Abstract
Antimony (Sb) usually occurs associated with arsenic, lead, and other metal elements in sulfide deposits, and most is currently being extracted from the Karst areas, southwest China. In these areas, the acid generated from the oxidative dissolution of sulfide minerals is neutralized by the abundant carbonates but little is known about the effect of this process on the geochemical behavior of Sb and other contaminants. In this study, physicochemical properties (pH, EC) and the trace elements (Sb, As, Cu, Pb, Zn, Sr, etc.) concentrations in waters from mining-impacted Karstic environments were determined in order to determine their distribution and migration potential. It was found that pH values ranged from 6.51 to 9.82, and EC values varied from 369 to 1705 μs·cm−1 in river water samples. Waters of various types such as adit waters, flotation drainage, leaching waters, and river waters all contained high concentrations of dissolved trace elements, reaching up to 5475 μg·L−1Sb, 1877 μg·L−1As, 10,371 μg·L−1Zn, 1309 μg·L−1 Pb, 46 μg·L−1 Cu, and 1757 μg·L−1 Sr. The elevated concentrations of dissolved Sr indicated that Sr could be considered as an indicator of oxidative dissolution of sulfide minerals in the Karst rivers. A proportion of the trace elements were removed in the streams in the vicinity of the mine due to adsorption onto particulate matter, whereas migration of trace elements in the downstream of mine area was attributed to dispersion in dissolved forms. Strontium and Sb have a strong hydrophilicity compared to the other elements analyzed; in contrast, Pb had a high affinity for suspended particulate matter (SPM). It was also found that downstream sediments had elevated concentrations of mining-derived trace elements, but there was a significant decrease in concentration of contaminants in aqueous phase, suggesting that contaminant behavior was conservative in the water-sediment systems under the oxic conditions prevailing in these waters. There was a good correlation between Sb and As in water-SPM-sediment system, indicating that Sb and As are homologous in water environment of the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimony (Sb) is a toxic element and a global environmental contaminant (Shotyk et al. 2005) that is found throughout the environment as a result of natural processes and human activities. Sb occurs naturally at trace levels in the environment with an estimated abundance of 0.2~0.3 mg·kg−1 in the earth crust (Filella et al. 2002). Sb primarily occurs in mineral form as stibnite (Sb2S3) and valentinite (Sb2O3) (Filella et al. 2002). Sb and its compounds can be found in many currently used products and materials including automobile brake-linings, flameproof retardants, lead hardener, glass, ceramics, pigments, batteries, textiles, electronics, etc., and this list is still increasing (Grigoratos and Martini 2015; Hockmann and Schulin 2012). These wide-ranging uses have led to considerable inputs of this element into the environment.
Typical Sb concentrations of unpolluted waters are less than 1 μg L−1 (Filella et al. 2002). Higher concentrations usually originate from anthropogenic activities. Particularly high Sb concentrations are found in the waters from mining areas. For example, up to 29,423 μg L−1 was detected in mine drains (Zhu et al. 2009) and 163 μg L−1 was found in surface waters (Fu et al. 2011) in the vicinity of Xikuangshan Sb mine, China, which is one of the world largest Sb mines. Casiot et al. (2007) reported that Sb concentration was as high as 32 μg L−1 in tailing drainage, even though the nearby Sb mine had been abandoned for 85 years. Both mine exploitation and metal smelting are important sources of Sb and other potential toxic trace elements to the surrounding environment (Tschan et al. 2009; He et al. 2012). As a consequence, Sb and other trace elements pollutants have recently attracted increasing attention from the scientific community, mainly due to its industrial applications, its potential toxicity to humans and the environmental geochemistry of these pollutants in areas of mining activities (Byrne et al. 2017; Rzymski et al. 2017; Migaszewski et al. 2016; Macgregor et al. 2015).
The number of studies on the mineralogical characterization of contaminants (Sb, As, Cu, Zn, Pb, etc.) in mine tailings, wastes, soils, and sediments, and on the release and transport of these contaminants from mine materials into streams, farmlands, and soils, have increased in the past few years (e.g., Mariussen et al. 2017; Sprague et al. 2016; Moore et al. 2016; Yang et al. 2015; Asaoka et al. 2012; Hiller et al. 2012; Wang et al. 2011; Zhang et al. 2009). Several researchers reported that pH is one of the most significant impact factors which affects the distributions of elements in its environment. In most conditions, Cu, Pb, and Zn have a higher affinity for solid materials when the pH values are increasing (Basta and Tabatabai 1992; de Matos et al. 2001), while Sb and As tend to be released into the aquatic environment when the pH values are alkaline (Masscheleyn et al. 1991). Processes which may affect the pH of solution may therefore impact on the solubility of Sb and other elements. Ashley et al. (2003) has suggested that Sb has high solubility under oxidized conditions. When stibnite undergoes oxidative dissolution, this leads to the formation of sulphate ions and the acidification of solutions. If the acid solutions are then neutralized by alkaline substances, the increased pH of solution would consequently enhance the solubility of Sb. There is a super-large Sb deposit in Guangxi, China (He et al. 2012), which is a typical karst area with abundant carbonate. These Sb ore fields have been extensively exploits since the early 1990s. Early research on local stream waters showed that most of the pH values were near neutral or alkaline, but it was evident that both stream waters and sediments were highly contaminated with mining-derived potentially toxic elements (Li et al. 2009; Zhou et al. 2005). In a Karst water system, it is superficial to use pH as a measure of the extent and nature of the environment impact of the mining activities. As mentioned above, the geological background of Karst is rich in carbonates which would neutralize the acid solutions resulted from oxidative dissolution of sulfide minerals. Since Sb, As, Cu, Pb, Zn, and Sr mainly occur in such sulfide ore deposits, it is important to determine how variation in pH value impacts the distributions of Sb and these other elements when the sulfides undergo oxidative dissolution. Therefore, this study will focus on an Sb multi-metalliferous mining area in a Karstic geological background, to (1) determine the distribution of Sb and other trace elements in a Karstic river system; (2) characterize the response of Karstic environment to mining activities; (3) elucidate the processes controlling the migration behaviors of Sb and other contaminants in a Karstic river system.
Sampling and methods
Description of the study area
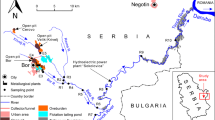
The study area, Dachang multi-metalliferous mine, is located in Nandan county, northwest of Guangxi Zhuang Autonomous Region, China (Fig. 1), and is part of the Karst area of Southwest China. The Dachang ore field is one of the most important nonferrous metal mining industrial bases and also has one of the most well-known super-large ore deposits in China (Tu 1988). The mineral resources of Dachang ore field mainly distributed in Dachang and Chehe town, Nandan county. The main ore fields are (1) a cassiterite-polymetallic sulfide ore field which includes Zn-Cu sulphide skarn deposits and Pb-Zn-Sb multi-metalliferous sulfide deposits and (2) a quartz-calcite veined tungsten-antimony ore field (He and Yang 2007).
The main surface water that flows through the mining area include three stream tributaries contaminated severely by mining activities and three other tributaries from areas which have not been impacted by mining activities. These tributaries then flow into the Diaojiang River which itself is a tributary of the Pearl River. Diaojiang River is also as the most significant level 1 tributary of Hongshui River, rising in the Chehe town, flowing through Nandan, Hechi, Duan, etc., and flowing into Hongshui River from northwest to southeast. Diaojiang watershed is situated in the geomorphological slope zone from Yunnan Guizhou plateau to Guangxi basin, and the terrain slopes downward from northwest to southeast. The strata of the Diaojiang watershed dates from the Carboniferous, Trias, Permian and Devonian periods, and is primarily comprised of limestone (Zhou et al. 2005).
Materials and methods
Sampling locations are shown in Fig. 1. Water and sediment were sampled at intervals along the main polluted streams, the adjacent non-impacted streams upstream of the point where they receive mining-impacted discharges, and the downstream sections of these streams where they flow into the Diaojiang River. According to the actual conditions of the study area, the sampling sites of water and sediment can be divided into six groups: (1) background stream water: upstream of the mining area (site1) and the three tributaries located downstream of the mining area which were not impacted by mining activities (sites 33, 34, 35, 37, 40); (2) adit water: draining from the mining adits (AW1, 2, 3, 4); (3) flotation drainage: draining from the mine flotation (sites 8, 17, 21); (4) leaching water: draining from the tailing leachate (LW); (5) river water: located in the vicinity of the mine to reflect the immediate impact of the mining activity (sites 2–31); and (6) downstream river water: located downstream of the mining area to enable characterization of the extent of natural attenuation processes (sites 32, 36, 38, 39, 41, 42). Accordingly, sediment samples were collected with water samples at the same sites. In addition, a tailing sample was collected from the tailing dump with the leachate sample.
Water samples were collected in duplicate at each site in 500-ml HDPE bottles, immediately filtered through 0.22-μm membrane filter paper to remove suspended particulate matter(SPM), acidified with HNO3, and then stored in a cool box (< 4 °C). The pH and electrical conductivity (EC) of the waters was measured in situ using a Hanna portable meter calibrated with standard pH solutions and an Orion conductivity meter, respectively. In the laboratory, the filter papers were air-dried at 25 °C and prepared for acid digestion prior to analysis to determine heavy metal concentrations. The sediment and tailing samples were sampled using a stainless steel trowel and placed in polythene bags. Prior to digestion, each sample was air-dried at 25 °C and then milled so that it passed through a 150-μm sieve. A portion of the tailings sample was passed through a 48-μm sieve before mineralogical analysis using X-ray diffraction (XRD).
Digestion and analysis of samples
Firstly, 0.01-g milled sample of sediment was placed in a PTFE crucible to which 1 ml HNO3 and 0.5 ml HF were added. The crucible was sealed with a stainless steel gland bush and heated at 170 °C (internal pressure: ca. 7.9 bar) for 12 h. The gland bush was then opened, and the solution was heated to dryness. A further 1 ml of HNO3 was added and evaporated to dryness. Finally, the residue was re-dissloved in 1 ml of HNO3 and diluted to 100 ml by de-ionized water (Milli-Q, 18.2 MΩ cm). The solution was decanted into polystyrene test tubes and analyzed by ICP-MS (Platform ICP, produced by Micromass) for trace elements. The digestion procedure for the filter paper samples with SPM was the same as that for sediments and the resulting solutions were also analyzed by ICP-MS for the same suite of elements.
The filtered water samples were directly analyzed by ICP-MS for the same suite of elements. Tailing mineralogy was measured by powder XRD (D/max-2200 PC, Rigaku Co., Japan) using Cu–Ka radiation filtered by Ni.
Reagents and quality control
De-ionized water produced by a Milli-Q system (Millipore, Bedford, MA, USA) was used throughout the sample preparation, digestion, and determination. Sub-boiling distilled (DST-1000, Savillex, USA) nitric acid and hydrofluoric acid were used for sample digestions. Multielement standard solutions (AccuTrace ICP-MS Calibration Standard, 10 μg ml−1 each element) for ICP-MS analysis were purchased from AccuStandard Incorporation, New Haven, USA.
Rigorous quality control procedures included analysis of reagent blanks, water blanks, filter paper blanks, replicate samples, and certified national reference material of sediment (GBW07305. China National Research Center of CRM’s). The precision of determination of elements was better than 5%.
Results
Basic physicochemical property of waters
Background water (sites 1, 33, 34, 35, 37, 40) samples were neutral to alkaline with pH values ranging from 7.2 to 8.0, and EC values were in the range 151–340 μs cm−1. Adit water (AW1, 2, 3, 4) samples were circumneutral (pH of 7.0–7.4), but EC values ranged between 299 and 1558 μs cm−1. These were higher because of the greater solubility of sulfidic minerals in oxidizing water (Zhou et al. 2017; Ashley et al. 2003). In contrast, however, the pH of flotation drainage (sites8, 17, 21) samples varied widely with values ranging from 7.2 to 12.1, and the EC value of those samples was also much higher, at 1183–2285 μs cm−1. The highest pH and EC values were determined for the sample from FD8. For river water samples in the vicinity of the mining area, pH values ranged from 6.51 to 9.82, and EC values varied from 369 to 1705 μs cm−1. In the downstream river water samples, the pH and EC values ranged from 7.3 to 7.9 and 425 to 808 μs cm−1, respectively. Although the EC values of waters significantly were less than that of flotation drainage, they were still higher than the background waters.
Figure 2a shows the distribution of pH and EC values along the main stream. An obvious variation of pH and EC values can be observed because of the influence of mining activities. The pH values at the mine area sites 2–31 are significantly decreased, while the EC values at the mine area are markedly increased compared to the upstream site 1 and the downstream sites 32–42. At site 42, the pH and EC values are closed to the background levels along the flow direction.
In general, the extent of transport for most of heavy metals in water-SPM systems is determined by adsorption/desorption on SPM in the water. Indeed, heavy metals are typically adsorbed to other phases or present in mineral forms within the SPM fraction. The concentrations of SPM at sites 8, 18, 19 and 22–25 (103-540 mg L−1) were significantly elevated compared to the other sites (1–60 mg L−1). The sites which had especially elevated SPM were located at the outfall of flotation drainage or at the downstream of mining activity, and these also led to the elevated SPM in river water samples in the vicinity of the mine area (sites 2–31). The variation in SPM along the main stream shows a similar pattern to the EC values, which is displayed in Fig. 2b.
Trace elements concentrations in waters
Concentrations of Sb and other elements in the study area are shown in Table 1. Concentrations of soluble Sb in background water samples ranged from 1 to 5 μg L−1 (mean 2 μg L−1) were much higher than the general levels (≤1 μg·L−1) (Filella et al. 2002). In contrast, the results showed that background values of As, Cu, Zn, and Pb in water samples from the study area were in good agreement with global background values (Badal and Kazuo 2002; Mandal and Suzuki 2002; Flemming and Trevors 1989; Warren 1981). Waters showed average Sr concentration of 661 μg L−1 (ranged from 104 to 1757 μg L−1) in this mine area was 4.2 times higher than the background values (155 μg L−1). In particular, content of Sr were up to 1704 μg L−1 and 1757 μg L−1 were detected in an adit water (AW4) and a river water (site7) sample from the mining area, respectively.
Site 1 is the background river which is located in upstream of mine area (Figs. 1 and 3). There is a small flotation plant situated in between sites 1 and 2 and we found that the water sample from site 2 contained the highest concentration of dissolved As (1877 μg L−1). Accordingly, dissolved As concentration of site 3 was also very high with 1369 μg L−1. From sites 2 to 6, the main stream receives drainage from two kinds of big mining and flotation plants. The only one leaching water collected from this area has also contained elevated dissolved As (1723 μg L−1); this water emerged at the bottom of very old mine tailings. There are two contaminated rivers flow into the main stream after site 7 and site 31, respectively. The results showed there were elevated concentrations of a range of metalloids and heavy metals in river water from the mining area (Fig. 3a–d: site 2~31). There were also elevated dissolved Sb in river water of mine area and of downstream. On the site 42, soluble Sb and As contents were 109 μg L−1 and 10 μg L−1, 55 times and 3 times higher than the background values, respectively; soluble Zn and Sr contents were 158 μg L−1 and 338 μg L−1, 8 times and 2 times higher than the background values, respectively, while soluble Cu and Pb contents were 1 μg L−1 and 0.09 μg L−1, were close to the background values, respectively. Furthermore, it also showed an obvious disturbance by the mine activities (Fig. 3d, Fe: site 2~32) according to the soluble Fe contents in the main stream. In addition, although it showed an obvious decreasing of SPM concentration along the flow direction (Fig. 2b) after site 31, which was much closed to the background level, the concentration of heavy metals and metalloids were much higher than the background values. At site 42, the SPM concentration was 0.57 mg L−1, but the Sb, As, Cu, Zn, and Pb concentrations of SPM were 1530 mg kg−1, 1170 mg kg−1, 1730 mg kg−1, 16,500 mg kg−1, and 2830 mg kg−1, respectively.
Trace elements concentrations in sediments
The sediment samples from flotation water had the significantly elevated metalloids and heavy metals concentrations. The highest sediment As, Sb, and Sr concentrations of 33,090 mg kg−1, 25,140 mg kg−1, and 1042 mg kg−1, respectively, were found at site 17 which in the vicinity of a flotation drainage from the Sb ore deposit. The highest sediment Zn concentration was 37,031 mg kg−1, was found at site 16 which was nearby a zinc ore mining and flotation plant. The highest sediment Cu concentration was 5703 mg kg−1 for sediment collected near a flotation drainage (site 8) of polymetaltic ore deposit. The highest sediment Pb concentration was 8164 mg kg−1 at site 22 which was close to a mining and flotation plant.
The distribution of metalloids and heavy metals in sediment along the flow direction of main stream was shown in Fig. 4. Those metalloids and heavy metals concentrations were similar to the background values at site 1, whereas there was a significantly increase of those contents in the mine area sites 2–32, with the maximum of Cu, Zn, As, Sb, Fe, Sr, and Pb content is 905 mg kg−1, 23,100 mg kg−1, 13,891 mg kg−1, 3559 mg kg−1, 88,918 mg kg−1, 423 mg kg−1, and 4090 mg kg−1, respectively (Fig. 4.). The sediment samples of downstream from sites 36–42 showed a decreasing of heavy metals concentrations. However, the distribution of heavy metals in sediment of downstream still showed Zn, As, Sb, Fe, and Pb concentrations as high as 3921–10,166 mg kg−1, 2163–5109 mg kg−1, 829–2591 mg kg−1, 26,038–66,429 mg kg−1 and 976–3177 mg kg−1, which were significantly higher than the background values. Elevated metalloids and heavy metal concentrations were detected even in the sediments of downstream of main stream, which may be caused by the long distance transport of the sediments affected by the mining activities in the vicinity of mine.
Discussion
Sources of contaminant elements in the water environment
Investigation of six groups of water in the study area indicated that there are three major sources of metalloids and heavy metals in the stream of study area: flotation water, adit water and leaching water.
In this study, we collected water samples from three different flotation plants. The pH values showed a strong alkalic characteristic in flotation drainage, which was due to the using of alkaline matter such as lime in the flotation of ore. The results showed that much higher Sb, Zn, and Pb, and the relatively lower As and Cu appeared in the flotation drainage (Table 1). This mainly resulted from that most of deposit type in study area is Pb-Zn-Sb multi-metalliferous sulfide ore, especially for the actively flotation plants. EC values indicated that flotation drainage contained a large number of heavy metal ions which are carried by tailings and toxically mineral processing reagents which include plenty of heavy metal salt. The highest dissolved Sb concentration (5475 μg L−1) was detected in the site 17 flowed into Sanchahe river (Fig. 5). Site 17 is the outfall of an Sb ore flotation. With the input of this flotation drainage, significant elevated dissolved Sb concentrations (sites 18–20) and anomalous elevated pH values were found in Sanchahe river (Fig. 5). Furthermore, the confluence of Sanchahe river and Pingcun river flow into the main stream after site 31, which resulted in an elevated dissolved Sb appeared at site 32 (Fig. 3a). In the main stream, the highest dissolved Zn (9799 μg L−1) and Pb (498 μg L−1) was observed in site 7 which is the downstream of a Pb-Zn ore mining and flotation drainage.
Adit water is always an important source of heavy metals in environment, e.g. the highest dissolve Sb concentration reached 9300 μg L−1 in the vicinity of the adit from an abandoned Sb mine, Slovakia (Hiller et al. 2012). In this study, elevated dissolved Sb concentrations were indeed found in adit water samples but the dissolved other heave metals and metalloids were relatively low (Table 1). This is because Sb is major constituent of minerals in this Sb-dominated mining area, while arsenic and other elements are present as impurties or minor components of these minerals (Macgregor et al. 2015; Li and Thornton 1993). It was obvious that AW3 and AW4 included the elevated dissolved Sb (AW3: 1410 μg L−1; AW4: 2901 μg L−1) were found in Chashan mine area (Fig. 6), which resulted in a significant increasing Sb at site 9 and 10 in Chashan river. Similarly, AW1 included 278 μg L−1of dissolved Sb was located in between sites 18 and 19 (Fig. 5), which also resulted in a little bit increasing Sb appeared in Sanchahe river.
There was only one leachate collected in the study area, which was sampled from the sink of mine tailings dump in Wuyi mine. The leachate sample showed a very high As concentration but low concentrations of Sb and other heavy metals (Table 1). This was related to mineral compositions of mine residues in tailings dump. Our X-ray diffraction patterns showed that the mine residue samples are dominated by quartz, illite, gypsum, kaolinite, montmorillonite, plagioclase, and iron minerals. Mine residues contained some of iron minerals (0.3~3.1%) in Wuyi mine. However, it has revealed that the host phases of arsenic in solids can be iron minerals (Fawcett et al. 2015). Under reducing condition, absorbed As can be released with the reductive dissolution of Fe-bearing sorbent (Herbel and Fendorf 2006; Langner and Inskeep 2000; Takahashi et al. 2004). And there was another study (Casiot et al. 2007) showed that As displayed higher mobility than Sb under simulated anoxic conditions. An additional factor to consider in this study, however, is that the Wuyi mine dump was very tall, at over 65 ft. Waters percolating into anoxic sections of the mine dump material and then emerging downslope may explain why elevated As was detected in the leachate from the Wuyi waste dumps. The Wuyi mine dump spans the area between sites 2 and 4 this may explain why significant amounts of dissolved arsenic flow into the upstream area (site2~4) of the mainstream (Fig. 3b). A very high concentration of Sr was also detected in the leachate sample (Table 1), and this may result from the high concentrations of carbonates dissolved because of the acidity generated from the surface sulfides oxidation in the waste dumps (Ritchie et al. 2013).
In addition, there were very high Zn concentration (1465 ± 2817 μg L−1) in the river water of mine area (Table 1), but there were not much higher concentration of Zn in the flotation water, adit water, and leaching water, which indicated that there were some other sources with high Zn content in this study area.
Response of pH values and trace elements to mining activities in the Karst waters
Cassiterite-polymetallic sulfide ore field and quartz-calcite veined tungsten-antimony ore field are the main types of deposit in the most important deposit in this study area (He and Yang 2007). The pH values of background water were alkalescence because of the abundant carbonates geological background. In the mine area, the acidity generated by the decomposition of primary sulfides was immediately neutralized by the abundant carbonates as the water samples were mostly neutral with very few of slightly acidic. As a result, the pH values of river waters were decreased in the mine area, while the EC concentrations were increased due to the probably chemical reaction between the acidity and carbonates (Fig. 2a). Moreover, the increase in heavy metals contents of the waters in the mine area was a consequence of sulfides oxidation and dissolution (Fig. 3) (Zhou et al. 2017; Hiller et al. 2012; Ashley et al. 2003).
The elevated of Sr were detected in the waters associated with mine activities. Sr is enriched in carbonate rocks resulting from its similar ion radius with Ca (Liu et al. 1984). As a typical carbonate rocks area, our background value of Sr (mean 155 μg L−1) was similar with average value of Sr (175 μg L−1) in the upstream of Pearl River (Liu et al. 2007), which were much higher than the one (mean 78 μg L−1) of world freshwater (Palmer and Edmond 1992). In this study area, however, contents of Sr ranged from 47 to 1704 μg L−1 in adit waters, from 594 to 1352 μg L−1 in flotation waters, from 220 to 1757 μg L−1 in the river water of mine area, and were 1264 μg L−1 in leaching water, respectively, which indicated that release of Sr in the Karst water environment of mine area should be resulted from the neutralization of carbonates and acidification drainage. Zhang et al. (2009) has also detected high contents of Sr with average value of 790 μg L−1 in river waters from a gold mine located in a Karst area, which was much higher than the background value of 160 μg L−1. We may conclude that Sr should be considered as an indicator for the oxidative dissolution of sulfide minerals in the Karst waters.
Distribution of Sb and other trace elements in the water-SPM system
SPM is an important carrier that heavy metals migrate in water, which is a significant factor for dissolving or transporting of heavy metals. The element which has a higher affinity to SPM is much easy to adsorb and the content of soluble fraction of this element in water fall sharply, whereas if the element which has a lower affinity to SPM is much easy to migrate in water with soluble fraction. An understanding of the distribution of elements as dissolved and SPM fractions in water is significant to characterize the migration of elements. In the study area, Sb and Sr were observed in the soluble fractions, whereas As, Cu, Zn, and Pb were observed in the SPM fractions in river (Fig. 7). Although As usually shows a similar behavior to Sb, and the presence of As and Sb in soluble fraction has been reported for other surface water, As observed in water-SPM system in this study showed some difference with other studies (Cidu and Frau 2009; Zhang et al. 2009; Cidu and Biddau 2007). Based on the distribution of As in water-SPM system in the different waters of study area (Fig. 8), the results showed that the difference is associated with the flotation drainage and river water. In both of the two kinds of waters, As has a high affinity for SPM fractions compared to Sb in the natural condition, as also reported by Casiot et al. (2007) and Masson et al. (2009). The reason could be that As show a tendency to bind heavy particles which come from the adsorbed or mineral phase of the flotation drainage. This viewpoint could also be supported by a higher As/Sb value in SPM (mean: 5.81) than in solution (mean: 1.04).
Transport of element in the aqueous environment was also expressed by the partition coefficient (Kd, unit: L·kg−1), which is the ratio of element contents in SPM (μg kg−1) to element contents in water (μg L−1). In the downstream of mine area, sites 36–42 were not affected by any other direct heavy metal sources, and the interaction of water-SPM-sediment here are sufficient, so the partition coefficient of element could reflect the hydrophilicity of heavy metal in water. The range and average Kd value varies for the heavy metals (Table 2), but a generally increasing order of (Sr, Sb) < (Zn, As, Cu) < Pb can be observed. It indicates that Sr and Sb have a strong hydrophilicity compared to other heavy metals, and that Pb has a high affinity to SPM compared to those heavy metals.
Geochemical behavior of Sb and other trace elements in the water-sediment system
Contaminated sediments are now being considered as one of the significant sources of heavy metals to aquatic systems; indeed a number of studies have reported release of heavy metals into aqueous phase (Sharifi et al. 2016; Fawcett et al. 2015; Wang et al. 2011; Masson et al. 2009). In this study, concentrations of Sb and other contaminants in sediment were in the higher ranges (Table 1) due to the mine impact compared of the background levels, but concentrations of these elements in the river water of downstream were not much higher. The results showed that elevated Sb and other contaminants in sediment collected from the downstream area (Table 1) might be the result of long distance transport of the sediments from mining area.
The distributions of As, Cu, and Pb in sediment along the main stream are showed in Fig. 4. The contents of these elements in downstream sediment (sites 36–42) are as high as 2163–8395 mg kg−1 of As (Fig. 4b), 263–580 mg kg−1 of Cu, and 976–3177 mg kg−1 of Pb (Fig. 4c), whereas the Fig. 3 showed As, Cu, and Pb concentrations in downstream water are only 8–15 μg L−1 (Fig. 3b), 1–2 μg L−1 and 0.06–0.59 μg L−1 (Fig. 3c). These results suggested that sediments cannot release As, Cu, and Pb in this study area. According to the pH values in water along with the main stream (Fig. 2a), it showed that there is an apparent alkalescence environment in the downstream river, which might be the reason of that Cu and Pb cannot be released from the sediments. It has been reported that Cu and Pb could be more mobilized in an acid condition, and the solubilities of Cu and Pb are notably higher when they are under acidic conditions (pH = 3.3~5.0) than under alkaline conditions (pH = 8.0) (de Matos et al. 2001; Chuan et al. 1996). There are some studies (Hiller et al. 2012; Masson et al. 2009; Milham and Craw 2009; Casiot et al. 2007) that suggested that As has the higher affinity than Sb for the stream sediments and hydrous ferric oxides (HFOs), which could explained that the downstream sediments contained up to 8395 mg kg−1 As and 2591 mg kg−1 Sb, while the downstream waters contained 15 μg L−1As and 211 μg L−1 Sb. Contents of Fe in the sediment samples of downstream was highly elevated, reaching as much as 66,429 mg kg−1 (Fig. 4d), which might resulted in that arsenic in downstream sediments was associated with Fe oxides, and the sediments cannot release As in the downstream with the conditions. In a natural water-sediment system, the affinity of As for the sediments was higher than the Sb (Casiot et al. 2007; Manaka et al. 2007).
At sites 36–42, soluble As contents range 8–15 μg·L−1, which is close to the value of As in unpolluted waters (Badal and Kazuo 2002). Compared to the higher As contents in the sediments of sites 36–42, the lower concentrations of As appear in the soluble fractions, which showed that the release of As from sediment to water is slowly. Arsenic usually occurs in sulfide minerals such as arsenopyrite, realgar, and orpiment and can be released from the oxidation of sulfide minerals (Badal and Kazuo 2002). If sulfide minerals are exposed to atmospheric O2, the reaction of oxidation of sulfide minerals commence vigorously, which can be attributed to oxidative dissolution of reduced S phases in oxic condition. Instead, the oxidation reaction of sulfide minerals would be slow while they are in the condition with little atmospheric O2. In the downstream of the study area, the oxidation is likely slow in sediment of long-term immersion. This characteristic could suggest that high levels of precipitated As is difficult to release for long-term submerged sediments.
Correlation between Sb and As in water-SPM-sediment system
Typical concentrations of dissolved Sb and As reported in the literature for unpolluted waters are less than 1 μg L−1 (Filella et al. 2002) and 10 μg L−1 (Badal and Kazuo 2002), respectively (Fig. 9a). In this study, concentrations of soluble Sb in background water samples ranged from 1 to 5 μg L−1 (mean 2 μg L−1) and As from 2 to 5 μg L−1 (mean 3 μg L−1). Background values of Sb in water of study area were higher than those reported in Filella et al. (2002) but this may be a result of the regional geology. Values of elements in background water are more related to the inner source of soils (Wang and Zhang 2007). The average concentration of Sb in soils from Guangxi is 2.12 mg kg−1, which is a factor of 2 higher than the mean value for Chinese soils (1.06 mg kg−1) and for world soils (1.0 mg kg−1) (Qi and Cao 1991). Furthermore, our study area is located in the antimony metallogenic belt (He and Yang 2007; Wang et al. 2004; Tu 1988). Therefore, the background water in this study area contains relatively higher Sb background values than in other areas. Concentrations of soluble As in unpolluted water samples ranged from 1 to 10 μg L−1 (Badal and Kazuo 2002), while background values of As ranged from 2 to 5 μg L−1 in our study area, which showed that the As value of the background water in the study area is consistent with the background values of other areas of the world.
Fig. 9 showed a good correlation between Sb and As in waters, SPMs, and sediments of the study area. In contrast, the good correlation between Sb and As was more significant in SPMs (Fig. 9b) and sediments (Fig. 9c) than in waters (Fig. 9a). It can be indicated that Sb and As are homologous in SPMs and sediments in the study area and further indicated that the Sb mining has caused significant impacts of Sb and As on the water-SPM-sediment system in the mine area.
Conclusions
Sb pollution, one of the typical environmental issues of China, was investigated in a karstic river system, southwestern China. Research results showed that there are three kinds of pollution sources in the study area: (1) flotation water drainage with the characteristics of much higher Sb, Zn, and Pb, and the relatively lower As and Cu; (2) adit water contained the elevated dissolved Sb; and (3) leaching water which was from the sink of mine tailings dump showed high level of As compared to the other pollutants.
With the impact of mining activities, pH values ranged from 6.51 to 9.82, EC values varied from 369 to 1705 μs cm−1, and the elevated Sr (mean: 661 μg·L−1) including contaminants were detected in the water samples. According to the response of pH values and Sr concentrations to mining activities, it could be concluded that Sr can be considered as an indicator of oxidative dissolution of sulfide minerals in the karstic rivers.
A proportion of the trace elements were removed in the streams in the vicinity of the mine due to adsorption onto particulate matter, whereas migration of trace elements in the downstream of mine area was attributed to dispersion in dissolved forms. In the water-SPM systems, Sr and Sb have a strong hydrophilicity compared to the other elements analyzed; contrastly, Pb had a high affinity for SPM. In the water-sediment system, downstream sediments have been contaminated by the elevated concentrations of pollutants, but a significant decreasing concentration of contaminants in aqueous phase was found, suggesting that contaminant behavior was conservative under the oxic conditions prevailing in these waters. Furthermore, there was a good correlation between Sb and As in water-SPM-sediment system, which indicated that Sb and As are homologous in the study area.
References
Asaoka S, Takahashi Y, Araki Y, Tanimizu M (2012) Comparison of antimony and arsenic behavior in an Ichinokawa River water-sediment system. Chem Geol 334:1–8
Ashley PM, Craw D, Graham BP, Chappell DA (2003) Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. J Geochem Explor 77:1–14
Badal KM, Kazuo TS (2002) Arsenic round the world: a review. Talanta 58(1):201–235
Basta NT, Tabatabai MA (1992) Effect of cropping systems on adsorption of metals by soils: II. Effect of pH. Soil Sci 153:195–204
Byrne P, Runkel RL, Walton-Day K (2017) Synoptic sampling and principal components analysis to identify sources of water and metals to an acid mine drainage stream. Environ Sci Pollut Res 24:17220–17240
Casiot C, Ujevic M, Munoz M, Seidel JL, Elbaz-Poulichet F (2007) Antimony and arsenic mobility in a creek draining an antimony mine abandoned 85 years ago (upper Orb basin, France). Appl Geochem 22:788–798
Chuan MC, Shu GY, Liu JC (1996) Solubility of heavy metals in a contaminated soil: effects of redox potential and pH. Water Air Soil Pollut 90(3):543–556
Cidu R, Biddau R (2007) Transport of trace elements at different seasonal conditions: effects on the quality of river water in a Mediterranean area. Appl Geochem 22:2777–2794
Cidu R, Frau F (2009) Influence of the fine-particle load in Mediterranean rivers and streams on the distribution of trace elements in filtered and non filtered aqueous fractions. Appl Geochem 24:611–623
de Matos AT, Fontes MPF, da Costa LM, Martinez MA (2001) Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environ Pollut 111:429–435
Fawcett S, Jamieson H, Nordstrom D, McCleskey R (2015) Arsenic and antimony geochemistry of mine wastes, associated waters and sediments at the Giant Mine, Yellowknife, Northwest Territories, Canada. Appl Geochem 62:3–17
Filella M, Belzile N, Chen Y-W (2002) Antimony in the environment: a review focused on natural waters I. Occurrence. Earth Sci Rev 57:125–176
Flemming CA, Trevors JT (1989) Copper toxicity and chemistry in the environment: a review. Water Air Soil Pollut 44:143–158
Fu Z, Wu F, Mo C-L, Liu B, Zhu J, Deng Q, Liao H, Zhang Y (2011) Bioaccumulation of antimony, arsenic, and mercury in the vicinities of a large antimony mine, China. Microchem J 97:12–19
Grigoratos T, Martini G (2015) Brake wear particle emissions: a review. Environ Sci Pollut Res 22:2491–2504
He H, Yang Z (2007) Application of the prospecting-information method to newly metallogenic prediction in the Dachang ore field, Guangxi. Miner Resour Geology 21:560–562 (in Chinese)
He M, Wang X, Wu F, Fu Z (2012) Antimony pollution in China. Sci Total Environ 421-422:41–50
Herbel M, Fendorf S (2006) Biogeochemical processes controlling the speciation and transport of arsenic within iron coated sands. Chem Geol 228:16–32
Hiller E, Lalinská B, Chovan M, Jurkovič L, Klimko T, Jankulár M, Hovorič R, Šottník P, Fľaková R, Ženišová Z, Ondrejková I (2012) Arsenic and antimony comtamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl Geochem 27:598–614
Hockmann K, Schulin R (2012) Leaching of Antimony from Contaminated Soils In: Competitive Sorption and Transport of Heavy Metals in Soil and Geological Media. CRC Press, Boca Raton 121
Langner HW, Inskeep WP (2000) Microbial reduction of arsenate in the presence of ferrihydrite. Environ Sci Technol 34:3131–3136
Li X, Thornton I (1993) Arsenic, antimony and bismuth in soil and pasture herbage in some old metalliferous mining areas in England. Environ Geochem Health 15:135–144
Li L, Zhang G, Liu H, Xiang M, Wei X (2009) Antimony and arsenic migration and environmental impacts on river draining in the Dachang multi-metalliferous mine area in Guangxi, China. Res Environ Sci 22:682–687 (in Chinese)
Liu YJ, Cao LM, Li ZL (1984) Element Geochemistry, vol 365. Science in China Press, Beijing (in Chinese)
Liu C-Q et al (2007) Biogeochemistry processes and surface-earth materials cycling—erosion and biological nutrients cycling in Karstic catchments, Southwest China. Science in China Press, Beijing 148 (in Chinese)
Macgregor K, MacKinnon G, Farmer J, Graham M (2015) Mobility of antimony, arsenic and lead at a former antimony mine, Glendinning, Scotland. Sci Total Environ 529:213–222
Manaka M, Yanase N, Sato T, Fukushi K (2007) Natural attenuation of antimony in mine drainage water. Geochem J 41:17–27
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mariussen E, Johnsen IV, Strømseng AE (2017) Distribution and mobility of lead (Pb), copper (Cu), zinc (Zn), and antimony (Sb) from ammunition residues on shooting ranges for small arms located on mires. Environ Sci Pollut Res 24:10182–10196
Masscheleyn PH, Delaune RD, Patrick WH (1991) Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol 25(8):1414–1419
Masson M, Schäfer J, Blanc G, Dabrin A, Castelle S, Lavaux G (2009) Behavior of arsenic and antimony in the surface freshwater reaches of a highly turbid estuary, the Gironde Estuary, France. Appl Geochem 24(9):1747–1756
Migaszewski ZM, Gałuszka A, Dołęgowska S (2016) Rare earth and trace element signatures for assessing an impact of rock mining and processing on the environment: Wiśniówka case study, south-Central Poland. Environ Sci Pollut Res 23:24943–24959
Milham L, Craw D (2009) Antimony mobilization through two contrasting gold ore processing systems, New Zealand. Mine Water Environ 28(2):136–145
Moore F, Dehbandi R, Keshavarzi B, Amjadian K (2016) Potentially toxic elements in the soil and two indigenous plant species in Dashkasan epithermal gold mining area, West Iran. Environ Earth Sci 75:268
Palmer MR, Edmond JM (1992) Controls over strontium isotope composition of river water. Geochim Cosmochim Acta 56:2099–2111
Qi W, Cao J (1991) Research on the background values of antimony in soils. Chin J Sci 22:209–210 (in Chinese)
Ritchie VJ, Ilgen AG, Mueller SH, Trainor TP, Goldfarb RJ (2013) Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chem Geol 335:172–188
Rzymski P, Klimaszyk P, Marszelewski W, Borowiak D, Mleczek M, Nowiński K, Pius B, Niedzielski P, Poniedziałek B (2017) The chemistry and toxicity of discharge waters from copper mine tailing impoundment in the valley of the Apuseni Mountains in Romania. Environ Sci Pollut Res 24:21445–21458
Sharifi R, Moore F, Keshavarzi B (2016) Mobility and chemical fate of arsenic and antimony in water and sediments of Sarouq River catchment, Takab geothermal field, Northwest Iran. J Environ Manag 170:136–144
Shotyk W, Krachler M, Chen B (2005) Antimony: global environmental contaminant. J Environ Monit 7:1135–1136
Sprague DD, Michel FA, Vermaire JC (2016) The effects of migration on ca. 100-year-old arsenic-rich mine tailings in cobalt, Ontario, Canada. Environ Earth Sci 75:405
Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K (2004) Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38:1038–1044
Tschan M, Robinson B, Schulin R (2009) Antimony in the soil-plant system - a review. Environ Chem 6:106–115
Tu (1988) Searches and studies for Superlarge mineral deposits. Golden Geology 1–2 (in Chinese)
Wang L, Zhang M (2007) Release behaviors of heavy metals from polluted soils with heavy metals of different sources. Res Environ Sci 20:134–138 (in Chinese)
Wang D, Chen Y, Chen W et al (2004) Dating the Dachang Giant tin - polymetallic deposit in Nandan, Guangxi. Acta Geol Sin 78:132–138 (in Chinese)
Wang X, He M, Xi J, Lu X (2011) Antimony distribution and mobility in rivers around the world's largest antimony mine of Xikuangshan, Hunan Province, China. Microchem J 97:4–11
Warren LJ (1981) Contamination of sediments by lead, zinc and cadmium: a review. Environ Pollut Ser B Chem Phys 2:401–436
Yang H, He M, Wang X (2015) Concentration and speciation of antimony and arsenic in soil profiles around the world’s largest antimony metallurgical area in China. Environ Geochem Health 37:21–33
Zhang GP, Liu C-Q, Liu H, Hu J, Han G, Li L (2009) Mobilisation and transport of arsenic and antimony in the adjacent environment of Yata gold mine, Guizhou province, China. J Environ Monit 11:1570–1578
Zhou Y, Song S, Zhang C, Yang X, Liu C (2005) Water environmental geochemical response of rivers to mines and mining activity-a case study of the Diaojiang River drainage system, Guangxi, China. Geol Bull Chin 24:940–944 (in Chinese)
Zhou J, Nyirenda M, Xie L et al (2017) Mine waste acidic potential and distribution of antimony and arsenic in waters of the Xikuangshan mine, China. Appl Geochem 77:52–61
Zhu J, Wu FC, Deng QJ, Shao SX, Mo CL, Pan XL, Li W, Zhang RY (2009) Environmental characteristics of water near the Xikuangshan antimony mine. Acta Sci Circumst 29:655–661 (in Chinese)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.41401568). The authors would like to acknowledge Dr. Margaret Graham, a reader in University of Edinburgh, for her instructive suggestions and valuable comments on the writing of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Severine Le Faucheur
Rights and permissions
About this article
Cite this article
Li, L., Liu, H. & Li, H. Distribution and migration of antimony and other trace elements in a Karstic river system, Southwest China. Environ Sci Pollut Res 25, 28061–28074 (2018). https://doi.org/10.1007/s11356-018-2837-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2837-x