Abstract
Century-old tailings at the Beaver Mine site, Cobalt, Ontario, Canada are migrating downstream to previously uncontaminated Kirk Lake, resulting in metal dispersal into the environment. These metal-rich tailings have high arsenic concentrations and exceed the Ontario regulatory standards for arsenic in soil (18 mg/kg) by up to 240 times. Arsenic concentrations in surface water exposed to the tailings and waste rock were found to be up to 268 times the acceptable limit of 5 µg/L for the protection of aquatic life. At the Beaver Mine Site, where the tailings have sat largely undisturbed for the last century, prevailing redox conditions play an important role in the behavior of arsenic and other metals. Surface enrichment of metals was evident in tailings located above the water table and at both the Beaver Mine and Kirk Lake sites. Arsenic enrichment was evident in clay layers within the tailings. A principal component analysis and an analysis of similarity suggest that the migration process did not significantly alter the metal composition of the tailings (ANOSIM p = 0.21), likely because the migration process is relatively rapid compared to leaching mechanisms that would alter metal composition during migration. Our results indicate that the transportation of these century-old, metal-rich, tailings is having minimal impact on reducing their metal concentrations and as these largely unmanaged tailings continue to be transported through the environment they pose a risk to previously uncontaminated lake and stream ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The town of Cobalt, Ontario, Canada, is the product of a silver rush that was sparked in the early 1900s. At the height of the silver rush in 1911, there were more than 40 mines operating in the Cobalt region and in that year alone over 29 million ounces (591 tonnes) of silver were produced (Knight 1922). This silver rush advanced Canadian prospecting, mining, and metallurgical technologies and, importantly, generated the financial backing for future mine development in Canada.
The mining rush that occurred in Cobalt produced 319,926,147 oz (9951 tonnes) of silver from 1904 to 1922 (Knight 1922). The Beaver Mine, our study site, operated from 1910 to 1922, during which time a total of 6,031,338 oz (188 tonnes) of silver were mined. Milling of the ore on site produced 179,349 tonnes of tailings at the Beaver Mine by 1923 (Anderson 1993). Mine tailings in Cobalt are of particular concern for two reasons. The first is the tailings were produced during a period of minimal environmental regulation. Therefore, any waste produced from extracting silver was disposed of from the mills and mines as quickly and conveniently as possible. This typically meant dumping the tailings and waste rock into nearby lakes or depressions. This widespread unmanaged dumping caused the dispersal of tailings throughout the area. The second, and arguably more important, concern is the high concentration of arsenic-bearing minerals found in the ore and tailings. Through the milling process, arsenic was enriched in the tailings and made more mobile through a combination of several factors, including reduced grain size, rapid redox changes (Manning and Goldberg 1997; Kwong et al. 2006), and oxidation of arsenic-bearing minerals (Mok and Wal 1990; Dumaresq 1993; Eapaea et al. 2007; Kreidic et al. 2011; Hiller et al. 2013).
Understanding arsenic behavior in nature is important because it has adverse effects on human and environmental health (Percival et al. 2004). The behavior of arsenic in nature is complex as it can rapidly change redox state in response to environmental conditions. The near-surface zone of the tailings is the main area for arsenic mobilization as this zone can be subjected to rapid changes in redox conditions, due to such events as rainfall, freezing, thawing, fluctuations in the water table and evaporation (Gaskova et al. 2003; Kwong et al. 2006; Lim et al. 2009; Paktunc 2013; Foli et al. 2012). These changes create an unstable redox regime in the upper tailings that promotes the rapid mobilization of arsenic (Gaskova et al. 2003; Kwong et al. 2006) and these near-surface tailings are the most susceptible to erosion.
The Beaver Mine tailings are approximately a century old and no reclamation work or reprocessing of the tailings has occurred. The study area is also isolated, and upstream, from other Cobalt tailings sites and, therefore, the metal contamination observed at the Beaver Mine site is not being altered by inputs from other tailing sites in the Cobalt region. An interesting property of the Beaver Mine tailings is that they are enriched in calcite, as this was the primary gangue mineral for the silver ore. Through calcite dissolution, surface waters flowing through the tailings have a neutral to alkaline pH and, therefore, no acid mine drainage occurs. As such, the study area provides an excellent opportunity to examine the effects of time and transport on tailings in an alkaline environment with little anthropogenic disturbance. The objectives of this study are to characterize the metal concentrations of tailings that have remained largely undisturbed over the last century and to examine what effect, if any, the migration of tailings from the Beaver Mine site to nearby Kirk Lake had on metal concentrations in the tailings. Understanding how migration is altering the metal concentration in the tailings in a field setting is important because most of the tailings in the Cobalt area are both unmanaged and actively migrating along waterways, with affected waters eventually ending up in Lake Temiskaming and the Ottawa River (Dumaresq 1993).
Study site
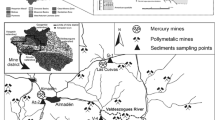
The Beaver Mine site (47° 21′ 43″ N, 79° 38′ 24″ W; elevation 350 masl) is located about 5 km south east of the town of Cobalt (Fig. 1). A layer of waste rock covers portions of the mine property to the south of the tailings deposit. The tailings occupy areas to the north and north east of the old mill foundation. Based on visual observations, the tailings’ material is mostly a medium to coarse-grained sand with small amounts (<10 %) of clay and silt. The tailings form dunes at the Beaver site and are readily susceptible to erosion by wind and water. Erosional features were evident at several locations at the Beaver tailings site, including recent washouts forming large channels and erosion along the banks of the stream that flows through the tailings. Little vegetation is growing on the Beaver Mine tailings except along the small stream, which runs through the tailings, where some Juncus canadensis J. Gay ex Laharpe (Canadian rush) is growing.
Map showing the location of tailings and waste rock at the Beaver Mine site and migrated tailings at Kirk Lake as of 2013. Inset maps show the location of the town of Cobalt in the Province of Ontario and the location of Kirk Lake in relation to Cobalt. Black triangles indicate the sampling locations of the tailings cores labeled as B and KL for tailings cores taken at the Beaver Mine Site and Kirk Lake, respectively. Black circles indicate the location where water samples were collected. Tailings accumulated at Kirk Lake before 1945 are shown as light gray stippling, whereas tailings accumulated since 1945 are shown as dark gray stippling
The stream flowing through the Beaver Mine tailings enters the site at two locations. The inlet stream, which enters the tailings from the surrounding forest, is the dominant source of flow that connects the Beaver site to Kirk Lake (47° 21′ 54″ N, 79° 37′ 48″ W; elevation 275 masl). A second smaller stream with very little flow enters the tailings from the base of a waste rock pile opposite the old mill foundation on the western side of the tailings. This waste rock stream wraps around the western tailings and joins the inlet stream in the middle of the tailings. After the confluence point, the stream flows north and dissipates into a marshy area of tailings vegetated with Canadian rush. At the northern boundary of the tailings, the water is channeled into the outlet stream where it flows to Kirk Lake.
Along the outlet stream from the Beaver site to Kirk Lake, considerable amounts of tailings were observed, mostly located where the stream widens and the water velocity decreases. The stream forms multiple channels at various points, but eventually the water ends up in the main channel that enters Kirk Lake. The migrated tailings at Kirk Lake form a delta style deposit and are again of medium to coarse sand size with small portions of clay and silt (<10 % based on visual inspection). In comparison to the Beaver tailings, the Kirk Lake tailings are much more vegetated, again primarily with Canadian rush. Some of the tailings transported to Kirk Lake have migrated beyond Kirk Lake to the southern bay of Crosswise Lake (Anderson 1993).
Methods
Tailings and water samples were collected at the Beaver Mine site and at Kirk Lake in the summer of 2013. Six tailings cores were sampled from the Beaver Mine site and three tailings cores at Kirk Lake. One additional tailings grab sample was collected from the stream between the two sites. The tailings samples were obtained using a hand corer which permitted samples to be taken from a maximum depth of 2 m. The tailings core samples were divided into 30 cm intervals and, when present, clay and sand layers were separated resulting in a total of 50 tailings samples. Grain size was determined visually for sand-sized grains and textural properties aided in the identification for clay-sized grains. The metal concentrations in all tailings and water samples were analyzed at Caduceon Environmental Laboratories in Ottawa, Ontario, Canada, following standard United States Environmental Protection Agency (EPA) protocols. All tailings samples were dried at room temperature and homogenized before analysis. Eight metals and metalloids of interest (arsenic, antimony, copper, cobalt, lead, zinc, silver and nickel) were chosen for the study due to their relationship with the ore in the Cobalt mining camp and their potential for harm to environmental and human health. Tailings samples were prepared following the EPA 6010b method for determination of metals of interest using inductively coupled plasma—atomic emission spectroscopy (ICP-AES), with the exception of antimony. Tailings samples analyzed for antimony were prepared following the EPA6020 method for determination using ICP—mass spectrometry. The detection limits of the analyses were: As (5 mg/kg); Sb (0.5 mg/kg); Cu (1 mg/kg); Co (1 mg/kg); Pb (5 mg/kg); Zn (3 mg/kg); Ag (0.2 mg/kg); Ni (1 mg/kg). Surface water samples were collected from seven locations in the streams that flow over the Beaver Mine tailings, Kirk Lake tailings and the waters of Kirk Lake (Fig. 1). All water samples were kept cool, void of oxygen and pre-filtered with a 0.45 µm filter to remove particulate matter. Arsenic was the primary metal of interest in surface waters, and was determined following the EPA 200.8 method using ICP-MS; the detection limit for arsenic in the water samples was 0.1 µg/L. Major ions and trace metals were also determined in the surface waters; their analytical method and detection limit are listed with their result in Table 2.
To examine the relationship between the geochemical assemblage in the Beaver and Kirk Lake tailings, a principal component analysis (PCA) was carried out. PCA is a multivariate statistical tool used in exploratory data analysis that uses on principal components (or factors) to explain the greatest amount of variance within the dataset. PCA is frequently applied in environmental data (e.g., soils, surface and groundwaters, sediments, contaminants) where numerous variables have complex inter-relationships. In our PCA, samples with similar axes loadings, based on their metal composition, will fall near each other in the PCA plot.
The power of this approach arises from its ability to distinguish groups of samples, which have different axes scores based on their elemental composition. Samples with similar axes scores, plotting together in groups, may have resulted from the samples having similar net geochemical behavior, similar geochemical processes exerted on them or sources that control the variance within that group. In this study, PCA is used to assess the effects of migration from the source of tailings at the Beaver Mine site to the deposition site at Kirk Lake. In completing the PCA, a broader spectrum of elements was used to better explain the variance within the dataset. For the tailings, zinc, antimony, arsenic, silver, cobalt, lead, and nickel were used. For water samples, more variables were included to reflect the complexity of reactions that can occur between dissolved ions in solution; iron, aluminum, copper, magnesium, potassium, calcium, silver, chlorine, sodium, antimony, arsenic, uranium, molybdenum, cobalt, sulfate, nickel, and pH were used. The samples were plotted based on their factor scores in relation to their composition of metals or elements, so samples enriched in certain metals will plot closer to that metal on the PCA plot.
To reduce the influence of the dominant metals before analysis metal concentration, data were centered to a mean of zero and standardized to unit variance. To test if the metal assemblage of the Beaver tailings was significantly different from the Kirk Lake tailings, an analysis of similarity (ANOSIM) was carried out. ANOSIM is a multivariate technique to test if samples within an assigned group are more similar to one another than they are to members of another predefined group. ANOSIM returns an R value between 1 and −1 where an R of 1 indicates that all samples within a group are more similar to one another than to any sample in another group and an R of −1 indicates that all samples within a group are more similar to samples in another group. The statistical significance of the R value is assessed based on 999 randomization tests where the groupings of the samples are randomized. To test specifically if arsenic concentrations differed between the Beaver Mine and Kirk Lake tailings, a non-parametric Mann–Whitney U test was performed. All statistical analyses were carried out using the vegan package (Oksanen et al. 2015) in the R statistical language (R Core Team 2015). Stratigraphic plots were created using C2 version 1.7 (Juggins 2010).
Results
Characterization of tailings
Metal and metalloid (hereafter referred to as metals) concentrations within the tailings reflect the mineralogy of the ore material they originated from. The tailings notably contained large amounts of arsenic, antimony, cobalt, and nickel, as high concentrations of these metals were typical of the calcite hosted silver-arsenide veins in the region (Fig. 2). The concentration of all target elements across all sites, with the exception of lead and zinc, exceeded the regulatory standards for soil in Ontario (Table 1; Ontario Ministry of the Environment 2011b). The tailings samples also exceeded the concentration of arsenic in soils unaffected by mining in the Cobalt area by up to 593 times; these background soil samples were found to range from 7.3 to 35 mg/kg, averaging 20.7 ± 8 mg/kg in a soil study of the mining camp by the Ontario Ministry of the Environment (2011a). The metal content at the Beaver Mine and Kirk Lake tailings is typical of tailings in the Cobalt mining camp. Depending on the sampling locations, and enrichment processes occurring, arsenic in the Cobalt mining camp tailings can range between 594 and 17,900 mg/kg (Hawley 1980; Dumaresq 1993). In terms of provincial soil standards, the regulatory standard for arsenic in Ontario is 18 mg/kg in soil (Ontario Ministry of the Environment 2011b), whereas arsenic in our study area ranged from 417 to 4330 mg/kg, which is up to 240 times the regulatory standard (Table 1). Arsenic greatly exceeded the concentrations of other elements of interest within the tailings, with the mean arsenic value, excluding the arsenic-rich clay layers, being 687 mg/kg and ranging from 417 to 2330 mg/kg. The mean copper, nickel and silver concentrations were slightly higher at the Beaver mine site compared to the migrated tailings at Kirk Lake (Table 1); however, this difference was not statistically significant.
Box plots for the ten tailings cores (mg/kg) of the eight principal elements associated with ore mineralogy in Cobalt, Ontario, Canada. The sample codes along the X-axis relate to the sampling locations (Fig. 1) at the Beaver Mine site (B) or Kirk Lake (KL)
An important relationship between grain size and metal concentration was observed with greater metal concentrations occurring in layers of clay-sized material compared to layers of sand-sized material (Fig. 3). At three of the ten sampling locations, clay layers were present and in each of these clay layers metal enrichment was observed. For arsenic, there was up to an order of magnitude enrichment in clay compared to the sand-rich layers. The highest arsenic values were found at site B6, which had two separate clay layers, with up to 4400 mg/kg in the clay layers. Sand layers adjacent to the clay were found to have depleted amounts of metals relative to sandy tailings not adjacent to clay layers. Metal enrichment in clay was most evident at the Beaver Mine site while at Kirk Lake enrichment was limited to arsenic, cobalt, and antimony.
Representative element concentration (mg/kg) versus depth for the elements associated with ore mineralogy in Cobalt, Ontario, Canada, taken from core B6 (a), KL1 (b), KL2 (c). Note the increase in all metal concentrations associated with clay layers at sample sites B6, and increase in arsenic, cobalt and antimony in the Kirk Lake sites KL1, and KL2
A surficial enrichment process was also observed at sampling locations that were well above the water table (sampling sites B4 and B5; Fig. 4). At both these sites, crusts were evident on the surface of the tailings and the tailings were dry throughout the length of the cores. Arsenic concentration with depth in these two cores showed surface enrichment in the upper 30 cm of the cores. The metalliferous crusts found on the surface of these sites were 300–500 mg/kg higher in arsenic than samples taken from below the upper 30 cm. The PCA analysis indicated substantial variability in the metal assemblage of tailings samples at the Beaver Mine site. This variability is driven primarily by ore heterogeneity, but enrichment processes in clays and surface sediments also contribute. The enrichment of these elements is ultimately a response of their geochemical properties to changing environmental (saturation) and redox conditions. Samples where enrichment occurred fall well away from the centroid of the values and in some cases outside the 95 % confidence interval of the distribution of the samples based on the estimated standard deviation (Fig. 5).
Increasing arsenic concentration (mg/kg) in surface sediments of non-saturated tailings cores B4 and B5. Surficial enrichment of arsenic is due to capillary action that causes pore waters (containing mobilized arsenic) to migrate upward, during periods of low precipitation and high temperatures. Evaporation then causes supersaturation and precipitation of the soluble constituents contained in the pore waters
Principal component analysis (PCA) of a the metal and metalloid concentrations of tailings samples collected from the Beaver Mine (circles) and Kirk Lake (triangles) sites and b water chemistry variables collected at the surface water sampling sites. The dashed line with arrows in b indicates the succession of samples along the flow path. Elements with a similar distribution in the samples are located closer together in the plots and the position of the tailings and water samples in the plots indicates their association with a particular elemental assemblage. The ellipses in the tailings PCA are the 95 % confidence ellipses for the Beaver Mine and Kirk Lake tailings
Impact of migration on metal concentrations
The migrated tailings sampled from Kirk Lake were the same medium to coarse-grained sandy material found at the Beaver Mine site. In contrast to the Beaver Mine tailings, the Kirk Lake tailings were less variable in their metal concentrations compared to the Beaver Mine site (Fig. 2; Table 1). The PCA also demonstrated that the Kirk Lake samples displayed less variability than the samples from the Beaver Mine. This decreased variability may be attributed to the transport processes that could physically dilute, leach, homogenize and disrupt the metal stratigraphy established at the Beaver site. In contrast, the Beaver site showed increased variability, likely due to the heterogeneity associated with the tailings stratigraphy and enrichment processes at the original deposition site. Although the migrated tailings are less variable in their metal concentrations, ANOSIM analysis indicated that there was no significant difference in any of the metals or between the Beaver Mine and Kirk Lake tailings sites (R = 0.06; p = 0.21). Furthermore, the Mann–Whitney U test indicated no significant difference in arsenic concentrations, the metal of greatest environmental concern, between the Beaver Mine and Kirk Lake sites (p = 0.57). This suggests that the migration process has not significantly altered the composition of these tailings.
Metal concentration in surface water
Water chemistry samples highlight the influence of waste rock and tailings on the surface waters flowing into Kirk Lake from the Beaver Mine site. PCA conducted on major ions and metals in the waters (Table 2), indicated that the geochemical assemblage of the waters from the uncontaminated inlet stream (site W1) was substantially altered by mixing with the much smaller stream, with higher metal concentrations, emanating from the waste rock pile (Fig. 5). After mixing of the two streams, the arsenic content of the sampled surface waters increases (Fig. 6), and the geochemical assemblage continues to become more similar to the waste rock waters, as it flows over the tailings along its path to Kirk Lake. Upon entering Kirk Lake, the water returns to an assemblage more similar to the inlet stream. Surface waters sampled from the forested inlet stream (site W1) prior to entering the Beaver Mine tailings site had a background arsenic concentration of 4 µg/L (Fig. 6), well below the safe drinking water standards for Canada (10 µg/L; Health Canada 2013) and below Canadian standards for the protection of aquatic life (5 µg/L; Environment Canada 2014). In contrast, waters entering the tailings from the waste rock stream (site W2) had arsenic values of 1340 µg/L, 268 times the acceptable limit for the protection of aquatic life and 335 times the arsenic concentration of the inlet stream. Mixing of these waters at the confluence point of the two streams (site W3), with the majority of the flow coming from the inlet stream, resulted in an arsenic concentration of 541 µg/L (Fig. 6). A short distance after the confluence, at site W4, the arsenic concentration rose to 755 µg/L before exiting the Beaver Mine tailings site. The stream water flows over a tailings-rich stream bed after leaving the Beaver site. Before entering the Kirk Lake tailings (site W6), the arsenic concentration of the stream water was 805 µg/L, indicating a continual addition of arsenic to the water from the tailings in the stream bed. Water samples taken directly from Kirk Lake had an arsenic concentration of 57 µg/L, which is still an order of magnitude greater than the Canadian objective for the protection of aquatic life, but strongly diluted from the more contaminated stream water.
Arsenic concentration in µg/L (bars) and pH (diamonds) of water samples taken at sampling locations seen in Fig. 1. Samples were taken before entering the Beaver Mine site (W1), at the Beaver Mine site (W2, W3, W4), entering and exiting Kirk Lake tailings (W5, W6) and in Kirk Lake (W7). Note the three orders of magnitude difference in arsenic concentration in the stream water before entering the mine site and the water exiting the waste rock pile
Discussion
This study demonstrates that these century-old tailings are still migrating from their original deposition area and transporting metal-rich material into new environments. Our results show that the transport of these tailings had minimal impact on their metal concentrations, suggesting that the transportation is relatively fast compared to potential leaching mechanisms that might reduce the concentration of some elements. Our results also highlight the important relationships between the surface waters, tailings and waste rock that help identify the major sources of contamination in waters exiting the Beaver Mine site that have implications for remediation strategies. In addition to our investigation of the migrating tailings, several geochemical processes were identified within the tailings that are important for assessing the tailings from an environmental perspective.
Metal profile of century-old tailings
The chemical profile of the tailings spatially and with depth is likely the result a century of weathering, through well-described redox reactions, and the heterogeneity of the ore that was processed. The distribution of metals in the tailings is strongly variable within the Beaver Mine tailings site. The spatial changes in the concentrations observed at different sites (Fig. 2) may be largely attributed to the material being processed and the amount of clay present at the site. For example, tailings sample sites B1, B2, B3 are closest to the Beaver mill and likely represent some of the earliest tailings that were processed from the richest ore at the mine. These sites have minimal amounts of clay but have high concentrations of arsenic and other metals. In contrast, B6 is the furthest tailings sampling site from the mill and shows arsenic concentrations that are greater than sites closer to the mill by at least 400 mg/kg, likely due to the high amounts of clay found there (Fig. 2). Metal enrichment in clay was also found at tailing sample sites KL1 and KL2 located at Kirk Lake (Fig. 3). At Kirk Lake, the enrichment in the fine-grained clay layers was limited to arsenic, cobalt and antimony, likely due to the saturated conditions encountered there year round. In these conditions, reductive dissolution can favor reduced species which, for these elements, are more mobile than their oxidized counterparts (Gaskova et al. 2003).
Changes in the metal concentrations of the tailings with depth are again, likely controlled by the heterogeneity of the ore grade, redox reactions, element associations and the movement of mobilized metals. At the Beaver Mine site, the deeper tailings represent the earliest tailings deposited. These tailings likely contained elevated arsenic and metal content, because the extraction methods were not as advanced during this time (Reid et al. 1922), and the ore being processed was of a higher grade. Elemental associations, particularly for silver, arsenic, cobalt, and nickel, can also explain anomalous zones within the tailings because these elements are typically enriched together, in both the ore and through geochemical processes occurring in the tailings. The increase in arsenic concentration at surface (Fig. 3) is likely due to the capillary processes described by Dumaresq (1993). In these sand size tailings, where water can move freely, the surface to approximately 90 cm depth represents the zone with the most redox changes, occurring during changes in weather, due to the wetting and drying of the tailings (Kwong et al. 2006). These fluctuations in the wet and dry conditions mobilize metals from the tailings into pore waters, with arsenic being one metalloid that mobilizes quickly in response to redox changes (Kwong et al. 2006; Casiot et al. 2007; Paktunc 2013). The mobilized metals in the pore waters migrate upward toward the tailings surface during periods of low rainfall and high temperatures, due to evaporation and capillary action. Evaporation causes supersaturation within the migrated pore waters and the elements in these waters precipitate soluble constituents in the near-surface sediments, often in the form of metalliferous crusts, where metal concentrations are found to be higher than the underlying tailings. This process of surface enrichment was most evident at tailings sites that were well above the water table because they are subject to evaporation without interference from groundwater.
The major exception to this pattern of surface enrichment was in clay layers, which contained the highest concentrations in our study, up to 4330 mg/kg of arsenic (Fig. 3). The increase in metal content within the clay horizons is due to the sorption potential of clay and the affinity of charged sites on the clay particle surfaces for arsenic, and other metals (Manning and Goldberg 1997). The clay layers at the Beaver Mine and Kirk Lake tailings represent an impermeable barrier for water, and cause infiltrating water to flow parallel to the clay as it travels through the tailings. The continual flow of water, containing mobilized metals, along these clay boundaries allows metals in these waters to be adsorbed onto sorption sites within the clay layers (Manning and Goldberg 1997; Eapaea et al. 2007). In support of this argument, it was also observed that where clay layers were encountered, the concentration of the metals decreased adjacent to the clay horizons (Fig. 3). A probable mechanism for arsenate (As3O4 3−) being adsorbed onto the clay surface is due to cation bridging by calcium. Calcium is abundant in the tailings and waters (>55,000 µg/L; Table 2), as calcite is the primary gangue mineral for the ores at the Beaver Mine (Knight 1922). Negatively charged adsorption sites at the clay interface are likely occupied by calcium, thereby creating positively charged bridges for mobilized arsenate to bind to, although further research would be required to examine the precise role of calcium in arsenic enrichment of clays.
Tailings migration
The migration of tailings caused by fluvial transport plays a major role in the movement and dispersal of contaminants in the environment (Kim et al. 2012). Understanding the extent of this migration is an important step in the assessment of sources of contaminant release within the impacted area (Kim et al. 2012). Anderson (1993) reported that 179,349 tonnes of tailings had been produced at the Beaver mill during its operation until 1923. The processed material was disposed of at the Beaver Mine site and nearly a century of tailings migration has resulted in a large tailings deposit downstream at Kirk Lake. Tailings have also migrated from Kirk Lake along Kirk Creek and resulted in a smaller deposit of tailings at the southern end of Crosswise Lake (Ontario Ministry of the Environment 2011a). The majority of the tailings at Kirk Lake are believed to have migrated in one large event, evidenced by the presence of a continuous clay layer in the pre-1945 tailings and its disappearance in the post-1945 tailings. This event likely occurred during a period of high water flow from the Beaver Mine tailings site, followed by a sustained period of minimal water disturbance (winter) to allow clay settling. The significance of this event is that if these tailings migrated quickly, the oxidative leaching that could occur during transport would not have had time to reduce the metal content of the tailings as they migrated between sites. This, in combination with the slow rate of leaching that would occur in alkaline conditions in a natural environment, may explain why there is no evidence for a significant decline in metal concentration between the undisturbed and the migrated tailings sites. Additionally, the leaching mechanisms occurring due to redox changes from drying and wetting of the tailings that would reduce metal concentrations are minimized because once the tailings are deposited at Kirk Lake they remain in a stable redox environment that is saturated year round. The increased water content in the Kirk Lake tailings has also promoted vegetation growth on the tailings at Kirk Lake. The roots from this vegetation growth further stabilize the tailings and prevent erosion. In their current location, at Kirk Lake, the migrated tailings appear to be in a more stable condition than at the Beaver Mine site. However, the migration process has not reduced the potential environmental hazard posed by these tailings, as they can still be transported to areas where conditions could be more favorable for leaching and metal release.
Surface waters
Surface water flowing across the tailings was generally three orders of magnitude higher in arsenic concentrations than our background surface water sample taken from the forested inlet stream (Fig. 6). The majority of the water entering the tailings was from the forested inlet stream (site W1), which had low metal concentrations with arsenic at this site below the Canadian objective of 5 µg/L for protection of aquatic life (Environment Canada 2014). Our surface water results suggest that the majority, roughly two thirds, of the arsenic input in surface waters exiting the Beaver mine site are from the waste rock stream (site W2), while the remaining one third of the arsenic is contributed from the tailings. As the stream empties into Kirk Lake, the metal concentrations measured in the stream water are diluted by Kirk Lake. Although Kirk Lake dilutes the metal concentration, the water in Kirk Lake is still an order of magnitude above the Canadian objective for the protection of aquatic life. The arsenic concentration of Kirk Lake is likely sufficient to alter the community structure of this ecosystem as some algae and aquatic plant species are known to have reduced growth rates at arsenic concentrations of 50 µg/L (Vocke et al. 1980; Environment Canada 2014). Without steps being taken to reduce the contaminant release from this site, it is plausible that the arsenic concentration of Kirk Lake will continue to affect aquatic life and habitat in Kirk Lake.
Conclusions
The migration of tailings from the Beaver Mine site to Kirk Lake has been identified as playing a role in the dispersal of contaminants into the environment. The Beaver Mine and Kirk Lake sites vastly exceeded regulatory standards for arsenic in soil (18 mg/kg) and water (5 µg/L) and are well above background arsenic concentrations found in the soils of the Cobalt area (up to 35 mg/kg). Importantly, this study demonstrated that the migration of the tailings from the Beaver Mine site to Kirk Lake did not significantly alter the metal concentration of the tailings. This indicates that when tailings migrate through streams the movement of these tailings in alkaline conditions is often too rapid to allow for any reduction in toxicity through leaching mechanisms. This has important implications for understanding the potential environmental harm from migrating tailings, given that faster flowing streams are more likely to transport tailings material compared to slower flowing streams or wetlands. Once transported to Kirk Lake, the tailings are deposited into a much more stable redox environment where leaching mechanisms, arising from rapid redox changes that would reduce metal concentrations, are minimized. However, there is still a need to limit future migration to avoid introducing further contaminated material into environments downstream of the original tailings deposits, because they have potential to be transported to areas where rapid redox changes occur frequently, which promote arsenic and metal release. Legacy environmental pollution, such as the Beaver Mine site, is an issue over much of the world and often presents unique challenges in management and remediation. It is important that these legacy sites are contained so that environmental damage does not continue to spread decades after the industrial activity has finished.
References
Anderson P (1993) Cobalt mining camp tailings inventory, Cobalt, Ontario. Unpublished Report. Ministry of Northern Development and Mines, p 196
Casiot C, Ujevic M, Munoz M, Seidel JL, Elbaz-Poulichet F (2007) Antimony and arsenic mobility in a creek draining an antimony mine abandoned 85 years ago (upper Orb basin, France). Appl Geochem 22:788–798
Dumaresq CG (1993) The occurrence of arsenic and heavy metal contamination from natural and anthropogenic sources in the Cobalt area of Ontario. M.Sc. Thesis, Carleton University, Ottawa, Canada
Eapaea MP, Parry D, Noller B (2007) Dynamics of arsenic in the mining sites of Pine Creek Geosyncline, Northern Australia. Sci Total Environ 379:201–215
Environment Canada (2014) Canadian environmental quality guidelines (CEGQ) under the Canadian Environmental Protection Act: water quality guidelines for the protection of aquatic life. Prepared by Environment Canada
Foli G, Nude PM, Amedjoe CG, Kyei L (2012) Arsenic leaching in mill tailings at the AngloGold Ashanit Obusi Mine, Ghana: management of contamination in the related water environment. West Afr J Appl Ecol 20(7):11–23
Gaskova OL, Bessonova EP, Bortnikova SB (2003) Leaching experiments on trace element release from the arsenic bearing tailings of Khuvo-Aksy (Tuba Republic, Russia). Appl Geochem 18:1361–1371
Hawley J (1980) The chemical characteristics of mineral tailings in the Province of Ontario, 1979. Ontario Ministry of the Environment
Health Canada (2013) Guidelines for Canadian drinking water quality—summary table. Prepared by Health Canada
Hiller E, Petrák M, Tóth R, Lalinská-Voleková B, Jurkovič L, Kučerova G, Radková A, Šottnik P, Vozár J (2013) Geochemical and mineralogical characterization of neutral/low sulphide/high carbonate tailings impoundment, Markusovce, eastern Slovakia. Environ Sci Pollut Res 20:7627–7642
Juggins S (2010) C2 version 1.6.7. Software for ecological and palaeoecological analysis and visualization. Department of Geography, University of Newcastle, Newcastle-upon-Tyne
Kim CS, Stack DH, Rytuba JJ (2012) Fluvial transport and surface enrichment of arsenite in semi-arid mining regions: examples from the Mojave Desert, California. J Environ Monit 14:1798–1813
Knight CW (1922) Geology of the mine workings of Cobalt and South Lorrain silver areas. Ontario Department of Mines. 31st Annual report, volume 31, Part 2
Kreidic N, Armiento G, Cibin G, Cinque G, Crovato C, Nardia E, Pacifico R, Cremisini C, Mottana A (2011) An integrated geochemical and mineralogical approach for the evaluation of arsenic mobility in mine soils. J Soils Sediments 11:37–52
Kwong YTJ, Beauchemin S, Hossain MF, Gould WD (2006) Transformation and mobilization of arsenic in the historic Cobalt mining camp, Cobalt, Ontario, Canada. J Geochem Explor 92:133–150
Lim M, Han G, Ahn J, You K, Kim H (2009) Leachability of arsenic and heavy metals from mine tailings of abandoned metal mines. Int J Environ Res Public Health 6:2865–2879
Manning BA, Goldberg S (1997) Adsorption and stability of As(III) at the clay mineral—water interface. Environ Sci Technol 31:2005–2011
Mok W, Wal CM (1990) Distribution and mobilization of arsenic and antimony species in the Coeur-d’Alene River, Idaho. Environ Sci Technol 24:102–108
NEMI (2015) National Environmental Methods Index. https://www.nemi.gov/home/
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) vegan: Community Ecology Package. R package version 2.2-1. http://CRAN.R-project.org/package=vegan
Ontario Ministry of the Environment (2011a) Final report: Cobalt-Coleman mining camp soil assessment. Prepared by Ontario Ministry of the Environment
Ontario Ministry of the Environment (2011b) Soil, ground water and sediment standards for use under part XV. I of the Environmental Protection Act. Prepared by the Ontario Ministry of the Environment
Paktunc D (2013) Mobilization of arsenic from mine tailings through reductive dissolution of goethite influenced by organic cover. Appl Geochem 36:49–56
Percival JB, Kwong YTJ, Dumaresq CG, Michel FA (2004) Transport and attenuation of arsenic, cobalt, and nickel in an alkaline environment (Cobalt, Ontario). Geological Survey of Canada Open File 1680
Reid FD, Denny JJ, Hutchison RH (1922) Milling and metallurgical practice in treatment of silver ores at Cobalt. Ontario Department of Mines. 31st Annual report, volume 31 Part 2
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Vocke RW, Sears KL, O’Toole JJ, Wildman RB (1980) Growth response of selected freshwater algae to trace elements and scrubber ash slurry generated by coal-fired power plants. Water Res 14:141–150
Acknowledgments
This paper was improved by the helpful comments from the editor and two anonymous reviewers. The authors have also benefited from the wealth of information and local knowledge collected by Carleton University’s Environmental Science Cobalt Field course. D.D.S and J.C.V are supported by Carleton University and an NSERC Discovery Grant to J.C.V.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests for this study.
Rights and permissions
About this article
Cite this article
Sprague, D.D., Michel, F.A. & Vermaire, J.C. The effects of migration on ca. 100-year-old arsenic-rich mine tailings in Cobalt, Ontario, Canada. Environ Earth Sci 75, 405 (2016). https://doi.org/10.1007/s12665-015-4898-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4898-1