Abstract
Phosphorus adsorption and release characteristics of surface sediments from Dianchi Lake were investigated through indoor simulation experiments. Kinetics and thermodynamics of adsorption and release of phosphorus in sediments were studied, and the influence of different phosphorus fractions on adsorption and release was analysed. Results show that the total phosphorus content in the sediments ranged from 843.96 to 8144.44 mg kg−1, which was 2–8 times as much as those in other lakes in China (e.g. Erhai Lake and Taihu Lake). The average values of different phosphorus forms in the sediment samples were ranked in the order of organic P (OP), calcium-bound P (Ca-P), metal oxide-bound P (Al-P), residual P (Res-P), Fe-bound P (Fe-P) and weakly absorbed P(NH4Cl-P). Compared with the case of other lakes in China, the sedimentary phosphorus adsorption capacity of Dianchi Lake was at a higher level, but its maximal release rate and release capacity were low, indicating a relatively low release risk. By comparing the equilibrium phosphate concentration in the sediments and the soluble reactive phosphorus in the overlying water, the risks of phosphorus release from sediments in Caohai, Lake Central and Southern Waihai were found to be relatively low in the short term, but were high in Northern Waihai. The release behaviour of sedimentary phosphorus in Dianchi Lake was mainly determined by NH4Cl-P, Fe-P and Al-P, among which NH4Cl-P and Fe-P served a greater function. Therefore, the contents of different fractions of phosphorus and their distribution characterization should be considered when characterizing the adsorption and release status of lake sedimentary phosphorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication of lakes can lead to widespread hypoxia and anoxia, loss of biodiversity, and increased frequency, spatial extent, and duration of harmful algal blooms. Phosphorus (P) has been regarded as the common limiting factor responsible for lake eutrophication. With the inputs of external phosphorus, the existing phosphorus could sink and accumulate in sediments. Phosphorus releases to the overlying water when physico-chemical factors are suitable and/or microbial activities are present in lake sediments (Rydin 2000; Hupfer et al. 1995). Recent studies indicate that in aquatic systems, not only various forms in which phosphorus occurs in sediment, but also the particle size with adsorbed phosphorus (physical P speciation) can control bioavailability of P (Andrieux-Loyer and Aminot 2001). The adsorption and release of phosphorus are important processes that affect the phosphorus fixed in the lake ecosystem. These processes are also important factors that adjust the dissolved phosphorus concentration in the overlying water (Froelich 1988; Lopez et al. 1996; Zhou et al. 2001; Xie et al. 2003). Dissolved phosphorus precisely considered to be bioavailable and hence a priority for control (Lewis et al. 2011).
The sorption and release of phosphorus in sediments are affected by temperature, pH (Wang et al. 2006), dissolved oxygen, redox conditions, microbes, sedimentary phosphorus forms (Katsev et al. 2006), water chemical composition, aquatic organisms, the contents and types of organic matter, salinity and so on (Abrams and Jarrell 1995; Jin et al. 2005). The total effect is dependent on the superposition of a variety of effects. Knowledge on TP content is inadequate to predict the capability for phosphorus release. Phosphorus speciation is an important parameter to assess the internal nutrient load in lakes (Kaiserli et al. 2002).
Research on phosphorus adsorption and release in surface sediments is beneficial in determining the interchange ability of phosphorus in sediments, its biological availability, the phosphorus cycle process in the water environment and the regeneration mechanism of phosphorus. The total nitrogen/total phosphorus (TN/TP) ratio of Dianchi Lake water is 12.79, and phosphorus is the common limiting factor responsible for water eutrophication. Especially, internal P loading can maintain high P concentrations and delay eutrophic lake recovery following abatement of external P sources. (Cooke et al. 1993). Most previous studies focused on the relevant environmental factors, but only a few focused on the properties and sources of the sediments. This study was aims to investigate the relationships of phosphate adsorption and release characteristics with phosphorus fractions, as well as to describe the adsorption and release characteristics of phosphate from Dianchi Lake’s surface sediments.
Materials and methods
Study area
Dianchi Lake (102°36′–102°47′E, 24°40′–25°02′N) is situated at Yunnan–Guizhou plateau of southwest China, which is the largest plateau lake in Yunnan Province and the sixth largest freshwater lake in China (Liu et al. 2011). Dianchi Lake is 309.5 km2 in area and is a typical rift lake with a storage capacity of 15.7 × 108 m3. The average depth of Dianchi Lake is 5 m and the maximum depth is 10.24 m (Wan et al. 2011). The long residence time of the water body and the widely distributed Cambrian phosphorite ores on its catchment led to the high phosphorus content of the sediments. In addition, because of the phosphatic slag generated from the exploitation of phosphorite, the industrial wastewater and the sewage runoff have contributed to the increase in the phosphorus content of the lake water. According to statistics, because of the large-scale phosphorite mining on the south shore, about 1.3 × 104 t of phosphatic slag goes into Dianchi Lake every year.
Sediment sampling and analysis
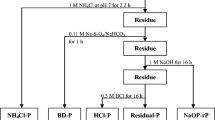
Based on the terrain, water quality, routine monitoring location, and dredged region of Dianchi Lake, 35 sampling sites were selected from four lake sections including Caohai, Northern Waihai, Lake Central and Southern Waihai, as shown in Fig. 1 (D4 sample has been dredged, so there was no sample). Surface sediment (0–10 cm) samples were collected with Petersen sampler in March 2013. The impurities (such as rocks, residuals of plants and animals) in the samples were discarded and then all samples were taken to the laboratory in air-sealed plastic bags and incubators. Sediment samples were freeze-dried, ground in a mortar and screened through a 100-mesh sieve for analysis of contents of TP and different P forms, namely, organic P (OP), calcium-bound P (Ca-P), metal oxide-bound P (Al-P), residual P (Res-P), Fe-bound P (Fe-P) and weakly absorbed P (NH4Cl-P). The corresponding overlying water samples were collected for analysis of the concentration of soluble reactive phosphorus (SRP).
Sedimentary phosphorus fractions
Total phosphorus (TP) was analysed using the (standards, measurements and testing) SMT separation method (Ruban et al. 1999, 2001). The procedure for the extraction of phosphorus fractions from sediments was based on the improved Psenner continuous extraction method (Koop et al. 1990). The procedure may be summarized as follows: (1) one gram of dry sediment was placed in a 100-mL acid-washed centrifuge tube with 50 mL of 1 M NH4Cl to extract NH4Cl-P. (2) The residue was combined with 50 mL bicarbonate–dithionate (BD) solution (0.11 mol L−1 NaHCO3− 0.11 mol L−1 sodium hydrosulphite) and stirred for 1 h at 40 °C to extract Fe-P. (3) The residue was combined with 50 mL 1 M NaOH and stirred for 16 h at 25 °C to extract Al-P. (4) The residue was combined with 50 mL 0.5 mol L−1 HCl and stirred for 16 h at 25 °C to extract Ca-P. (5) The residue was combined with 50 mL 1 mol L−1 NaOH and digested for 2 h to extract Res-P. The extraction was immediately centrifuged at 4000 r min−1 for 10 min and then filtered through 0.45 μm GF/C filter membranes. The solutions were then analysed for phosphorus using molybdenum antimony spectrophotometry (SEPA 2002). (6) Organic phosphorus (OP) was obtained by calculating the difference between TP and inorganic phosphorus (IP).
Phosphorus adsorption kinetic experiments
Sediment samples (0.5 g in dry weight) were placed in a series of 100 mL polyethylene centrifuge tubes with 50 mL 1 mg L−1 phosphorus solutions (KH2PO4). The suspensions were placed in a shaker at 25 ± 1 °C and 200 rpm. Subsequently, the centrifuge tubes were taken out of the shaker after 5, 30 min, 1, 2, 4, 8, 12 and 24 h. The suspensions were centrifuged immediately at 5000 rpm for 10 min and filtered through 0.45 μm GF/C filter membranes. Finally, the concentration of SRP was analysed and the adsorption capacity was calculated.
Phosphorus adsorption thermodynamic experiments
Two concentration ranges were investigated in this experiment. The low concentration ranged from 0 to 0.5 mg L−1 (i.e. 0, 0.05, 0.1, 0.2 and 0.5 mg L−1), and the high concentration ranged from 1 to 20 mg L−1 (i.e. 1, 2, 5, 10, 15 and 20 mg L−1). Sediment samples (0.5 g each) were placed in a series of 100 mL polyethylene centrifuge tubes, and 50 mL phosphorus standard solutions (KH2PO4) of various concentrations were added. The conditions of the experiments were the same as those of the adsorption kinetics experiments. After 24 h of equilibration, the solutions were centrifuged at 5000 rpm for 10 min and filtered through 0.45 μm GF/C filter membranes. Then, the phosphorus concentrations were analysed.
Phosphorus release kinetic experiments
Sediment samples (0.5 g each) were placed in a series of 100 mL polyethylene centrifuge tubes with 50 mL 0.02 mol L−1 KCl. The suspensions were placed in a shaker at 25 ± 1 °C and 200 rpm. Subsequently, the centrifuge tubes were taken out of the shaker after 0.5, 1.5, 3, 5, 6, 11, 20 and 24 h. The suspensions were centrifuged immediately at 5000 rpm for 15 min and filtered through 0.45 μm GF/C filter membranes. Then, the concentration of solubility orthophosphate (SRP) was measured.
Phosphorus release potential experiments
Sediment samples (with soil–water ratios of 1:25–20,000) were placed in a series of 100 mL polyethylene centrifuge tubes with 50 mL 0.02 mol L−1 KCl. The suspensions were placed in a shaker at 25 ± 1 °C and 200 rpm. After 120 min of equilibration, the solutions were centrifuged at 5000 rpm for 15 min and filtered through 0.45 μm GF/C filter membranes. Then, the phosphorus concentrations were analysed.
Results and discussion
Sedimentary phosphorus fractions
From the results, the value of TP content in surface sediments from Dianchi Lake ranged from 843.96 to 8144.44 mg kg−1 (mean 2171.81 mg kg−1), which is about 2–8 times as much as those in other lakes in China (Wang et al. 2012; Yang et al. 2013). The effects of phosphorus fractions in lake sediments for promoting lake eutrophication can be more efficiently evaluated based on phosphorus fractions, rather than total phosphorus (Kaiserli et al. 2002), the phosphorus fractions of sediment determine the sediment’s bioavailability and interface exchange capability (Ruttenberg 1992). Different values of combined phosphorus reflect different phosphorus pollution levels and release potentials of the sediments. The values of different phosphorus forms in Dianchi Lake sediments are shown in Table 1. The different phosphorus forms are ranked in the following order, starting with the form with the highest value: OP > Ca-P > Al-P > Res-P > Fe-P > NH4Cl-P.
Phosphorus adsorption kinetic process
Phosphorus adsorption kinetics on the surface sediments from Dianchi Lake are shown in Fig. 2. The adsorption of phosphorus on sediments has been widely reported as a multiple kinetic process, which involves at least two processes: a fast initial reaction and a slow reaction (Xie et al. 2003). The fast initial reaction was completed in the first 0.5 h and the slow reaction followed (0.5–4 h). The majority of phosphorus adsorptions on the surface sediments of Dianchi Lake were almost completed in 4 h. After 4 h, the adsorbed phosphorus was about 85 % of the maximum adsorption capacity. The sorption rates within the first 5 min were the highest in the whole adsorption process, ranging from 496.12 to 1175.92 mg (kg h)−1. The maximal phosphorus adsorption rate (V max) of Dianchi Lake sediments from different lake sections are ordered as follows (highest–lowest): Lake Central [1028.89 ± 74.86 mg (kg h)−1] > Southern Waihai [903.44 ± 139.19 mg (kg h)−1] > Northern Waihai [852.45 ± 226.32 mg (kg h)−1] > Caohai [729.29 ± 202.08 mg (kg h)−1], as shown in Table 2. The particle size of the sediments could influence the exchange process of phosphorus on the water–sediment interface. The finer particles of sediment had stronger adsorption capability for phosphorus and re-suspension capability. The sediment particles were very small and had strong adsorption capability for phosphorus in Lake Central, which has a large specific surface area. Therefore, the maximal phosphorus adsorption capacity of sediments in Lake Central was higher than those in other sections.
Phosphorus adsorption–desorption equilibrium concentration
Phosphate adsorption–desorption equilibrium concentration or equilibrium phosphate concentration (EPC 0) is frequently used to describe the adsorption characteristics of phosphorus on the water–sediment interface (Kerr et al. 2011). When the concentration of SRP in water is higher than the EPC 0, phosphorus will be adsorbed by the sediment. When the concentration of phosphorus in water is lower than the EPC 0, phosphorus will be desorbed from the sediment (Jarvie et al. 2005). EPC 0 has been commonly used when evaluating the behaviour of phosphorous on the water–sediment interface (Lottic and Stanley 2007).
In the concentration range of the experiment (initial concentration of P < 0.5 mg L−1), phosphorus sorbed by Dianchi Lake sediments and the concentration of phosphorus in water showed a good linear relationship. The data were well fitted by the linear Eq. (1) (p < 0.05, R 2>0.85).
where Q is the amount of phosphorus adsorbed on the sediment (mg kg−1), C 0 is the initial concentration of phosphorus in the solution (mg L−1), NAP is the content of native adsorbed phosphorus (mg kg−1) and m is the slope (L kg−1).
Phosphorus adsorption–desorption isotherms of the sediments from Dianchi Lake are shown in Fig. 3. When the initial phosphorus concentration was low, phosphorus would be desorbed from the Dianchi Lake sediments. As the initial phosphorus concentration increases, phosphorus would be sorbed on the Dianchi Lake sediments.
The EPC 0 is an important parameter to evaluate whether sediment is released or phosphorus is adsorbed. The EPC 0 is the equilibrium concentration in solution when the apparent adsorbed amounts of phosphorus on sediment falls to zero (House and Denison 2000). Increased surface area would drop the value of the zero equilibrium P concentration (EPC 0) with an increase in surface area and consequently enhances adsorption capability (Pan et al. 2002). The EPC 0 of Dianchi Lake sediments ranged from 0.0049 to 0.3644 mg L−1 (mean 0.0320 mg L−1). The maximum and minimum concentrations of SRP in the overlying water were 0.0980 mg L−1 and 0.0149 mg L−1, respectively. In Caohai, Lake Central and Southern Waihai, the concentrations of SRP in the overlying water was much higher than the EPC 0 in the sediments. In these areas, phosphorus was adsorbed on sediments and the phosphorus release risk was low. In Northern Waihai, the concentration of SRP in the overlying water was lower than the EPC 0, and phosphorus might be released into the water (Fig. 4).
Phosphorus adsorption isotherm
The Langmuir isotherm was employed to simulate the experimental data. This mode could be described as follows:
where Q is the amount of phosphorus adsorbed on the sediment (mg kg−1), C is the equilibrium concentration in the solution (mg L−1), Q max is the maximum adsorption capacity of adsorbent (mg kg−1) and K was the adsorption energy coefficient (L mg−1).
The Langmuir isotherms were well fitted by the phosphorus adsorption isotherm experimental data (p < 0.05, R 2 > 0.75). The fitting results are shown in Fig. 5. The Langmuir isotherm indicated that the sorption rate of phosphorus on the sediment was affected by the concentration of phosphorus in the solution and the active sites of the adsorbent (Liu and Shen 2008). With an increase in the initial concentration of phosphorus in the solution, the driving force from the concentration gradient increased and the amount of phosphorus adsorbed on sediment also increased. However, as the reaction progressed, the active sites of the adsorbent became saturated and the sorption rate decreased. The maximal phosphorus adsorption capacities of Dianchi Lake sediments (phosphorus initial concentration is 0.5–20 mg L−1) ranged from 535.69 to 41,466.41 mg kg−1 (mean 3084.29 mg kg−1). The maximal phosphorus adsorption capacities (Q max) of Dianchi sediments in different lake sections ordered from highest to lowest is as follows: Southern Waihai (5639.24 ± 1887.11 mg kg−1) > Lake Central (2032.89 ± 712.51 mg kg−1) > Northern Waihai (2011.90 ± 592.38 mg kg−1) > Caohai (1180.85 ± 581.48 mg kg−1), as shown in Table 3.
As shown in Table 4, the NAP and Q max of Dianchi Lake were both higher than those of south China plain lakes and other plateau lakes (Wang et al. 2005). Sediments in Dianchi Lake had high adsorption capacity for phosphorus because they had high amounts of kaolinite and hydromica, which show high affinity to phosphorus.
Phosphorus release kinetic process
The release of phosphorus from sediments is a complicated kinetic process, which usually comprises a fast initial reaction step and a slow reaction step (Lee-Hyung et al. 2003). The release of phosphorus mainly occurred within 8 h for the sediments in Dianchi Lake. The concept of release rate (the amount of phosphorus released by unit mass of sediment during unit time) was introduced to investigate the release kinetics of phosphorus further (Wang et al. 2006). The release rate of sedimentary phosphorus in Dianchi Lake was fastest during the first 0.5 h. The maximal phosphorus release rate was between 0.08 and 8.33 mg (kg h)−1 [mean 1.56 mg (kg h)−1]. The maximal phosphorus release rate (V max) for sediments from different lake sections sequenced from highest to lowest in the following: Northern Waihai [2.47 ± 2.27 mg (kg h)−1] > Caohai [2.22 ± 1.03 mg (kg h)−1] > Southern Waihai [0.95 ± 0.28 mg (kg h)−1] > Lake Central [0.49 ± 0.29 mg (kg h)−1], as shown in Table 5.
To analyse the release kinetics process of sedimentary phosphorus quantitatively, the first-order rate equation (Hou et al. 2003) was used for modelling.
where Q t is the amount of released phosphorus at time t (in dry weight, mg kg−1), Q max was the amount of released phosphorus at equilibrium (in dry weight, mg kg−1), k was the rate constant of phosphorus release and t was the releasing time (min). The kinetics of phosphorus release on Dianchi sediments was well fitted by the first-order rate equation, with the constants and correlation coefficient R 2 larger than 0.70, reaching a significant correlation (p < 0.05). Q max is an important parameter of sedimentary phosphorus release kinetics. The Q max of phosphorus in Dianchi surface sediments ranged from 0.14 to 11.76 mg kg−1 (mean 2.12 mg kg−1). The average values of Q max of sedimentary phosphorus from the different lake sections are ordered as Caohai (3.32 ± 2.10 mg kg−1) > Northern Waihai (2.83 ± 2.82 mg kg−1) > Southern Waihai (1.43 ± 0.50 mg kg−1) > Lake Central (0.99 ± 0.52 mg kg−1). The vegetation community is more flourishing in Caohai than in other parts of the lake, leading to more biological residues. The decomposition of phytodetritus is usually not complete, and the leftovers are buried layer upon layer in the sediments. Aerobic decomposition occurs at the surface, but, in the sublayers, anoxic and anaerobic conditions are formed from top to bottom. Under this oxygen-deficient condition and with the action of microorganisms, free-state PO4 3− ions are released to the water. Additionally, Caohai and Northern Waihai are the recipients of most rivers, including Xinyunliang River, Baoxiang River and Panlong River, which are the rivers with large flow and play a major role in agriculture irrigation, flood discharge and pollutant acceptance. The surface sediments in these areas receive more external pollutants. With the effects of biological mineralization and chemical reaction, free-state phosphorus (usually PO4 3−P) are released continuously and stored in the sediment porewater. A concentration gradient of PO4 3−P is formed between the sediments and the overlying water, and phosphorus release occurs on the water–sediment interface according to the molecular diffusion law under specific environmental conditions (Fig. 6).
Compared with sediments from other lakes, the maximal release rate and capacity of Dianchi sedimentary phosphorus were relatively low (Jin et al. 2006) (Table 6). It has been reported that when the value of Ca/(Fe + Al) in metal content is larger than 0.7, the sediment is calcium-bound (Ding et al. 2010). The value of Ca/(Fe + Al) in Dianchi sediments ranged from 0.78 to 1.46, indicating that the sediments of Dianchi Lake were typically calcareous. The mean annual temperature of Dianchi lake water is around 17 °C, and the pH ranges from 7.59 to 8.80. Phosphate in the lake water precipitates by adsorbing on CaCO3 or co-precipitating with CaCO3. The co-precipitation effect is enhanced by increasing the temperature and pH (in the range of 8.0–10.0) (Boström et al. 1988; Hu et al. 2007). This mechanism restrained the release of Ca-P from Dianchi sediments.
Phosphorus release potential
The method used to measure the phosphorus release potential from sediment in this research is one-step extraction. The sedimentary phosphorus release potential was determined by the released amount of phosphorus at 1:100 soil–water ratio. In fact, the phosphorus equilibrium concentration on the water–sediment interface differed with different soil–water ratios (Aminot and Andrieux 1996). In general, smaller soil–water ratio is in favour of phosphorus release from sediments. Therefore, one may use the infinite dilution method to measure the release potential of sedimentary phosphorus (IDE-P). More dilution resulted in a greater release of phosphorus from sediments, as shown in Fig. 7. The released amount of phosphorus peaked at 1:20,000 ratio and then reached an equilibrium in further dilution (Morin and Morse 1999). The IDE-P of Dianchi sediments fall between 32.64 and 419.00 mg kg−1. The average values of IDE-P in sediments from different lake sections are ordered from highest to lowest in the following: Caohai (157.96 ± 94.11 mg kg−1) > Northern Waihai (113.45 ± 87.64 mg kg−1) > Southern Waihai (80.16 ± 33.69 mg kg−1) > Lake Central (67.17 ± 30.01 mg kg−1). The concentration and release potential of sedimentary phosphorus were lower in Lake Central, which is far away from anthropological activities. The phosphorus release potential was relatively high in river estuary areas where the rivers are close to the Kunming and flows through the urban area, mainly because of the large concentration gradient between the sediments and inflow water.
Influence of different phosphorus fractions on the adsorption process
The adsorption and release processes of sedimentary phosphorus are not only related to the phosphorus concentration in the overlying water, but are also involved with other factors, such as specific environmental conditions and sedimentary phosphorus fractions. The NAP in Dianchi sediments was significantly and positively correlated with Ca-P content (p < 0.05), as shown in Table 7, because many phosphorite mines are located on the south lakeshore of Dianchi and phosphorite ores are abundant in calcium content. The sedimentary inorganic phosphorus in Dianchi Lake was mainly calcium-bound, and Ca-P is a relatively stable fraction of sedimentary phosphorus and contributes to a permanent burial of phosphorus in sediments. Other adsorption characteristic parameters had no obvious correlations with sedimentary physical and chemical properties. Therefore, to investigate the phosphorus adsorption characteristics comprehensively, the content of native adsorbed phosphorus, as well as the content of maximal and total maximal adsorbed phosphorus, should be analysed.
The influence of different phosphorus fractions on the release process
Different fractions of combined sedimentary phosphorus reflect the contamination characteristics of different historical periods. Different phosphorus species are diverse in bioavailability and can indirectly indicate the release potential of sedimentary phosphorus. Different phosphorus fractions play a significant role in the eutrophication of the overlying water. The release mechanism and released amount of sedimentary phosphorus are related to the existing forms of internal phosphorus, the transformation ability of metal-bound phosphorus and the phosphorus exchange on the sediment–water interface. The sedimentary phosphorus is mainly inorganic orthophosphate. Once conditions conducive to the dissolution of insoluble phosphate precipitate, such as Ca-P, Al-P and Fe-P, appear, the release of phosphorus will happen. The determinants of phosphorus release for lakes with different geographical environments vary. The phosphorus release from surface sediments of Dianchi Lake was mainly determined by NH4Cl-P, Fe-P and Al-P, among which NH4Cl-P and Fe-P served a greater function. This result is consistent with other research findings (Tiren and Pettersson 1985). NH4Cl-P is a form of phosphorus regarded to be easily released. Fe-P is rather labile and almost active. It will be released under reductive conditions. Furthermore, when the redox potential and salinity are reduced, Fe-P will be activated and released into the water. This part of phosphorus would be either consumed in bio-utilization or combined with other ions in water (such as Ca2+ and Al3+). Therefore, when the Fe, Al and Ca contents in the sediments change because of anthropological contamination, phosphorus will recombine between different forms, realizing the transformation of different phosphorus fractions (Table 8).
Conclusions
The sorption and release of phosphorus in sediments are affected by many factors. The study shows that the relationships of phosphate adsorption and release characteristics with phosphorus fractions, the adsorption and release characteristics of phosphate from Dianchi Lake’s surface sediments. The main results of the research were listed as follows:
-
1.
The maximal phosphorus adsorption rate (V max) and maximal phosphorus adsorption capacities (Q max) of Dianchi sediments in Waihai were considerably larger than those in Caohai.
-
2.
The comparison of the equilibrium phosphate concentration (EPC0) in the surface sediments and the SRP in the overlying water of Dianchi Lake demonstrated that in the short term, there was a low risk of phosphorus release from sediments in the lake sections of Caohai, Lake Central and Southern Waihai, but a high risk from sediments in Northern Waihai.
-
3.
Compared with other lakes in China (e.g. Erhai Lake and Taihu Lake), a higher level phosphorus adsorption capacity was found in Dianchi Lake. However, the maximal release rate and capacity of phosphorus of Dianchi Lake sediments were at a lower level, indicating a relatively low release risk. Phosphate in the lake water precipitates by adsorbing on CaCO3 or co-precipitating with CaCO3. The co-precipitation effect is enhanced by increasing the temperature and pH (in the range of 8.0–10.0). This mechanism restrained the release of Ca-P from Dianchi sediments.
-
4.
The NAP in Dianchi sediments was significantly and positively correlated with Ca-P content, Ca-P is a relatively stable fraction of sedimentary phosphorus and contributes to a permanent burial of phosphorus in sediments.
-
5.
The release behaviour of sedimentary phosphorus in Dianchi Lake was mainly determined by NH4Cl-P, Fe-P and Al-P among which NH4Cl-P and Fe-P played a larger role. Based on our findings, we are of the opinion that when characterizing the adsorption and release status of lake sedimentary phosphorus, not only TP content should be considered, but also the contents of different fractions of phosphorus and their distribution characterization.
References
Abrams MM, Jarrell WM (1995) Soil-phosphorus as a potential non-point source for elevated stream phosphorus level. J Environ Qual 24:132–138
Aminot A, Andrieux F (1996) Concept and determination of exchangeable phosphate in aquatic sediments. Water Res 30(11):2805–2811
Andrieux-Loyer F, Aminot A (2001) Phosphorus forms related to sediment grain size and geochemical characteristics in French coastal areas. Estuar Coast Shelf Sci 52:617–629
Boström B, Andersen JM, Fleischer S et al (1988) Exchange of phosphorus across the sediment–water interface. Hydrobiology 48:229–244
Cooke GD, Welch EB, Martin AB et al (1993) Effectiveness of Al, Ca, and Fe salts for control of internal phosphorus loading in shallow and deep lakes. Hydrobiology 253(1–3):323–335
Ding SM, Bai XL, Fan CX et al (2010) Caution needed in pretreatment of sediments for refining phosphorus-31 nuclear magnetic resonance analysis: results from a comprehensive assessment of pretreatment with ethylendiaminetetraacetic acid. J Environ Qual 39:1668–1678
Froelich PN (1988) Kinetie control of dissolved phosphate in natural rivers and estuaries: a primer on the phosphate buffer mechanism. Limnol Oceanogr 33(4):649–668
Hou LJ, Liu M, Jiang HY (2003) Ammonium adsorption by tidal flat surface sediments from the Yangtze Estuary. Environ Geol 45:72–78
House WA, Denison FH (2000) Factors influencing the measurement of equilibrium phosphate concentrations in river sediments. Water Res 34:1187–1200
Hu J, Shen Q, Liu YD et al (2007) Mobility of different phosphorus pools in the sediment of Lake Dianchi during cyanobacterial blooms. Environ Monit Assess 132:141–153
Hupfer M, Gachter R, Giovanoli R (1995) Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci 57(4):305–324
Jarvie HP, Jurgens MD, Willianms RJ et al (2005) Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Hertfordshire Wye. J Hydrol 304:51–74
Jin XC, Wang SR, Pang Y et al (2005) The adsorption of phosphate on different trophic lake sediments. Colloids Surf A 254(1–3):241–248
Jin XC, Wang SR, Bu QY (2006) Laboratory experiments on phosphorous release from the sediments of 9 lakes in the middle and lower reaches of Yangtze River region, China. Water Air Soil Pollut 176(1–4):233–251
Kaiserli A, Voutsa D, Samara C (2002) Phosphorus fractionation in lake sediment—Lakes Volvi and Koronia, N Greece. Chemosphere 46(8):1147–1155
Katsev S, Tsandev I, Heureux IL et al (2006) Factors controlling long-term phosphorus efflux from lake sediments: exploratory reactive-transport modeling. Chem Geol 234(1–2):127–147
Kerr JG, Burford M, Olley J et al (2011) Phosphorus sorption in soils and sediments: implication for phosphate sorption in soils and sediments: implications for phosphate supply to a subtropical river in southeast Queensland, Australia. Biogeochemistry 102:73–85
Koop K, Boynton WR, Wulff F et al (1990) Sediment-water oxygen and nutrient exchanges along a depth gradient in the Baltic Sea. Mar Ecol Prog Ser 63:65–77
Lee-Hyung K, Euiso C, Michael KS (2003) Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere 50:53–61
Lewis WM, Wurtsbaugh WA, Paerl HW (2011) Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ Sci Technol 45:10300–10305
Liu Y, Shen L (2008) From Langmuir kinetics to first-and second-order rate equations for adsorption. Langmuir 24(20):11625–11630
Liu JL, Wang RM, Huang B et al (2011) Distribution and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in wild fish species from Dianchi Lake, China. Environ Pollut 159:2815–2822
Lopez P, Lluch X, Vidal M et al (1996) Adsorption of phosphorus on sediment of the Balearic Island (Spain) related to their composition. Estuar Coast Shelf Sci 42(2):185–196
Lottic NR, Stanley EH (2007) Benthic sediment influence on dissolved phosphorus concentrations in a headwater stream. Biogeochemistry 84:297–309
Morin J, Morse JW (1999) Ammonium release from re-suspended sediments in the Laguna Madre estuary. Mar Chem 65:97–110
Pan G, Krom MD, Herut B (2002) Adsorption–desorption of phosphate on airborne dust and riverborne particulates in East Mediterranean seawater. Environ Sci Technol 36(16):3519–3524
Ruban V, Brigault S, Demare D et al (1999) An investigation of the origin and mobility of phosphorus in freshwater sediments from Bort-Les-Orgues Reservoir, France. Environ Monit 1:403–407
Ruban V, Muntan H, Quevauviller Ph et al (2001) Harmonized Protocol and certified reference material for the determination of phosphorus in freshwater sediment—a synthesis of recent works. Fresenius J Anal Chem 370:224–228
Ruttenberg KC (1992) Development of a sequential method for different forms of phosphorus in marine-sediments. Linnol Oceanogr 37(7):1460–1482
Rydin E (2000) Potentially mobile phosphorus in Lake Erken sediment. Water Res 34(7):2037–2042
SEPA (2002) Monitoring and analytical method of water and wastewater (4th edn) (in Chinese). Environmental and Scientific Press of China, Beijing
Tiren T, Pettersson K (1985) The influence of nitrate on the phosphorus flux to and from oxygen depleted lake sediments. Hydrobiologia 120:207–223
Wan X, Pan XJ, Wang B et al (2011) Distribution and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in wild fish species from Dianchi Lake, China. Environ Pollut 159:2815–2822
Wang SR, Jin XC, Zhao HC et al (2005) Phosphate adsorption characteristics onto the sediments from shallow lakes in the middle and lower reaches of the Yangtze River (in Chinese). Environ Sci 26(3):38–43
Wang SR, Jin XC, Bu QY et al (2006) Effects of particle size, organic matter and ionic strength on the phosphate sorption in different trophic lake sediments. J Hazard Mater 128:95–105
Wang SR, Ni D, Jiao LX (2012) Space-time variety of organic matter and nutrient in surface sediments from Poyang Lake (in Chinese). J Environ Eng Technol 2(1):24–28
Xie LQ, Xie P, Tang HJ (2003) Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms: an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake. Environ Pollut 122:391–399
Yang WL, Jiang GC, Wang ZQ (2013) Chemical forms and distribution of phosphorus in surface sediments of lake Hongze (in Chinese). Earth Environ 41(1):43–49
Zhou Q, Gibson CE, Zhu YM (2001) Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK. Chemosphere 42:221–225
Acknowledgments
This research was supported by the National Major Science and Technology Program for Water Pollution Control and Treatment (No. 2012ZX07102). The authors are grateful to all the group members for their help during the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Deng, W., Xu, X. et al. Phosphorus adsorption and release characteristics of surface sediments in Dianchi Lake, China. Environ Earth Sci 74, 3689–3700 (2015). https://doi.org/10.1007/s12665-015-4723-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4723-x