted that the addition of MTBE enhanced the rate to some extent at maximum load conditions and resulted in minimum BTE loss (maximum of 6.7%) when compared with the neat diesel and D50-B50. Comparable outcome was likewise seen by Rakopoulos et al. (2012) where three loads were viewed as whose brake thermal efficiency of DEE with different rates of (5, 10, 15, 20, and 25%) in its mixes with diesel fuel are considered and the outcomes demonstrated lessening in BTE as for increment in the level of DEE because of the reduction in calorific estimation of the whole blend when compared with baseline diesel where maximum BTE was 31% at high load.
Brake-specific fuel consumption
Brake-specific fuel consumption (BSFC) is characterized by the amount (mass stream rate) of the fuel per unit of created brake power. Additionally, building up certain brake power, the fuel amount quantified represents BSFC. Brake-specific fuel consumption indicates the efficiency with which an engine converts the supplied energy to useful work output and for any engine, a lower value for BSFC is always desirable where lower brake-specific fuel consumption can be achieved by the higher calorific value of the fuel. The maximum brake-specific fuel consumption for diesel was 0.45 kg/kWh, 0.59 kg/kWh for biodiesel, 0.47 kg/kWh for D50-B50, 0.48 kg/kWh for D50B45MTBE5, 0.475 kg/kWh for D50B40MTBE10, 0.484 kg/kWh for D50B35MTBE15, and 0.49 kg/kWh for D50B40MTBE20 at minimum BMEP. From the Fig. 3, it was vivid that the BSFC decreases as the BMEP increases which was mainly because the power development plays a dominant role when compared to fuel consumption as the engine load increases. The general increasing trend of biodiesel and D50-B50 with respect to that of the neat diesel was about 31.1 and 15.5% higher respectively and also the increasing trend of ternary blends at low and medium loads consuming a different amount of fuel which was as high of about 5.5% when compared to that of diesel. This was due to the lower calorific values of the fuel blends to keep up a similar output of the obtained power, and more fuel should be provided to the engine and it was observed that the amount of CIME sprayed or injected was more than that of the other blend test fuels due to the higher viscosity and density of the CIME blend thus, injecting higher amounts of CIME into the combustion chamber (Mosarof et al. 2016). Alptekin (2017) have stated that the inherent oxygen content of any fuel would decrease the heat content value and which intern resulted in higher fuel consumption. At maximum load, there was also an increase in brake mean effective pressure where 5%MTBE contained blend showed better results than the remaining blends at maximum loading operations and 15% MTBE, 20% MTBE had almost same fuel consumption rates. Sezer (2011) also got a similar trend of BSFC where the percentage of specific fuel consumption where 43.5% was observed for dimethyl ether and 23.6% BSFC for diethyl ether which was mainly to maintain the same output power where more fuel is injected into the combustion chamber.
Brake-specific energy consumption
For the most part, brake-specific energy consumption (BSEC) is the proportion of vitality obtained by consuming fuel for an hour to the real energy acquired at the wheels which are dimensionless.
Brake-specific energy consumption (MJ/kWh) = brake-specific fuel consumption (kg/kWh) × net calorific value of fuel (MJ/kg).
It is demonstrative how viable the energy acquired from the fuel is achieving the wheels. The impact of test energizes on BSEC for various ternary mixes was shown in Fig. 4. The BSEC was higher when the engine running on pure biodiesel at all loads. This was due to the inefficient burning of fuel at lower compression. At lower loads, the combustion was decayed because of the lower pressures inside the cylinder and thereby the more measure of fuel was infused into the ignition chamber to deliver the required power yield. BSEC of ternary blended fuels was increasing with increase in the concentration of MTBE percentage for all values of BMEP and BSEC had a decreasing trend as load increases. The increasing of engine load will increase the cylinder pressure and temperature which causes decreasing the tendency in the BSEC. The maximum brake-specific energy consumption for diesel was 19.055 MJ/kwh, 22.42 for biodiesel, 19.11 MJ/kWh for D50-B50, 19.248 MJ/kWh for D50B45MTBE5, 19.322 MJ/kWh for D50B40MTBE10, 20.04 MJ/kWh for D50B35MTBE15, and 20.32 MJ/kWh for D50B40MTBE20 at minimum brake mean effective pressure (BMEP). At 25% engine load, the MTBE5% blended fuel has 1.2% higher BSEC than neat diesel which is minimal when compared with the other ternary blends and even same at 100% engine load. Pure biodiesel fuel has higher BSEC compared to neat diesel for all operating conditions as heat released was lower for CIME and MTBE blends because of the lower calorific values. The delay in the start of ignition of ternary blends resulted in the rise of BSEC because of the downturn of the burning procedure (Jaichandar and Annamalai 2013). Property enhancement of the Calophyllum oil like diminishment in thickness, autoignition temperature of the atomized fuel, resistance to autoignition capability, fuel’s fire point which was the motivation behind why BSEC of ternary mixes is lower than that of CIME. In any case, the BSEC esteems increment marginally at the higher pressures proportion because of fuel dilution by Sezer (2011) where BSEC was dependent on the amount of fuel consumed.
Emission characteristics
The combustion attributes of diesel engine can be assessed by observing the pressure being developed inside the cylinder and the rate at which the amount of heat being released which are explained below.
Unburnt hydrocarbon emissions
The deviation of UBHC emissions for distinct blends of test fuels was shown in Fig. 5. UBHC emission trends to diminish for all tests with the gradual increment of BMEP due to its enhanced combustion. The maximum HC emission for diesel was 0.82 g/kWh, 0.26 g/kWh for biodiesel, 0.42 g/kWh for D50-B50, 0.43 g/kWh for D50B45MTBE5, 0.51 g/kWh for D50B40MTBE10, 0.58 g/kWh for D50B35MTBE15, 0.76 g/kWh for D50B40MTBE20 at minimum brake mean effective pressure (BMEP). For neat diesel, the maximum HC emission observed at all engine loads. Delay in the start of the ignition due to which favors more opportunity for the fuel vapors to diffuse and an unreasonable part of fuel controlled in the lean flame out region which caused an expansion in HC emissions. Also, if neat diesel in contrast with CIME and blend which consist equal amount of diesel and biodiesel are considered, pure diesel had maximum HC of 0.82 g/kWh, CIME had 0.26 g/kWh and when engine running with D50-B50 blend emitted 0.43 g/kWh of HC in its exhaust gases. This was mainly due to the lower volatility of biodiesel compared with pure diesel which contributed in a larger difference in HC emission. However, the HC emissions slightly increased when the engine was run with ternary blend blends in contrast with CIME (Ashok et al. 2017). At all the loads, as the MTBE concentration increased, the UBHC emissions were also increased with a positive result which was less than that of HC emitted by pure diesel fuel. This satisfactory reduction happened due to the complete burning of the blended fuel with the effect of MTBE which was basically an oxygenate. Due to peak pressures in the cylinder during the power stroke at maximum brake mean effective pressures, the hydrocarbon molecules are burned effectively. The maximum HC was observed in MTBE20% blended fuel at all engine loads of about 44% greater than MTBE5% blend and 7.27% less than that of neat diesel. This in resemblance with one of the researches done by Liu et al. (2017) where diesel and polyoxymethylene dimethyl ethers (PODE3–4)/diesel blends with 10–30% PODE3–4 where the high ignitability of PODE3–4 promotes the combustion in over-mixing zones and advancement of combustion mainly at over-mixing zones was due to the ease of ignition of PODE3–4 where 4.6% reduction in UBHC was seen.
Carbon monoxide emissions
Formation of carbon monoxide takes place when the fuel burns in an insufficient supply of air and at a low temperature. Figure 6 depicts the change of CO with respect to BMEP for all the test blends. The maximum CO emission for diesel was 11.998 g/kWh, 10.44 g/kWh for biodiesel, 11.46 g/kWh for D50-B50, 11.23 g/kWh for D50B45MTBE5, 11.42 g/kWh for D50B40MTBE10, 11.73 g/kWh for D50B35MTBE15, 11.85 g/kWh for D50B40MTBE20 at minimum BMEP. It was inferred that carbon monoxide emissions decrease with increase in engine brake mean effective pressure. Neat Calophyllum inophyllum biodiesel has shown 15.4% lower CO as that of neat baseline diesel at 100% engine load. The O2 content and higher resistance to autoignition produced complete combustion for CIME fuel operation which resulted in lower CO emission (Ashok et al. 2017). It was inferred that the CO emitted by all MTBE fuel mixes was lower than the relating baseline diesel fuel case where CO emissions got decreased with the increase in the volumetric concentration of oxygenate. Whereas in contrast with pure diesel, this reduction happens due to the complete burning of the blended fuel with the effect of MTBE. Also, MTBE blends caused slower dissipation, slower and poorer fuel-air blending due to the higher latent heat resulted in the increase in the CO emission among the ternary blends with an increase in the concentration of MTBE in the test blend. The maximum CO was observed in MTBE20% blended fuel at all engine loads of about 3.3% greater than MTBE5% blend and 1.18% less than that of neat diesel. Decisively, the CO follows indistinguishable conduct from the emitted HC, a reality all in all ascribed to the same physical and chemical systems influencing nearly, similarly, a significant lessening in CO discharges for all ternary mixed fuel contrasted with baseline diesel. It was observed by Sivakumar et al. (2010) that there was complete burning related with MTBE blends. Attributable to the extra oxygen content and abbreviated ignition start qualities of MTBE blends resulted in the reduction of UBCO. UBCO outflow for diesel with 4% MTBE at idling was 0.02% having 0.03% for baseline diesel.
Carbon dioxide emissions
The variation of CO2 emission for the test fuels at different fuel blends was shown in Fig. 7. Due to the improvement in the combustion process inside the combustion chamber, the rate of CO2 formation got increased. A considerable reduction in CO2 emissions for a higher concentration of ternary blended fuel at all BMEP, but at lower percentages, the rate of CO2 formation was less when compared with baseline diesel. The maximum CO2 emission for diesel was 10.2%, 10.8% for biodiesel, 10.4% for D50-B50, 10.2% for D50B45MTBE5, 9.76% for D50B40MTBE10, 10.3% for D50B35MTBE15, and 9.77% for D50B40MTBE20 at maximum brake mean effective pressure (BMEP). It was revealed in Fig. 7 that the CO2 emissions were increased because more amount of fuel was injected due to an increase in the engine load. For the ternary blends of CO2 emissions, the CO2 formed was less than that of the pure diesel. If the molecular structure and the number of carbon atoms were considered, MTBE has less number of carbon atoms as that of the pure diesel and hence the amount of CO2 formed during the combustion of the blended blend was observed to be less than that of baseline diesel. Here, it was to be noted that the CO2 reduction was mainly due to the low carbon content of the MTBE additive but not due to any other combustion factors that affect the rate of CO2 formation. Similar results were obtained when Sezer (2011) performed various experiments on a diesel engine using dimethyl ether and diethyl ether as fuels under various equivalence ratios and the results were compared to conventional diesel fuel. The experimental results under different engine speed revealed that the CO2 emission is reduced by 39.21 and 5.8% for DME and DEE fuels at maximum engine speed and this is attributed to the simple molecular structure of ethers and its low carbon content which has resulted in low CO2 emission.
Nitrogen oxides emission
At bring down temperature, the nitrogen molecule is stable where the mono-atomic dissociation of nitrogen takes place at extremely higher temperatures. This dissociated nitrogen molecule being highly reactive and to reach equilibrium, it reacts with the nascent oxygen and forms nitrogen oxides. This formation of NOx can be explained by a three-step Zeldovich mechanism as follows (Nanthagopal et al. 2016):
One of the most important factors in the emission of oxides of nitrogen was the in-cylinder temperature and the oxygen content of the fuel. The higher the combustion temperature and oxygen concentration, the higher was the formation of NOx. Other factors like compression ratio, cylinder geometry, inlet air temperature, and pressure and chemical properties of fuel play an important role in the formation of NOx. Figure 8 shows the variation of NOx with respect to brake mean effective pressure for all the test fuels. The maximum NOx emission for diesel was 11.87 g/kWh, 15.69 g/kWh for biodiesel, 14.40 g/kWh for D50-B50, 11.59 g/kWh for D50B45MTBE5, 10.99 g/kWh for D50B40MTBE10, 10.18 g/kWh for D50B35MTBE15, and 7.67 g/kWh for D50B40MTBE20 at maximum BMEP. It has been observed that nitrogen oxides emissions increase with an increase in engine BMEP as engine running at very lean mixtures as engine rpm increases which are favorable for the formation of NOx. Also, the NOx emission increased about 40% when the diesel engine fuelled with CIME fuel compared to conventional diesel. Rahman et al. (2013) have suggested that the faster burn rate of the biodiesel during the uncontrolled combustion phase along with lower radiation heat transfer and variable flame temperature results in higher emissions of oxides of nitrogen. CIME has higher cetane index compared to pure diesel which reduces the ignition delay period and helps achieve better combustion with higher NOx formation (Monirul et al. 2016). For the ternary blends of inference can be drawn for ternary MTBE blends that the NOx formed were lower than the comparing ones of the perfect CIME and baseline fuel blend. The lessened premixed part of combustion where nitrogen oxides predominantly form, just mostly exceeded by the additional fuel-bound oxygen bringing more ‘zones’ close to stoichiometric conditions and little to the lean were the attributes for less NOx formation (Lamani et al. 2017). The ternary blends demonstrated a little higher decrease, which might be due to its latent heat which resulted in the cooling effect which majorly reduced NOx despite an increase in oxygen content in the fuel due to MTBE content in it. Shih (1998) stated that the formation of NOx was very sensitive to the cylinder charge temperature and the addition of MTBE resulted in the decrease of exhaust gas temperature and thus reduced the NOx up to 25% at an equivalence ratio of 0.5 and mentioned the reasons behind.
Smoke emission
The indication of incomplete combustion is the smoke formation which occurs primarily due to deficiency of air in combustion-rich zone, the viscosity of the fuel being very high, poor atomization, and accumulation of excess fuel inside the cylinder. Figure 9 demonstrates the variety of smoke at different loads for all the tested fuel blends. The maximum smoke emission for diesel in terms of its opacity was 72.8%, 63.2% for biodiesel, 66.4% for D50-B50, 75.7% for D50B45MTBE5, 81.4% for D50B40MTBE10, 82.7% for D50B35MTBE15, and 83.4% for D50B40MTBE20; at minimum brake mean effective pressure (BMEP), the smoke emissions for all fuel blends were increased with increase in engine load. For constant speed engine, smoke emission increased with increase engine BMEP as the fuel supplied per stroke is relatively more. Interestingly, the higher smoke emission was observed for diesel than the baseline fuel at 100% engine load. At maximum brake power, the smoke emission was 72.8% for diesel fuel, whereas for neat Calophyllum inophyllum biodiesel, it was 63.2%. The inherent oxygen content of CIME caused complete combustion and lower smoke emission (Nanthagopal et al. 2017). Poorer spray characteristic properties resulted in higher smoke opacity of MTBE at all ranges of BMEP which recorded lower values of 3.8, 10.56, 11.97, and 12.7% than the baseline blend respectively for 5, 10, 15, and, 20% of MTBE concentration at 100% load; regardless of the way that the oxygenates bring down smoke discharges, the fundamental explanation behind MTBE to show higher smoke, in examination with diesel fuel, was poor atomization and lacking time for oxidation were the reasons behind. Similar results of increase in smoke was observed by Venu and Madhavan (2017) where the blend of methanol-diesel-jatropha with 5% volumetric concentration of DEE and ethanol-diesel-jatropha with DEE of 5% showed increase in smoke opacity where it was mentioned that this rise was due to the low in-cylinder temperature and the spray characteristic diminishment due to the higher viscosity.
Combustion characteristics
The combustion characteristics determine the mechanisms or the rate of reactions taking place inside the combustion chamber when the fuel was burnt to produce heat energy where these characteristics mainly depend on the intermolecular structures, chemical, and physical properties. The combustion parameters are discussed below.
In-cylinder gas pressure
The cylinder pressure against crank angle diagrams at 50 and 100% loads for the neat diesel fuel at maximum load was shown in Fig. 10. Where biodiesel (CIME), B50-D50, and other four ternary blend blends with MTBE concentrations of 5, 10, 15, and 20%, concentrating on their part around high-temperature TDC where the peak pressure variation with the degree crank angle (− 300 to 400° with TDC). Generally, in CI engines, the mass fraction burned mainly decides the peak pressures developed inside the cylinder where initially it depends on the rate at which the combustion takes place and the fuel which is being burnt in the uncontrolled combustion stage (Fig. 11). Also, the pressure developed increases gradually in the cylinder due to more amount of fuel being consumed when load increases. The combustion starts earlier for CIME fuel contrasted with diesel because of its inborn oxygen content. From Fig. 10, the attainment of peak in-cylinder gas pressure was greatly influenced by delay period and the amount of fuel involved when uncontrolled combustion phase starts. As expected, higher peak cylinder pressure was observed for diesel fuel compared to CIME and D50-B50 blend fuel blends which were highly dependent on the calorific value of the fuel (Ashok et al. 2017). For ternary blended blends, it can be inferred that the beginning of burning happens later with an expanding the level of MTBE in the mix, while the peak pressure is delayed and is lower. Also, the premixed part of combustion was observed to decline as the concentration of oxygenating increased which was due to the lower pressure during the combustion. The maximum peak pressure for the tested fuels by Sivakumar et al. (2010) depicted that for the MTBE concentration of 4%, the peak pressure observed was 71.5 bar which in contrast with diesel showed 74.8 bar. He mentioned that the reduction in the premixed part of combustion of the oxygenated blend was the reason behind the fall of peak cylinder pressure.
Heat release rate
Heat release rate was calculated from the in-cylinder pressure as per the first law of thermodynamics. For 100% of respective BMEP with respect to the crank angle for the distinct blends plotted are shown in the Fig. 12. The combustion and the events taking place at different stages is an indication of the total rate of heat release (HRR) where the initial and second peaks define the premixed phase of combustion and mixed-phase combustion. The rate at which the heat is being released of pure CIME was comparatively very less and this rise was proportional to the volumetric quantity of the diesel (D50-B50 blend). The shorter delay period in the ignition due to the higher cetane index which is a direct measure of ignition quality resulted in the lower hear release of CIME. There are also factors like higher resistance to flow, surface tension and very poor atomization of the fuel droplets can affect the heat released during the combustion of CIME (Nanthagopal et al. 2017). A negative release of heat was observed initially for all the test blends which indicated the evaporation of the mixture then followed by a positive rate of heat release due to delay period post abrupt combustion at the air-fuel premixing phase and diffusion combustion phase where mixing velocity controls the rate at which the fuel was burnt. At 50% OF BMEP, a longer delay in the start of combustion of ternary MTBE-blended blends which affected the maximum pressure generated and the rate at which the heat energy is released. The hike in the delay period with different concentrations of MTBE in the ternary blended blend can be explained by the vaporization of MTBE which caused injection of the fuel inside the low-temperature region particularly at lower BMEP. D50-B30-MTBE20 showed the highest value of 46.41 J/° CA which was 7.21% more than that of the baseline test blend which was potential because of higher unpredictability and low idle latent heat properties of MTBE causing abundance fuel collection amid the premixed part of combustion post higher energy release per unit time at maximum BMEP. Peak HRR of ternary blends approaches near TDC which can be explained based on latent heat, cetane index which prolonged ignition delay. MTBE15% blend with enriched O2 content and their higher resistance to atomization which reduced the premixed combustion phase reasoned lower heat energy release per unit time. One of the experiments done by Sivakumar et al. (2010) demonstrated comparative outcomes that the heat energy release rate was 31.7 J/° CA for 4% of MTBE, while it was 33.81 J/° CA for baseline fuel blend. The diminished extent of HRR for the MTBE mixed diesel energizes could be because of the enhanced start of combustion and the better burning.
Ignition delay
Ignition delay (ID) is the length between the beginning of fuel injection (SOI) and the beginning of combustion (SOC) as far as CA deg. Some of the fundamental factors which influence the delay period were physical/chemical characteristics, cetane index, chamber temperature, and its pressures. Figure 13 demonstrates a variety of ignition delay with brake mean effective pressure for every blend. As the load got increased, the delay period decreased which was because of higher peak pressures. Ignition delay for neat diesel was observed to be 16° CA, 14.3° CA for biodiesel, 14.8° CA for D50-B50, 16.78° CA for D50B45MTBE5, 17.6° CA for D50B40MTBE10, 17.7° CA for D50B35MTBE15, 23.2° CA for D50B40MTBE20 at minimum brake mean effective pressure (BMEP). Ignition delay of neat CIME and D50-B50 was lower than the neat diesel due to the rich O2 content in the CIME which was adequate to ensure the complete burning of fuel during the main combustion than neat diesel. For all ternary blends, ignition delay was increased as the concentration of MTBE increases in the blend than that of the pure diesel throughout the engine load. This was mainly due to physical delay (Yang et al. 2014) where it was anticipated that the varying proportions of MTBE in the MTBE-blended diesel fuel would result in differing physical properties, with a consequent effect on the physical time delay associated with spray droplet formation and vaporization where longer ignition delay lead to larger premixed part of combustion and less mixing controlled burning part. Due to its lower viscosity, the MTBE blend was expected physically to produce altered spray geometry with a short breakup length and this will result in enhanced mixing (Donahue and Foster 2000). Also, the high volatility of the MTBE in the blend will also help promote the formation of the premixed gaseous mixture. On the other hand, evaporation of the diesel spray was considered to limit mixing: if so, the key parameter for the spray evaporation will be the enthalpy of vaporization rather than the fuel volatility. This results in the prolongated physical ignition delay. A generally more prominent resistance from decomposition and the propensity for utilization of OH by MTBE are relied upon to influence both the physical and chemical aspects of the ignition delay. Donahue and Foster (2000) investigated the effect of 20% of MTBE concentration in diesel at different timing valve close (TVC) signal where the start of injection takes place. They observed that the delay period was increased where they stated that the delay at 75% of the load was mainly due to the lower peak pressures due to the lower heating values of the MTBE additive.
Mass fraction burnt and mean gas temperature
The diesel engine combustion process is also examined by means of mass fractions burned and its mean gas temperature formation with respect to crank angle. These two parameters are very much essential to investigate the fuel properties on diesel engine characteristics. The mass fractions burned of all tested fuels are estimated using Weibe function which is presented below (Stone and Green-Armytage 1987)
Where
- x :
-
= mass fraction burnt (%)
- θ:
-
= crank angle
- θ0:
-
= crank angle at the start of combustion
- Δ θ:
-
= combustion duration (crank angle degrees)
- a :
-
= duration parameter which indicates the completeness of combustion
- m :
-
= form factor which characterizes the combustion rate (0.35–0.46)
The variations of mass fractions burned with respect to crank angle for 100% loading condition is presented in Fig. 14. It is witnessed that the concentrations of CIME and MTBE in the ternary and binary blends play a pivotal role in mass fractions burned. The D50-B50 fuel has shown higher mass fractions burned at the same crank angle as compared to all other fuels. This might be due to the reduction in the net calorific value of the blend which has resulted in higher mass fractions burned. On the other hand, the increase in the concentration of MTBE in ternary blends decreased the burning fractions and 20% of MTBE has shown lower mass fraction burned.
The mean gas temperature can be derived from the heat release rate, in-cylinder pressure, and fuel injection; the graph represents the interconnected relationship between all the factors which help give a satisfactory combustion. The variation of mean gas temperature with respect to crank angle for all tested fuels is shown in Fig. 15. It is also evident that the diesel fuel has a higher mean gas temperature when compared to all other fuels due to its higher net calorific value. The presence of oxygen content in the blends because of the addition of MTBE reduced the mean gas temperature and the reduction in mean gas temperature is higher with a higher concentration of MTBE in the ternary blends.
Conclusion
A single cylinder direct injection CI engine was fueled with diesel, pure CIME, and ternary blended diesel-biodiesel-MTBE (oxygenated additive). Performance, emission, and combustion characteristics of the ternary blend blends of 5, 10, 15, and 20% have been studied post the results were analyzed by comparing with the neat baseline diesel fuel blend. The primary conclusions of this experimental study are summarized as follows:
-
These ternary blends have lower brake thermal efficiency compared to pure diesel. Least reduction in brake thermal efficiency of 3% was found for 5% of MTBE of concentration at all the loads and BTE decreases as the concentration increases due to the lower calorific values of the overall blend.
-
Increase in BSFC and BSEC of 6 and 4.1% at higher load is noted for the 5% of MTBE blends with respect to neat diesel fuel in order to maintain the constant output power.
-
The unburned hydrocarbon (BSHC) and CO emissions got reduced for all blend blends where maximum reductions are 63.9 and 6.4% respectively at maximum load for 5% of MTBE concentration because of the enhanced combustion taking place inside the combustion chamber due to the rich oxygen content.
-
The carbon dioxide emissions followed the same trend as that of baseline diesel fuel with almost the same amount of emission for a lower concentration of MTBE but were reduced almost by 4.2%.
-
However, the opacity of the smoke was increased by 3.9–12.7% as the MTBE concentration got increased which was due to diminished spray characteristics and the cooling effect of MTBE.
-
Significant reduction in NOx emission was observed for all ternary blends compared to neat diesel at minimum brake mean effective pressure where 20% of MTBE achieved maximum reduction of 3.37%. This was due to the reduced part of premixed combustion and the cooling effect taking place due to its low latent heat of vaporization of MTBE reducing the blend temperature.
-
In the combustion characteristics, the peak pressure, heat release rate, and ignition delay of 5% MTBE blend is 71.1 bar, 41.9 J/°, and 16.78°. MTBE blends have shown lower in-cylinder gas pressure and heat release rate at 50 and 100% engine loads. This peak in-cylinder gas pressure decreases with the addition of MTBE to CIME fuel because of the longer ignition delay which reduced the premixed part of combustion which affected the peak pressure and heat release rate.
The experimental results obtained for various concentrations of MTBE with diesel–biodiesel blends are compared with similar kind of works and the same has been presented in Table 7. The earlier studies of ternary blends of diesel-biodiesel with DEE and biodiesel–bioethanol were taken for comparative analysis. It is noted that the addition of 5–10% of DEE with diesel-biodiesel blends showed significant improvement in BTE, BSFC, and NOx emissions. However, there is no specific trend in CO, HC, and smoke emissions. On the other hand, the binary blends operation with 5% of bioethanol with diesel-biodiesel blends improved the performance, combustion, and emission characteristics.
Therefore, from the present work, it may be concluded that the ternary blends of diesel-biodiesel-MTBE are the promising alternative fuels for a diesel engine for significant reductions in exhaust emissions where technology must be made cost-effective to implement in the market on a wide scale basis. Hence, a lot more research works need to be carried out in ternary blend composition along with material compatibility studies in diesel engine before adapting the ternary blend as regular fuel for diesel engine applications.
Abbreviations
- CI:
-
Compression ignition
- CIME:
-
Calophyllum inophyllum methyl ester
- DEE:
-
Diethyl ether
- MTBE:
-
Methyl tertiary-butyl ether
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- BTE:
-
Brake thermal efficiency
- BSFC:
-
Brake-specific fuel consumption
- BSEC:
-
Brake-specific energy consumption
- B100:
-
100% Biodiesel (Calophyllum inophyllum)
- D50B50:
-
50% diesel + 50% biodiesel
- D50B45DEE5:
-
50% diesel + 45% biodiesel + 5% diethyl ether
- D50B40DEE10:
-
50% diesel + 40% biodiesel + 10% diethyl ether
- D50B45MTBE5:
-
50% diesel + 45% biodiesel + 5% methyl tertiary-butyl ether
- D50B40MTBE10:
-
50% diesel + 40% biodiesel + 10% methyl tertiary-butyl ether
- HC:
-
Hydrocarbons
- NOx :
-
Oxides of nitrogen
- ppm:
-
Parts per million
References
Alagu K, Nagappan B, Jayaraman J & GnanaDhas AA (2018). Impact of antioxidant additives on the performance and emission characteristics of CI engine fuelled with B20 blend of rice bran biodiesel. Environ Sci Pollut Res, pp.1–11
Ali OM, Mamat R, Masjuki HH, Abdullah AA (2016) Analysis of blended fuel properties and cycle-to-cycle variation in a diesel engine with a diethyl ether additive. Energy Convers Manag 108:511–519
Alptekin E (2017) Evaluation of ethanol and isopropanol as additives with diesel fuel in a CRDI diesel engine. Fuel 205:161–172
Arcoumanis C, Bae C, Crookes R, Kinoshita E (2008) The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: a review. Fuel 87(7):1014–1030
Arumugam A, Ponnusami V (2019) Biodiesel production from Calophyllum inophyllum oil a potential non-edible feedstock: an overview. Renew Energy 131:459–471
Ashok B, Nanthagopal K, Jeevanantham AK, Bhowmick P, Malhotra D, Agarwal P (2017) An assessment of Calophyllum inophyllum biodiesel fuelled diesel engine characteristics using novel antioxidant additives. Energy Convers Manag 148:935–943
Atabani AE, da Silva César A (2014) Calophyllum inophyllum L.—a prospective non-edible biodiesel feedstock. Study of biodiesel production, properties, fatty acid composition, blending and engine performance. Renew Sust Energ Rev 37:644–655
Atabani AE, Silitonga AS, Ong HC, Mahlia TMI, Masjuki HH, Badruddin IA, Fayaz H (2013) Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew Sust Energ Rev 18:211–245
Awad OI, Mamat R, Noor MM, Ibrahim TK, Yusri IM, Yusop AF (2018) The impacts of compression ratio on the performance and emissions of ice powered by oxygenated fuels: a review. J Energy Inst 91(1):19–32
Cataluña R, Dalávia D, Da Silva R, Menezes E, Venturi V, Wagner R (2011) Acceleration tests using gasolines formulated with di-TAE, TAEE and MTBE ethers. Fuel 90(3):992–996
Cerri T, D’Errico G, Onorati A (2013) Experimental investigations on high octane number gasoline formulations for internal combustion engines. Fuel 111:305–315
Chauhan BS, Kumar N, Cho HM (2010) Performance and emission studies on an agriculture engine on neat Jatropha oil. J Mech Sci Technol 24(2):529–535
Das D, Kumar A, Yadav A (2018) Evaluation of performance, emission and combustion characteristics of a CI engine fueled with karanja biodiesel and diethyl ether blends. Biofuels 9(1):89–94
Donahue RJ, Foster DE (2000) Effects of oxygen enhancement on the emissions from a DI diesel via manipulation of fuels and combustion chamber gas composition (No. 2000–01-0512). SAE Technical Paper
Jaichandar S, Annamalai K (2013) Combined impact of injection pressure and combustion chamber geometry on the performance of a biodiesel fueled diesel engine. Energy 55:330–339
Jiaqiang E, Liu T, Yang WM, Li J, Gong J, Deng Y (2016) Effects of fatty acid methyl esters proportion on combustion and emission characteristics of a biodiesel fueled diesel engine. Energy Convers Manag 117:410–419
Kajitani S, Usisaki H, Clasen E, Campbell S, & Rhee KT (1994). MTBE for improved diesel combustion and emissions. SAE Technical Paper No. 941688
Kalsi SS, Subramanian KA (2017) Effect of simulated biogas on performance, combustion and emissions characteristics of a bio-diesel fueled diesel engine. Renew Energy 106:78–90
Lamani VT, Yadav AK, Narayanappa KG (2017) Influence of low-temperature combustion and dimethyl ether-diesel blends on performance, combustion, and emission characteristics of common rail diesel engine: a CFD study. Environ Sci Pollut Res 24(18):15500–15509
Lešnik L, Vajda B, Žunič Z, Škerget L, Kegl B (2013) The influence of biodiesel fuel on injection characteristics, diesel engine performance, and emission formation. Appl Energy 111:558–570
Liu H, Wang Z, Zhang J, Wang J, Shuai S (2017) Study on combustion and emission characteristics of polyoxymethylene dimethyl ethers/diesel blends in light-duty and heavy-duty diesel engines. Appl Energy 185:1393–1402
Miyamoto N, Ogawa H, Nurun NM, Obata K, & Arima T (1998). Smokeless, low NOx, high thermal efficiency, and low noise diesel combustion with oxygenated agents as main fuel(No. 980506). SAE technical paper
Monirul IM, Masjuki HH, Kalam MA, Mosarof MH, Zulkifli NWM, Teoh YH, How HG (2016) Assessment of performance, emission and combustion characteristics of palm, jatropha and Calophyllum inophyllum biodiesel blends. Fuel 181:985–995
Mosarof, M. H., Kalam, M. A., Masjuki, H. H., Alabdulkarem, A., Ashraful, A. M., Arslan, A., ... & Monirul, I. M. (2016). Optimization of performance, emission, friction and wear characteristics of palm and Calophyllum inophyllum biodiesel blends. Energy Convers Manag, 118, 119–134
Nanthagopal K, Ashok B, Raj RTK (2016) Influence of fuel injection pressures on Calophyllum inophyllum methyl ester fuelled direct injection diesel engine. Energy Convers Manag 116:165–173
Nanthagopal K, Ashok B, Tamilarasu A, Johny A, Mohan A (2017) Influence on the effect of zinc oxide and titanium dioxide nanoparticles as an additive with Calophyllum inophyllum methyl ester in a CI engine. Energy Convers Manag 146:8–19
Nanthagopal K, Ashok B, Saravanan B, Patel D, Sudarshan B, Ramasamy RA (2018) An assessment on the effects of 1-pentanol and 1-butanol as additives with Calophyllum Inophyllum biodiesel. Energy Convers Manag 158:70–80
Natarajan M, Frame EA, Naegeli DW, Asmus T, Clark W, Garbak J, ... & Wallace JP (2001). Oxygenates for advanced petroleum-based diesel fuels: Part 1. Screening and selection methodology for the oxygenates. SAE Technical Paper No. 2001–01-3631
Ong HC, Masjuki HH, Mahlia TMI, Silitonga AS, Chong WT, Leong KY (2014) Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers Manag 81:30–40
Ong ZC, Mishani MBM, Chong WT, Soon RS, Ong HC, Ismail Z (2017) Identification of optimum Calophyllum inophyllum bio-fuel blend in diesel engine using advanced vibration analysis technique. Renew Energy 109:295–304
Praveen A, Rao GLN, & Balakrishna B (2017). Performance and emission characteristics of a diesel engine using Calophyllum inophyllum biodiesel blends with TiO2 nanoadditives and EGR. Egyp J Petroleum
Rahman SA, Masjuki HH, Kalam MA, Abedin MJ, Sanjid A, Sajjad H (2013) Production of palm and Calophyllum inophyllum based biodiesel and investigation of blend performance and exhaust emission in an unmodified diesel engine at high idling conditions. Energy Convers Manag 76:362–367
Rajamohan S, Kasimani R. (2018a). Analytical characterization of products obtained from slow pyrolysis of Calophyllum inophyllum seed cake: study on performance and emission characteristics of direct injection diesel engine fuelled with bio-oil blends. Environ Sci Poll Res, 1–6
Rajamohan S, Kasimani R. (2018b). Studies on the effects of storage stability of bio-oil obtained from pyrolysis of Calophyllum inophyllum deoiled seed cake on the performance and emission characteristics of a direct-injection diesel engine
Rakopoulos DC, Rakopoulos CD, Giakoumis EG, Dimaratos AM (2012) Characteristics of performance and emissions in high-speed direct injection diesel engine fueled with diethyl ether/diesel fuel blends. Energy 43(1):214–224
Ramakrishnan P, Kasimani R, Peer MS, Rajamohan S (2018) Assessment of n-pentanol/Calophyllum inophyllum/diesel blends on the performance, emission, and combustion characteristics of a constant-speed variable compression ratio direct injection diesel engine. Environ Sci Pollut Res 25(14):13731–13744
Ren Y, Huang Z, Miao H, Di Y, Jiang D, Zeng K et al (2008) Combustion and emissions of a DI diesel engine fuelled with diesel-oxygenate blends. Fuel 87(12):2691–2697
Roy MM (2008) Investigation of methyl tertiary butyl ether—diesel combustion and odorous emissions in a direct-injection diesel engine. Proceed Instit Mech Eng Part D: J Automobile Eng 222(2):251–263
Roy MM, Tsunemoto H, Ishitani H (2000) Effect of MTBE and DME on odorous emissions in a DI diesel engine. JSME Int J Series B Fluids and Thermal Eng 43(3):511–517
Sezer I (2011) Thermodynamic, performance and emission investigation of a diesel engine running on dimethyl ether and diethyl ether. Int J Therm Sci 50(8):1594–1603
Shameer PM, Ramesh K (2017) FTIR assessment and investigation of synthetic antioxidant on the fuel stability of Calophyllum inophyllum biodiesel. Fuel 209:411–416
Shih LKL (1998). Comparison of the effects of various fuel additives on the diesel engine emissions SAE Technical Paper No. 982573
Silitonga AS, Hassan MH, Ong HC, Kusumo F (2017) Analysis of the performance, emission and combustion characteristics of a turbocharged diesel engine fuelled with Jatropha curcas biodiesel-diesel blends using kernel-based extreme learning machine. Environ Sci Pollut Res 24(32):25383–25405
Sivakumar V, Sarangan J, & Anand RB (2010). Performance, combustion and emission characteristics of a CI engine using MTBE blended diesel fuel. In Frontiers in Automobile and Mechanical Engineering (FAME), 170–174
Srihari S, Thirumalini S, Prashanth K (2017) An experimental study on the performance and emission characteristics of PCCI-DI engine fuelled with diethyl ether-biodiesel-diesel blends. Renew Energy 107:440–447
Stone CR, Green-Armytage DI (1987) Comparison of methods for the calculation of mass fraction burnt from engine pressure—time diagrams. Proceed Instit Mech Eng Part D: Trans Eng 201(1):61–67
Topgül T (2015) The effects of MTBE blends on engine performance and exhaust emissions in a spark ignition engine. Fuel Process Technol 138:483–489
Venu H, Madhavan V (2017) Influence of diethyl ether (DEE) addition in ethanol-biodiesel-diesel (EBD) and methanol-biodiesel-diesel (MBD) blends in a diesel engine. Fuel 189:377–390
Vijayakumar C, Ramesh M, Murugesan A, Panneerselvam N, Subramaniam D, Bharathiraja M (2016) Biodiesel from plant seed oils as an alternate fuel for compression ignition engines—a review. Environ Sci Pollut Res 23(24):24711–24730
Viswanathan K (2018) Experimental investigation on emission reduction in neem oil biodiesel using selective catalytic reduction and catalytic converter techniques. Environ Sci Poll Res. 1–2
Yang SY, Naser N, Chung SH, & Al-Qurashi K (2014). Ignition delay and soot oxidative reactivity of MTBE blended diesel fuel. SAE Technical Paper No. 2014-01-1266
Yokota H, Nakajima H, & Kakegawa T (1998). A new concept for low emission diesel combustion (2nd rep.: reduction of HC and CO emission, and improvement of fuel consumption by EGR and MTBE blended fuel). SAE Technical Paper No. 981933
Zhu L, Cheung CS, Zhang WG, Huang Z (2011) Combustion, performance and emission characteristics of a DI diesel engine fueled with ethanol–biodiesel blends. Fuel 90(5):1743–1750
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bragadeshwaran, A., Kasianantham, N., Ballusamy, S. et al. Experimental study of methyl tert-butyl ether as an oxygenated additive in diesel and Calophyllum inophyllum methyl ester blended fuel in CI engine. Environ Sci Pollut Res 25, 33573–33590 (2018). https://doi.org/10.1007/s11356-018-3318-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3318-y