Abstract
The variation trend and growth rate of P were analyzed by the concentration of the phosphorus fraction on surface sediment of Dongting Lake from 2012 to 2016, to reveal the cumulative effect of P in the actual environment. Meanwhile, the adsorption kinetics and adsorption isotherm were employed to examine the P-release possibility of sediment, which predicts the yearly released sediment phosphorus in Dongting Lake. The actual growth rate of TP (Total Phosphorus) is 53 mg·(kg·year)−1 in East Dongting Lake, 39 mg·(kg·year)−1 in South Dongting Lake, and 29 mg·(kg·year)−1 in West Dongting Lake, while the sum of the phosphorus fraction growth rates has little difference from the rate of TP in sediments of the three areas of Dongting Lake. Furthermore, the Elovich model and the Langmuir crossover-type equations are established to present the adsorption characteristic of sediment in Dongting Lake; the result shows that the sediments play a source role for phosphorus in East and South Dongting Lake from zero equilibrium phosphorus concentration (EPC0) in the present situation, but an adsorption effect on TP is shown in West Dongting Lake. When the conditions of environment change are ignored, the maximum P-sorption level in sediments of East Dongting Lake will reach in 2040 according to the actual growth rate of sediments, while that in West Dongting Lake and South Dongting Lake will be in 2046 and 2061, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main determinants of eutrophication are phosphorus and nitrogen, however, the phosphorus is the first limiting nutrient element influencing algal population and density in the Lake (Monbet et al. 2009; Michaeld et al. 2009). Most of the phosphorus comes from exogenous input and endogenous pollution in lakes (Kaiserli et al. 2002). The mainly cause of eutrophication is endogenous P, which is released in sediment after cutting off the external pollution (Redshaw et al. 1990; Xu et al. 2013). But the interfacial exchange and bioavailability of phosphorus are affected by the P fraction in sediment. Different phosphorus forms in sediments have different effects on the eutrophication of the water body (Pan et al. 2007). Meanwhile, it are also valuable parameters judging phosphorus migration and ecological effects on sediments (Vallja et al. 2013; Huang et al. 2004). The research of variation laws of phosphorus is of great significance under the condition of cutting off the external input, to reveal effectively the behavior characteristic, migration law, and eutrophication of phosphorus (Xiang and Zhou, 2010). P in sediment can enter the overlying water by the following ways: desorption, dissolution, mineralization, and microbe release (Baldwin 1996; Gonsiorczyk et al. 1998), resulting in an increase of dissolved phosphorus contents in water. The processes of phosphorus exchange at the sediment-water interface are divided into two parts; one is the adsorption reaction between sediment and interstitial water, and the other is diffusion or convection between the overlying water and interstitial water (Richardson 1985; Nichols 1983). The process of P exchange in sediments can be described and explained by adsorption kinetics and sorption isotherms; meanwhile, the relevant parameters of the P-sorption model, such as P-sorption rate, maximum adsorption capacity (Qmax), and zero equilibrium phosphorus concentration (EPC0), are calculated to determine the possibility of P release in sediments. EPC0 is a universal tool assessing the P-exchange behavior at the sediment-water interface used usually to describe the P behavior characterized in sediments and judge the state of phosphorus forms (Jasong et al. 2011a, b; Lottig and Stanley 2007). The research suggests that if the soluble active phosphorus (SRP) concentrations of overlying water are significantly lower than the EPC0 of sediments, the sediments would release P into the overlying water as a P release source. On the contrary, it would absorb P from the overlying water, which served as a sink of phosphorus (Jarvie et al. 2005).
Dongting Lake is the second freshwater lake in China, which belongs to a typical Lake Taking in – Sending out water. The nutrients, such as the total nitrogen, the total phosphorus, ammonia nitrogen, and nitrate nitrogen, may increase in overlying water due to the economic development around Dongting Lake and the construction of water conservancy projects, resulting in the risk of water eutrophication being increasingly higher and even the appearance of slight eutrophication in some areas of the lake (Huang et al. 2013). Many researchers on analyzing composition, spatial distribution and source characteristic of nitrogen and phosphorus in overlying water and surface sediments of Dongting Lake had been conducted in recent years, which also involved the factors of explain why the nutrients in overlying water of some areas have been overweight, but the eutrophication has been not happened. (Wang et al. 2014; Wang et al. 2013). In the last two decades, water environment improvement projects have been carried out to protect the ecological environment of Dongting Lake, resulting in the cutoff of external pollution source input. Then, the nutrients in the sediment play an important effect on eutrophication in Dongting Lake. The variation laws of phosphorus forms on surface sediment of Dongting Lake are analyzed with regression analysis according to monitoring data of P fractions from 2012 to 2016, which preliminarily judges the possible risk of phosphorus in sediment. Simultaneously, adsorption kinetics and adsorption isotherms are adopted to analyze the sorption-release mechanism of P in sediment and judge the possibility of phosphorus release in sediment of Dongting Lake by the P-sorption rate, EPC0, and Qmax, which will predict the year of P release in sediment. The aim of this study is to prevent eutrophication of water bodies and provide the basis for preserving the ecological environment in Dongting Lake. Furthermore, the results can be used for reference in adsorption of phosphorus in sediment of other freshwater lakes.
Materials and methods
Research and data source
Dongting Lake (Wang et al. 2016), which is the second largest freshwater lake in China, is located in the northeast Hunan Province, with an extensive catchment area from 28° 44′ 0″ N to 29° 35′ 0″ N and 112° 0′ 0″ E to 113° 05′ 0″ E. It drains a catchment area of 2820 km2, belonging to a typical lake which is taking in and sending out water. It is shaped like the letter U, divided into three regions, namely the East Dongting Lake, the South Dongting Lake, and the West Dongting Lake. The lake has Xiangjing River, the Zi River, the Yuan River, and the Xinqiang River as east input, and the Songzi estuary, the Taiping estuary, and the Ouchi estuary as three bleeders of Yangtze River water flow.
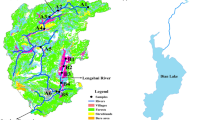
In this work, analysis data source contains two ways. Firstly, the data of P forms in sediment from 2012 to 2016 was provided by the Ecological and Environmental Monitoring Center of Dongting Lake of Human Province. Secondly, the sediment is collected to be used in the adsorption kinetics experiment and adsorption isotherm experiment in September 2014. Samples were selected in accordance with different environmental conditions (topography, aquatic plant coverage, and water depth, among others) from the East Dongting Lake (D1, D2, D3, D4, D5, D6), the South Dongting Lake (D7, D8, D9, D10), and the West Dongting Lake (D11, D12, D13, D14) (Fig. 1).
Adsorption kinetics
To study adsorption kinetics, 150 mL capacity conical flasks by taking 50mL with initial P concentrations of 1mg/L. The pH of the slurries was maintained at 7.5–8.0 by adjusting with 0.01 mol/L NaOH and 0.01 mol/L HCl. Then, 0.5 g dried sediment of different sample sites was added to these conical bottles. Next, the suspensions were placed in a stable-temperature air shaking bath at 20 ± 0.5 °C. The suspensions from the overlying water were collected at 0, 10, 20, 30, 60, 120, 240, 480, 720, 1440, and 2880 min, and then filtered through a 0.45-μm membrane to analyze phosphate concentration by phosphor molybdenum blue spectrophotometry (Editorial Board of the State Environmental Protection Administration, 1989). Each sample was set to the parallel test; the relative standard deviation of each sample is lower than 5%.
Adsorption equilibrium
To study adsorption equilibrium, the adsorption isotherm experiment consisted of a series of initial P concentrations of 0, 0.05, 0.1, 0.5, 1.0, 5, 10, 20, and 50 mg/L obtained by adding different quantities of 50 mg/L KH2PO4 solution to the Erlenmeyer flask, and 0.5 g dry sediment was added into the conical flask. Then, the suspensions were placed in a stable-temperature air shaking bath at 20 ± 0.5 °C for a 2880-min equilibration period. After 2880 min of equilibration, the suspensions were collected and then filtered through a 0.45-μm membrane. The P concentration after filtering is analyzed by phosphor molybdenum blue spectrophotometry. Each sample was set to a parallel test; the relative standard deviation of each sample is lower by 5%.
Statistical analysis
The adsorption capacity (Q) has been introduced to describe the sediment adsorption effect of phosphorus, which predicts the absorbed phosphorus quality per unit mass of sediment. The equation for the calculation of Q is as follows:
where co is the initial P concentration in the solution (mg/L), ce is the equilibrium concentration of P in the solution (mg/L), V is the volume of the solution (L), and W is the mass of the dry sediment (g).
The kinetics processes of phosphate adsorption on sediments could be described by the first-order reaction kinetics model, second-order adsorption kinetics model, parabolic diffusion model, and the Elovich model (Padmesh et al. 2005; Ho and Yuh log han 2006; Mcdowell and Sharpley 2003). The Elovich model is expressed as follows:
where q is the adsorption capacity of P adsorbed by the sediment (mg/g), t is the adsorption time (h), a is the initial adsorbing rate, and b is the adsorption coefficient, which indicates the strength of the phosphate adsorbed on the sediment.
The problem of phosphorus in sediment-water allocation can be reflected by the isothermal adsorption model, such as the Langmuir equation and the Freundlich isotherm model. However, compared with adsorption process of P in the natural environment system, strange swings that adsorption isotherms appear in natural water was found by long-term practice (O’Connor et al. 1980). Therefore, this isothermal adsorption model needs adjustment to match the actual situation. Considering the influence of P uptake in the sediments, the Langmuir crossover-type equations were used for the fitting in this study. The modified Langmuir equation is expressed as follows (Wang et al. 2005):
where Q is the adsorption capacity of P at equilibrium (mg/g); Qm is the maximum P-sorption capacity in the sediment, which is the indicator of P-sorption capability in sediment and reflects the capacity factor of sediment-adsorbed phosphate; k is the adsorption equilibrium constant; ce is the equilibrium concentration of P in the aqueous phase (mg/L); EPC0 is the zero equilibrium P concentration of the sediment, which is the intersection between the adsorption isotherm and axis of ce (mg/L); and NAP is the phosphorus concentration originally incorporated in the sediment and desorbed under experimental conditions (mg/kg).
Result and discussion
Variation laws of total phosphorus
TP is the sum of the phosphate forms in the sediment, which can reflect the degree of contamination and the risk of release of phosphorus in sediments (Zhang et al. 2012). The contents of TP on surface sediment from 2012 to 2016 in the three lake districts of Dongting Lake are depicted in Fig. 2. The concentrations of TP varied greatly in sediments of the three lake areas, whose degree for various lake districts is East Dongting Lake > West Dongting Lake > South Dongting Lake. There are two reasons for the variance of TP in sediments of the three lake areas. Human activities are frequent and intensive due to a large population around East Dongting Lake and Xiangjiang River, which cause enormous nutrient input from pesticides, fertilizers, livestock, and domestic sewage, and where soil erosion is serious in watershed catchment basins. There are large amounts of suspended sediment in Xiangjiang River, while sand has a strong capacity for P sorption, resulting in much phosphorus from the river carried into the lake (Huang et al. 2013; Tian et al. 2016). Moreover, the physicochemical property of sediment can also make a difference in the phosphorus content of different space. The main component of sediments in East Dongting Lake is muddy-sandstone and tuffaceous, and the sediment particles are finer than those of other regions, which cause the P-sorption capacity in the sediment of the East area to be stronger than those of the other regions. Phosphorus contents of surface sediment in the East Dongting Lake are evaluated to moderate the level of pollution by the Ontario Guidelines for Sediment Quality Assessment (Persaud et al. 1993), while P contents are at safe levels in the West and South Dongting Lake. However, the concentration of TP in sediments of the three regions in Dongting Lake was increased year by year, which indicates that sediments rendering the starvation state of P still absorb phosphorus from the overlying water. This phenomenon is related to the construction of the Three Gorges Project. The particulate matter in the Yangtze River downstream of the dam has been reduced by 60% compared with the previous ones since the impoundment of the Three Gorges Project, which simultaneously makes reservoir inflow decrease and water-flow velocity slow down in Dongting Lake, causing the dissolved phosphorus in the overlying water to settle down and be stored in sediment (Tian et al. 2016).
To further describe variation laws of TP from 2012 to 2016, TP in 5 years is linear fitted by the equation (TP = a + b · T) as presented in Table 1. R2 ranges from 0.966 to 0.982, which shows a pronounced correlation. The actual growth rate of TP in sediments is 53 mg·(kg·year)-1 in East Dongting Lake, 39 mg·(kg·year)-1 in South Dongting Lake and 29 mg·(kg·year)-1 in West Dongting Lake, 29 mg/(kg∙year). The main cause of discrepancy is concerned with sediment particles. Experiment research testifies that the finer the sediment particles, the stronger the adsorption capacity and resuspension ability for phosphates (Huang 2011). Regardless of the external conditions of the change, the density of P in the East Dongting Lake will be the level of heavy pollution in 2037 by the current adsorption rate, whereas the phosphorus concentrations in West and South Dongting Lake will achieve moderate pollution levels in 2016 and 2017, respectively. In general, phosphorus in sediments of Dongting Lake has been at a dangerous level according to the current content of phosphates.
Variation laws of phosphorus fraction
The contents of P fractions from 2012 to 2016 in three lake districts of Dongting Lake are presented in Fig. 3. The contents of four P forms represent a trend of growth with year in the three regions of Dongting Lake, while the order of growth rates of P fractions in sediments was HCl-P > Res-P > OP > NaOH-P. This difference was mainly caused by the Three Gorges Project, sewage interception project, and agricultural non-point source pollution. Wei found that the phosphorus component of the suspended particles was mainly composed of spontaneous apatite phosphate in the Yangtze River (Wei et al. 2010), while Dongting Lake belongs to the water-carrying lake in the Yangtze River side. The flow velocity of water will get slow in the lower reaches of the Yangtze River due to the Three Gorges Project, and apatite phosphorus of suspended particles settles down in sediment of Dongting Lake, which makes the HCl-P content the highest in the whole lake. NaOH-P of sediment is derived mainly from domestic sewage and industrial wastewater (Ruban et al. 1999), while the Intercepting Sewage Project in recent years has been implemented in Dongting Lake watershed to prevent wastewater from directly entering the lake. Therefore, the main source of NaOH-P in sediment of Dongting Lake is cut off, which leads to the growth rate of NaOH-P being the slowest in whole P forms. The study suggests that 60% of OP in sediment comes from agricultural pollution (Ruban et al. 1999). Agriculture is developed around the Dongting Lake area, but agricultural effluent is rarely treated and directly enters the lake, which leads to a faster growth of OP in sediment. However, there is a difference in the growth rate of the same P forms in the three areas of Dongting Lake. The growth rates of NaOH-P and Res-P in East Dongting Lake were significantly higher than those in South and West Dongting Lake, while the growth rates of HCl-P and OP have little difference between the three areas of Dongting Lake. Research suggests that the growth rate of P fractions in sediment is affected by factors such as geo-environment, hydrodynamic characteristics, and growth of aquatic organisms (Yang et al. 2017). This will lead to the growth of the same P forms held discrepant in the three regions of Dongting Lake due to variance of physicochemical properties in sediment and dissolved phosphorus in overlying water.
To further explain the variable law of P fractions of Dongting Lake with years, linearly fitting for the average concentrations of P forms is shown in Table 2. Phosphorus morphologies from 2012 to 2016 in sediment of Dongting Lake suit the linear fitting equation with the range of R2 being 0.879–0.981, except the NaOH-P and Res-P of sediment in South Dongting Lake, as presented in Table 2. The study concluded that the sum of the growth rates for the forms of phosphorus is not significantly different from that of TP growth in the sediment of the same region. According to the results of the Pardo and Ruban research, the relationship between various P fractions includes two equations: IP = NaOH-P + HCl-P and TP = IP + OP + Res-P (Pardo et al. 2004; Ruban and Rauret 2001). As shown in the previous discussion, there is a difference in the increasing rate of the same elements in different areas, the concrete as follows: the growth rate of P forms in sediments of East Dongting Lake is the fastest compared with those of the other two regions, except the adsorption rate of OP in West Dongting Lake, being slightly higher than that in East Dongting Lake. HCl-P and Res-P do not belong to bioavailable phosphorus of sediment, which contributes less to the concentration of dissolved phosphate in overlying water of the lake (Rydin 2000), while NaoH-P and OP in sediment are bioavailable phosphorus, and their content range presents the pollution level of P in sediment and the capacity for its endogenous release (Xiang and Zhou 2010). This research indicates that the bioavailable phosphorus is 37–44% of the TP in sediment of Dongting Lake, while the adsorption rate of sediments for OP ranged from 5.905 to 11.324 mg/(kg·h) and that for NaOH-P increased from 6.326 to 10.772 mg/(kg·h). At this pace, the accumulation of OP and NaOH-P will increase gradually in the sediment as time goes by; therefore, their effect on eutrophication cannot be ignored during the later period, especially East Dongting Lake where the growth of OP and NaOH-P in sediments is faster than those of other regions.
Sorption kinetics of phosphorus
The adsorption kinetics of sediment for phosphorus can be reflected by the adsorption rate, which is defined as the adsorption amount of sediment to P per unit time. As shown in Table 3, the P adsorption rate in the three regions of Dongting lake exists difference during the same period, and that of the same area are various in different periods. The maximum adsorption rate of sediment for P grew fastest within 10 min in East Dongting Lake and West Dongting Lake; their rates reached the values 79.8 and 78.6 mg/(kg·h), respectively, while the maximum velocity of adsorption was 63.6 mg/(kg·h) from 10 to 20 min in sediment of South Dongting Lake. The range of the adsorption rate of P tends to be stable in sediment of the three lake areas after 1440 min, which is 0.004–0.017 mg/(kg·h) in East Dongting Lake, 0.004–0.008 mg/(kg·h) in South Dongting Lake, and 0.008–0.013 mg/(kg·h) in West Dongting Lake, respectively. The actual adsorption rate of P in sediment is 0.006 mg/(kg·h) in East Dongting Lake, 0.003 mg/(kg·h) in South Dongting Lake, and 0.004 mg/(kg·h) in West Dongting Lake from previous research. Therefore, the relationship between the actual adsorption rate and experimental adsorption rate in different lake areas is as follows: East Dongting Lake’s actual rate is situated in the experimental adsorption rate interval, while the rate is lower than the minimum experimental adsorption rate in the South and West Dongting Lake. Huang and Wang found that the dissolved phosphorus concentration of overlying water is 0.09–0.13 mg/L in East Dongting Lake, 0.08–0.09 mg/L in South Dongting Lake, and 0.09–0.12 mg/L in West Dongting Lake (Huang et al.; Wang et al. 2016), whereas the initial P content was 0.1 mg/L in this kinetics experiment. Thus, the main reason for the above phenomenon can be related to the P content in overlying water.
The modified Elovich model is employed to further describe the kinetics characteristic of adsorption for P in sediment, and the fitted result is presented in Fig. 4 and Table 4. The adsorption process of sediment for phosphorus is divided into two stages: one is rapid adsorption and the other is slow adsorption, as presented in Fig. 4, which is similar to the general finding about sorption kinetics of P for sediment (Chen et al. 2014). There are two stages of phosphate adsorption in the three areas of Dongting Lake. The rapid stage of P adsorption occurs within 60 min; then, the slow sorption stage appears between 60 and 240 min. The adsorption amount of P for sediment changes smaller after 240 min, which means the stage seems to be in process of adsorption equilibrium. The range of R2 of the fitted curve about the Elovich model is 0.887–0.912, all higher than 0.8, which indicates fitted equations of sediment adsorption reach extremely significant levels, as presented in Table 4. The results indicate a in sediment at different sample sites is reduced from West Dongting Lake to East Dongting Lake, and to South Dongting Lake.
Analysis of phosphorus release
The Langmuir crossover-type equations are introduced to describe the relationship between equilibrium concentrations of phosphorus and adsorbed amount of sediment for P with various initial contents of P; results as fitted curve are shown in Fig. 5. The R2 range of curve fitting is 0.912–0.991, which indicates the adsorbing behavior of P can be described by the Langmuir crossover-type equations adsorption isotherm in the sediments of the three lake regions. The point of intersection between sorption isotherms and the x-axis is not at the origin coordinates but at the positive x-axis; meanwhile, as the initial P content increased in overlying water, the equilibrium adsorption capacity in sediment for P accelerated first and then became flat with a change in trend. An investigation of its reason showed that the concentration gradient of P between the overlying water and the surface of the sediment increased with the initial phosphate content enlarging, which strengthened the mass transfer process of P between the solution, sediment surface, and sediment pore space and accelerated the combination of phosphorus and adsorption sites, leading to a severe increase of P-sorption capacity in sediment (Plazinski et al. 2009). The adsorption site of the surface sediment will stay constantly with the increase of the initial P concentration in the solution, which leads to the balance of the P-sorption amount of the sediment. Meanwhile, the force of electrostatic repulsion is produced between the negative charge of the surface sediment and the phosphate ion in the solution, which may even cause the P-sorption contents’ decline (Huang et al. 2012).
The results of the calculation about parameters of the Langmuir crossover-type equations and NAP are presented in Table 5. The range of Qmax in sediment is 1.238–1.862 mg/g in East Dongting Lake, 1.734–2.032 mg/g in South Dongting Lake, and 1.739–1.907 mg/g in West Dongting Lake. The maximum P-sorption level in sediments of East Dongting Lake will be reached in 2040 according to the actual adsorption rate, while that in West Dongting Lake and South Dongting Lake are estimated to be 2046 and 2061, respectively.
EPC0 is the equilibrium concentrations of sediment on adsorption-desorption equilibria of phosphorus, which is used to judge whether the sediment is acting as a source or sink of phosphate, revealing the flux of soluble phosphorus in the overlying water (Jasong et al. 2011a, b). The range of EPC0 is 0.161–0.302 mg/L in East Dongting Lake, 0.234–0.260 mg/L in South Dongting Lake, and 0.006–0.15 mg/L in West Dongting Lake, with their corresponding EPC0 average of 0.272, 0.247, and 0.01 mg/L, respectively. According to recent researches (Huang et al. 2013; Wang et al. 2016), the content of dissolved phosphorus in overlying water is 0.09–0.13 mg/L in East Dongting Lake, 0.08–0.09 mg/L in South Dongting Lake, and 0.09–0.12 mg/L in West Dongting Lake. In comparison, the dissolved phosphate concentration of overlying water is obviously lower than the EPC0 in East and South Dongting Lake, that is, in the desorption region of the sorption isotherm, which determines the preliminarily sediment of East and South Dongting Lake generally acts as the P source, while the dissolved P content is higher than the EPC0 in the overlying water of West Dongting Lake, which indicated sediment has an adsorptive effect to dissolve phosphate. However, sediments have a weak adsorption function to overlying water phosphorus in the three regions of Dongting Lake, which is still the gathering place of phosphate. The East and South Dongting Lake do not match with the actual, except West Dongting Lake compliance, in comparison with the evaluation results of EPC0. Sondergaard found that it is one of the main sources of P in sediment that the primary producer can transform the dissolved phosphate in overlying water into organic matter by the relevant effect, finally sinking into the sediment in the bottom of the lake when they died (Sondergaard et al. 2001). According to the circulation theory of the phosphorus in the lake (Liu 2016), when the exchange velocity between the sediment and aquatic organisms is greater than the release rate between the sediment and overlying water, the P content will be increased in the sediment of the lake.
Conclusion
(1) The sediment still has an adsorption effect on dissolved phosphorus of overlying water in Dongting Lake. Their adsorption rates are 53 mg/(kg·year) in East Dongting Lake, 39 mg/(kg·year) in West Dongting Lake, and 29 mg/(kg·year) in South Dongting Lake. Without considering the external conditions of the change, the density of P in East Dongting Lake will be at the level of heavy pollution in 2037 by the actual P-sorption rate, whereas the P concentration in West and South Dongting Lake will achieve the moderate pollution level in 2016 and 2017, respectively. In general, P in sediments of Dongting Lake has been at a dangerous level judging by the current P content.
(2) The P form contents represent a trend of growth with year in the three regions of Dongting Lake; meanwhile, the sum of P forms’ growth rate is not significantly different from that of TP growth in the sediment of the same region. The growth rate of OP is 5.905–11.324 mg/(kg·h) in Dongting Lake, and that of NaOH-P is 6.326–10.772 mg/(kg·h). Furthermore, the accumulation of OP and NaOH-P will increase gradually in the sediment as time goes by; therefore, their effect on eutrophication cannot be ignored during the later period, especially East Dongting Lake.
(3) The kinetic characteristic of P-sorption in sediment is described by the Elovich model. The rapid stage of P adsorption occurs within 60 min, and then, slow P sorption appears between 60 and 240 min. The P-sorption amount of sediment changes smaller after 240 min, and the stage seems to be in the process of adsorption equilibrium after 1440 min. Compared with the actual P-sorption rate in Dongting Lake, East Dongting Lake’s actual rate is situated in the experimental adsorption rate interval, while the rate is lower than the minimum experimental P-sorption rate in the South and West Dongting Lake.
(4) The P-sorption isothermal curve can be described by the Langmuir crossover-type equations. The maximum P-sorption level in sediments of East Dongting Lake will be reached in 2040, according to the actual P-sorption rate, while those in West Dongting Lake and South Dongting Lake are estimated to be 2046 and 2061, respectively. According to comparable results between EPC0 and dissolve P of the overlying water, East and South Dongting Lake’s sediments act as role of P source in most case, while West Dongting Lake’s sediment has adsorptive effect to dissolve P.
References
Baldwin DS (1996) The phosphorus composition of a diverse series of Australian sediments. Hydrobiologia 335(1):63–73
Chen CY, Xu XM, Deng WM et al (2014) Characteristics of phosphorus adsorption on surface sediments of Dianchi Lake. Acta Sci Circum 34(12):3065–3075 (in Chinese)
Editorial Board of the State Environmental Protection Administration (1989). Water and wastewater monitoring and analysis methods [M]. China Environmental Science Press. (in Chinese)
Gonsiorczyk T, Casper P, Koschel R (1998) Phosphorus-binding forms in the sediment of an oligotrophic and a eutrophic hardwater lake of the Baltic Lake District (Germany). Water Sci Technol 37(3):51–58
Huang Q H, Wang D H, Wang C X, et al (2004) Vertical variation of the phosphorus form in the sediments of Meiliang Bay and Wuli Lake of Taihu Lake[J]. China Environ Sci 24(2):147–150
Ho, Yuh log han (2006) Review of second-order models for adsorption systems. ChemInform 136(3):681
Huang L-d, Chai R-s, Zong X-b et al (2012) Characteristics of phosphorus sorption kinetics on sediments at different initial phosphorus concentrations. J Zhejiang Univ (Agric Life Sci.) 38(1):81–90 (in Chinese)
Huang DZ, Wan Q, Li-Qiang LI et al (2013) Changes of water quality and eutrophic state in recent 20 years of Dongting Lake. Res Environ Sci 26(1):27–33 (in Chinese)
Huang L D (2011) Factors affecting phosphorus sorption by lake sediments. Zhejiang University
Jasong K, Michele B, Jon O et al (2011) Phosphorus sorption in soils and sediments: implications for phosphate supply to a subtropical river in southeast Queensland, Australia. Biogeochemistry 102(1–3):73–85.
Jarvie H P, Jürgens M D, Williams R J, et al (2005) Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Herefordshire Wye[J]. J Hydrol 304(1–4):51-74
Kaiserli A, Voutsa D, Samara C (2002) Phosphorus fractionation in lake sediments—lakes Volvi and Koronia, N. Greece. Chemosphere 46(8):1147–1155
Lottig NR, Stanley EH (2007) Benthic sediment influence on dissolved phosphorus concentrations in a headwater stream. Biogeochemistry 84(3):297–309
Liu JJ (2016) Study on forms, release rules and control measure of internal phosphorus and nitrogen in Chaohu. Heifei University of Technology, Lake
Monbet P, Mckelvie ID, Worsfold PJ (2009) Dissolved organic phosphorus speciation in the waters of the Tamar estuary (SW England). Geochim Cosmochim Acta 73(4):1027–1038
Michaeld SC, Ivanj F, Stephena N (2009) Soil and sediment phosphorus fractions in a forested watershed at Acadia National Park, ME, USA. Forest Ecol Manag 258(10):2318–2325
Mcdowell RW, Sharpley AN (2003) Phosphorus solubility and release kinetics as a function of soil test P concentration. Geoderma 112(1–2):143–154
Nichols DS (1983) Capacity of natural wetlands to remove nutrients from wastewater. Journal 55(5):495–505
O'Connor DJ, Connolly JP (1980) The effect of concentration of adsorbing solids on the partition coefficient. Water Res 14(10):1517–1523
Pan C R, Wang J Q, Zheng Z X, et al (2007) Forms of phosphorus and nitrogen existing in sediments in Chaohu Lake. J Ecol Rural Environ 23(1):43–47
Padmesh TVN, Vijayaraghavan K, Sekaran G, Velan M (2005) Batch and column studies on biosorption of acid dyes on fresh water macro alga Azolla filiculoides. J Hazard Mater 125(1–3):121–129
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. International & Comparative Law Quarterly (2):494–495
Pardo P, Rauret G, López-Sánchez JF (2004) Shortened screening method for phosphorus fractionation in sediments: a complementary approach to the standards, measurements and testing harmonised protocol. Anal Chim Acta 508(2):201–206
Plazinski W, Rudzinski W, Plazinska A (2009) Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv Colloid Interface Sci 152(1–2):2–13
Richardson CJ (1985) Mechanisms controlling phosphorus retention capacity in freshwater wetlands. Science 228(4706):1424–1427
Redshaw CJ, Mason CF, Hayes CR, Roberts RD (1990) Factors influencing phosphate exchange across the sediment-water interface of eutrophic reservoirs. Hydrobiologia 192(2–3):233–245
Ruban V, Rauret G (2001) European Commission BCR information report. Eur Commission 2001:1–25
Rydin E (2000) Potentially mobile phosphorus in Lake Erken sediment. Water Res 34(7):2037–2042
Ruban V, Brigault S, Demare D, Philippe AM (1999) An investigation of the origin and mobility of phosphorus in freshwater sediments from Bort-Les-Orgues Reservoir, France. J Environ Monit Jem 1(4):403–407
Sondergaard M, Jensen PJ, Jeppesen E (2001) Retention and internal loading of phosphorus in shallow, eutrophic lakes. TheScientificWorldJOURNAL 1(1–3):427–442
Tian Q, Li-Qiang L I, Fu-Ping O U, et al (2016) Temporal-spatial distribution and speciation of nitrogen and phosphorus in Dongting Lake. J Hydroecol
Vallja L, Duka S, Cullaj A (2013) Development of a sequential extraction method for different forms of phosphorus in Bovilla lake sediments. Albanian J Agric Sci 12(4)
Wang C, Zhang Y, Shi H et al (2016) Macrozoobenthic community structure and bioassessment of water quality in Lake Dongting, China. J Lake Sci 28(2):395–404 (in Chinese)
Wang S, Jin X, Zhao H et al (2005) Phosphate adsorption characteristics onto the sediments from shallow lakes in the middle and lower reaches of the Yangtze River. Environ Sci 26(3):38–43 (in Chinese)
Wang Y, Jiang X, Li YF et al (2014) Spatial and temporal distribution of nitrogen and phosphorus and nutritional characteristics of water in Dongting Lake. Res Environ Sci 27(5):484–491 (in Chinese)
Wang, C. R., Hong, L. I., & Yuan, X. P (2013) Temporal-spatial distribution of nitrogen and phosphorus in fishery waters of the Dongting Lake. Resourc Environe Yangtze Basin 22(7), 928–936. (in Chinese)
Wei JF, Chen HT, Liu PX et al (2010) Phosphorus forms in suspended particulate matter of the Yangtze River. Adv Water Sci 21(1):107–112 (in Chinese)
Xu D, Chen Y, Ding S, et al (2013) Diffusive gradients in thin films technique equipped with a mixed binding gel for simultaneous measurements of dissolved reactive phosphorus and dissolved iron. Environmental Science & Technology 47(18):10477–10484
Xiang S, Zhou W (2010) Phosphorus existing forms and distribution characteristic in Lake Poyang sediments. J Lake Sci 22(5):649–654 (in Chinese)
Yang G, Qin Y W, Han C N, et al (2017) Distributions of phosphorus fractions in surface sediments of Mingjiang Mainstreams. Environ Sci (2018)0250–3301. (in Chinese)
Zhang B, Fang F, Guo J, Chen Y, Li Z, Guo S (2012) Phosphorus fractions and phosphate sorption-release characteristics relevant to the soil composition of water-level-fluctuating zone of Three Gorges Reservoir. Ecol Eng 40(3):153–159
Acknowledgements
The authors would like to thank the Ecological and Environmental Monitoring Center of Dongting Lake of the Human Province in China and Lu S Y, who is a researcher in the Chinese Research Academy of Environmental Sciences.
Funding
This work was financially supported by the Ministry of Science and Technology of China, Survey Project of toxic and hazardous chemicals and water environment in typical lake of China (No. 2015FY110900-06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Zhu, G., Yang, Y. Variation laws and release characteristics of phosphorus on surface sediment of Dongting Lake. Environ Sci Pollut Res 25, 12342–12351 (2018). https://doi.org/10.1007/s11356-018-1777-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1777-9