Abstract
In this work, the adsorption of chemical oxygen demand (COD) and biological oxygen demand (BOD) from treated sewage with low-cost activated carbon prepared from date palm shell waste by chemical activation method was studied. Different parameters affecting the adsorption process such as carbon dose, pH, contact time, agitation rate, and temperature were studied. Adsorption equilibrium was attained after 150 min at pH 6.0 with agitation rate of 400 rpm at 25 °C. The results showed that COD removal percentage of 95.4 and 92.8% for BOD was obtained with carbon dosage of 0.1 g/100 ml of solution. The experimental batch equilibrium results follow linear, Langmuir, and Freundlich isotherm models. The experimental data was fitted to a pseudo-second-order kinetics model controlled by pore diffusion. Thermodynamic parameter values of ΔH 0, ΔG 0, and ΔS 0 were calculated. The obtained data indicated that the adsorption was spontaneous, endothermic nature and reflects an increased randomness and degree of disorderliness at the activated carbon/sewage interface during the adsorption process investigated in this study. Concentrations of different impurities were reduced to very small value by investigated adsorption process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater (such as sewage) is often contaminated with different hazardous materials such as organic and inorganic compounds. Therefore, the treatment of such wastewater to an environmental acceptable limit is very important from environmental and economic views. The current problems in treatment processes of such wastewater arise from the increasing pollution by hazardous compounds which are difficult to decompose biologically (Olafadehan and Aribike 2000). These organic pollutions originate from many sources like human waste, industrial effluents, paper, and vegetable matter. During the decomposition of such organic pollutants, the consumption of the dissolved oxygen may be at a rate greater than that can be replenished, leading to depletion of oxygen and having serious consequences for the biota. Contamination of wastewater by large amounts of suspended matters reduces the light amounts that require to photosynthetic organisms. Organic pollutant materials may be fertilizers, hydrocarbons, plasticizers, phenols, carbohydrates, biphenyls, pesticides, etc. (Ali and Aboul-Enein 2004; Damià 2005; Ali et al. 2012). Traditional processes for the treatment of wastewater from such organic materials can be chemical, biological, or physical methods.
Adsorption techniques are most widely used among the physico-chemical processes that have proven useful for wastewater treatment (Hararah et al. 2010; Matsuo and Nishi 2000; Eckenfelder 1999). It is one of the most efficient and important method, especially if the adsorbent used does not require any further pretreatment step, cheap and available (Hyung and Kim 2008; Ling et al. 2010; Adeniji et al. 2010). Adsorbent used must be solid with high surface area and porosity, inertness and stability to withstand chemical, thermal and climatic changes, cost effective and good physico-chemical properties, and the adsorption process does not result in the formation of any hazardous materials (Ghodale and Kankal 2014; Eckenfelder 1999; Attallah et al. 2013; Hamed et al. 2016). This process is suitable for the treatment process of drinking water because it has many advantages in the removal of trace organic materials (Bleiman and Mishael 2010).
So, activated carbon which has the above characteristics has been regarded as a good adsorbent for the treatment of wastewater (Mohammad-Khah and Ansari 2009; Bansode et al. 2004). It can be used on large scale to remove the hazardous organic materials and adsorb odor and taste from wastewater (Olafadehan et al. 2012; Olafadehan and Aribike 2000). Activated carbon has high adsorption capacity, higher surface area, and microporous structure (Kavita and Namacivayam 2008). Therefore, it is one of the most widely used adsorbents for the treatment of wastewater (Kavita and Namacivayam 2007) and for removal of chemical oxygen demand (COD) and BOD content with high efficacy (Ghodale and Kankal 2014).
Generally, activated carbon can be prepared from a large number of low-cost sources and agricultural residues (date palm residue, corn cob, apricot stone and rice husk) (Tsai et al. 1998; Guo et al. 2003). Date palm wastes are the most attractive agricultural wastes for the production of activated carbon, especially in Mediterranean countries, where it has high carbon content and quite availability (Ahmed and Theydan 2014; Amor and Ismail 2015). Preparation and applications of activated carbon from biomass wastes for the removal of pollutants have been reported by many researchers (Yahya et al. 2015; Alslaibi et al. 2013; Nor et al. 2013; Demirbas 2009; Foo and Hameed 2009).
In the last decades, increasing awareness of the environmental impact of organic loads represented by COD and BOD increases the demand for the wastewater treatment prior to discharge into natural waters (Aluyor and Badmus 2008). Many studies have been investigated to study the treatment process of wastewater by activated carbon to reduce COD and BOD contents (Aluyor and Badmus 2008; Ghodale and Kankal 2014; Mall et al. 1994).
In the present work, date palm-activated carbons have been prepared from date palm and investigated as the possible adsorbents for the reduction of both COD and BOD from treated sewage. Several parameters such as adsorbent dosage, pH, contact time, agitation speed, and temperature were optimized. Adsorption isotherm and kinetics of the adsorption process were investigated, and thermodynamic parameters were calculated.
Experimental
Sampling and analytical methods
Samples of treated sewage were collected from the wastewater treatment facility in Sakakah City, Al Jouf Province, KSA. The treated sewage was analyzed for chemical oxygen demand (COD), biological oxygen demand (BOD), total suspended solids (TSS), total phosphorus (TP), total Kjeldahl nitrogen (TKN), and ammonia (NH3). Physico-chemical analyses were carried out according to standard methods for examination of water and wastewater (APHA 2005). The overall average values (for 10 samples) of COD, BOD, and TSS were 38, 14, and 24 mg/l, respectively. The ratio of BOD/COD is about 0.37. The average values of TKN, NH3, and TP were 23, 5, and 4 mg/l, respectively (Table 1).
COD and BOD concentrations in different samples were analyzed in laboratory according to the methods prescribed in Guide Manual for Water and Wastewater Analysis by CPCB. Open reflux method is used for the determination of COD, and titrimetric method is used for determination of BOD in laboratory before and after treatment with the prepared activated carbon. At equilibrium, the amounts of COD and BOD adsorbed by activated carbon at time t, at q t (mg/g), and at equilibrium q e (mg/g) were calculated using the following relationships
where q t shows the amounts of COD and BOD adsorbed by activated carbon at time t. C o, C t , and C e represent COD and BOD concentrations in the solution (mg/l) at initial, time t, and equilibrium, respectively. V is the volume of solution (l), and m is the mass of activated carbon (g).
To determine the concentration of COD in the sewage sample, 50 ml of sewage sample was placed into a 500-ml refluxing flask. Five to 7 glass boiling beads was added to serve as anti-bumping aid followed by the addition of 1 g HgSO4, and then very slowly add 5 ml of concentrated H2SO4/Ag2SO4, with mixing to dissolve HgSO4 in solution. The function of HgSO4 is to bind or complex chlorides. Next, accurately add and mix 25 ml of 0.004167 M K2Cr2O7 to avoid possible loss of volatile materials, cool, and while mixing add 70 ml of concentrated H2SO4-Ag2SO4 solution. After thorough mixing, the flask was attached to the reflux condenser (APHA 2005; Olafadehan et al. 2012). Then, the mixture was gently heated in a water bath and refluxes for 2 h, cooled, and washed down condenser with distilled water. Washing down of the interior of the condenser and flask twice, with approximately 25 ml portions of distilled water, was carried out (APHA 2005; Olafadehan et al. 2012). Disconnect reflux condenser and dilute mixture to about twice its volume with distilled water of approximately 350 ml with distilled water. Then, further cooling to room temperature was done. Two to three drops of Ferroin indicator was added and stirred with a magnetic stirring bar. The resulting mixture was rapidly titrated with 0.025 M FeSO4(NH4)2SO4·6H2O (FAS) (APHA 2005; Olafadehan et al. 2012). The end point is very sharp and may be reached rapidly with first sharp color change from blue-green to reddish brown. The same procedure was repeated for other samples. Also, the blank run was done; it was prepared using 50 ml of distilled water by treating it with the same reagents as the samples to determine the blank value. The concentration of COD of respective samples was calculated using the following equation (APHA 2005)
where
- A :
-
volume (ml) of FeSO4(NH4)2SO4·6H2O used for blank
- B :
-
volume (ml) of FeSO4(NH4)2SO4·6H2O used for sample
- M :
-
Molarity of FeSO4(NH4)2SO4·6H2O
- 8000:
-
milliequivalent weight of oxygen × 1000 ml/l.
Preparation of activated carbon
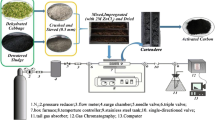
Different amounts of date palm shell waste were collected from some fields in Skaka region, Al Jouf Province, washed, and then dried under the sun for 7 days in order to remove the moisture content. Dry pieces were crushed to 6–11 mm. Furthermore, after drying, the date palm shell was separated, collected, cleaned, and prepared to be carbonized in the tube furnace. Before the carbonization step, dried shell sample was firstly weighed and then carbonized in a furnace with limited supply of oxygen for durations of about 4 h monitored from 100 to 700 °C in the presence of dehydrating agent ZnCl2 (20% by weight), followed by heating in absence of air (Hazzaa and Hussein 2015; Fiuza et al. 2015).
After the chemical activation process, the produced activated carbon was washed with excess amount distilled water to remove the remaining amounts of zinc chloride and ash. Prepared activated carbon was filtered, drained, and spread on a tray at room temperature. Then, the produced carbon was dried at 100 °C for 2 h, weighted, and crushed to size of 100 mesh.
To characterize the prepared activated carbon, different chemical test methods were used. The iodine number is determined by treatment known weight of produced activated carbon with a standard iodine solution under laboratory conditions (Olafadehan et al. 2012). The treated carbon solution was then filtered to separate the solid carbon from treated solution. The remaining iodine in the filtrate (solution) was measured by titration method with standardized 0.1 M sodium thiosulfate solution using starch indicator solution. The calculated Iodine value was 970.
The important characteristics of the produced activated carbon were as follows: surface area 0.7 × 105 m3/kg, particle density 163 kg/m3, and moisture content 6.7%.
Batch mode for treatment of sewage samples
Firstly, the original sewage samples were filtrated through sand filter to remove and reduce the TSS content. Batch adsorption experiments were carried out at room temperature (25 °C ± 1) using different weights of the produced activated carbon mixed with 100 ml of treated sewage in 250 ml Erlenmeyer. The mixtures were agitated continuously for 150 min, unless otherwise cited. The resulting solution was filtered, and the concentrations of both COD and BOD were determined, where the effect of activated carbon dose, pH of the solution, contact time, agitation speed, and temperature was investigated.
For batch adsorption process investigation, a good shaking for mixing the two phases was achieved using a thermostatic mechanical shaker of the type Julabo SW-20 C, Germany. The hydrogen ion concentration for the solutions was measured using a digital pH meter of Hanna Instruments type, Padova, Italy.
Results and discussion
Effect carbon dose
The effect of produced activated carbon dose prepared from date palm shell waste on percentage removal (%R) of COD and BOD from treated sewage was studied at pH 6.0 for 150 min at 25 °C with agitation rate of 400 rpm (Fig. 1). The activated carbon dose was increased from 0.01 to 0.3 g, with aqueous phase constant volume of 100 ml. As in the Fig. 1, the removal percentage (%R) increased with increasing in the carbon dose and this may be attributed to the increase in the function groups and the total surface area of the prepared activated carbon, and therefore, more active sites on the activated carbon were available for the adsorption of COD and BOD from the aqueous solution (Zhang et al. 2012).
Equilibrium was reached at about 0.1 g/100 ml carbon dose for the optimum percentage removal (%R) of 95.4 and 92.8% for COD and BOD, respectively. After equilibrium, further increases of carbon dose result in constant removal of COD and BOD, and accordingly, activated carbon dose of 0.1 g/100 ml is considered an optimal dose for the removal of COD and BOD from treated sewage in our work.
Effect of pH
The effect of pH of the aqueous solution is regarded as one of the important parameters affecting the adsorption technique. Adsorption of COD and BOD from treated sewage by 0.1 g/100 ml activated carbon was carried out at pH range of 2.0–9.0 for 150 min and at 25 °C, as in Fig. 2. The result obtained showed that the removal percentage of COD and BOD increased at low pH till pH 6.0, and with further increase in pH value, the removal percentage was decreased.
The higher value of the adsorption percentage (%R) observed at pH < 6 may be attributed to that at low pH value positive charge sites are created on the activated carbon surface, and it is resulted in a significantly high electrostatic attraction between positively charge surface of the activated carbon and organic compounds (COD and BOD). On the other hand, the decrease in the value of (%R) at pH > 6 may be attributed to increasing hindrance to the diffusion of organic compounds due to abundance of OH- ions (Ghodale and Kankal 2014; Sadhasivam et al. 2007).
Effect of contact time
The effect of contact time is an important factor in the study of economical sewage treatment processes. Therefore, the effect of mixing time on the removal of COD and BOD from treated sewage by 0.1 g/100 ml activated carbon at agitation rate of 400 rpm at pH = 6 and at 25 °C was studied in time periods from 15 to 210 min as shown in Fig. 3. The uptake of COD and BOD by activated carbon was very rapid at first 150 min to reach about 95.4% COD and 92.8% BOD, where the removal percentage increase with the increasing in the contact time and reaches a constant high value at 150 min. After 150 min (which regarded as equilibrium time), %R of COD and BOD adsorbed from treated sewage by activated carbon prepared from date palm wastes did not significant change with time.
The initial increase in the removal percentage, (%R), till 150 min, may be attributed to the fact that at the initial contact time period, the large number of unoccupied active sites is available for adsorption of both COD and BOD. The smooth and independent nature of curves, as in Fig. 3, indicates the formation of monolayer of COD and BOD cover on the outer surface of the activated carbon (Aluyor and Badmus 2008). Therefore, 150 min contact time was used for all further studies.
Effect of agitation rate
The relation between the agitation rate and adsorption percentage of COD and BOD from treated sewage by 0.1 g/100 ml activated carbon prepared from date palm waste was investigated in range 100 to 700 rpm agitation speed at pH 6 for 150 min and 25 °C. Figure 4 shows that as the agitation rate increase, the adsorption percentage of COD and BOD increased till agitation speed of 400 rpm. At agitation speed of 400 rpm, maximum values for adsorption percentage has obtained (95.4% COD and 92.8% BOD) but at higher agitation rate, the removal percentage remained nearly unchanged.
Adsorption isotherms
Equilibrium and kinetics are two important physico-chemical aspects for the evaluation of the using of activated carbon on the adsorption process of COD and BOD. In this work, the relation between COD and BOD adsorption capacities (q e) of activated carbon and the residual amounts (C e) of COD (or BOD) at equilibrium was analyzed with different isotherm models at a constant temperature. The equations for the three adsorption isotherm types are represented as
where (x/m) shows the amount of COD (or BOD) adsorbed by unit of activated carbon (mg/g), q o and k are Langmuir constants reflecting, respectively, the adsorption capacity (mg/g) and energy of adsorption (L/mg).
where k is the Freundlich constant and (1/n) is a measure of the adsorption capacity and intensity (Mohan and Karthikeyan 1997).
The relations between (x/m) against C e for the liner isotherm were plotted to determine the better type of the isotherm model fits the adsorption process of COD (or BOD) by activated carbon (Fig. 5). C e/q e against C e was plotted for the Langmuir isotherm, as in Fig. 6. Straight lines were obtained (R 2 > 0.99) confirming that Langmuir isotherm model is applicable for adsorption of both COD and BOD from treated sewage by activated carbon. The relation between log(x/m) against log C e was also plotted, as in Fig. 7, for the Freundlich isotherm.
The constants 1/n and k can be calculated from the slope and the intercept in Fig. 7, respectively. The parameters calculated from the Langmuir and Freundlich models are summarized in Table 2. The results obtained shows that the investigated adsorption isotherms models are best and suitable to describe adsorption process of COD and BOD by activated carbon. The higher values of R 2 (> 0.9) obtained for the investigated adsorption isotherms models show that the adsorption process of COD and BOD from treated sewage by prepared activated carbon in this work can be well explained by the models of Linear, Langmuir, and Freundlich isotherms (Aluyor and Badmus 2008).
From the data obtained in Table 2, the n values of the Freundlich model are > 1 and therefore favorable multilayer adsorption is expected. For Langmuir isotherm model, the maximum monolayer coverage capacity (q 0) was calculated to be about 41.3 mg/g for COD and 20.2 mg/g for BOD, K (Langmuir isotherm constant) is 4.9 l/mg for COD and 1.95 l/mg for BOD, and the R 2 values are 0.99 for both COD and BOD indicating that the adsorption data is fitted well to Langmuir isotherm model which is used for homogeneous surfaces. The Freundlich isotherm model supposes that neither homogeneous site energies nor limited levels of adsorption. Also, it can result from overlapping patterns of different Langmuir-type adsorption phenomena that take place at different sites (Maiti et al. 2008).
Kinetic adsorption modeling
In order to study the mechanism of the investigated adsorption process, different kinetic models have been investigated. For the experimental data obtained, two of suggested models were applied and studied under optimum conditions to determine the best fitted model. The pseudo-first-order rate and pseudo-second-order equations were investigated. The following pseudo-first-order equation can be applied (Maiti et al. 2008; Badmus et al. 2007).
Integrating of Eq. (8) with the boundary conditions of t = 0, q t = 0, and t = t, q t = q t gives Eq. (9).
where q e and q t are the amounts of COD (or BOD) adsorbed at equilibrium and at time t (mg/g), respectively, t is the contact time (min) and K 1 is the pseudo-first-order rate constant (/min).
The calculated values of both k 1and q e were determined from the slopes and intercepts, respectively, of the straight lines obtained in plots of log (q e − q t ) versus t, as in Fig. 8. The calculated data obtained for this system are illustrated in Table 3.
To describe the kinetic of some investigated adsorption process, Eq. (8) cannot be applied. Therefore, a pseudo-second-order kinetic model can be investigated to describe this type of adsorption. Hence, this model reduces to the following equation
Integrating of Eq. (8) with the boundary conditions of t = 0, q t = 0 and t = t, q t = q t , gives the following equation
By plotting of t/q t versus t, as in Fig. 9, the values of k 2 and q e were calculated and listed in Table 3. It can be seen that the values of correlation coefficients (R 2 = 0.99) for pseudo-first order are lower than that obtained for the pseudo-second-order kinetics (R 2 ≥ 0.998).
Depending on the correlation coefficients values of the results with the pseudo-second-order kinetic model, which provides the best fit to the obtained experimental data, chemisorption reaction is the main adsorption mechanism for the investigated adsorption process of COD and BOD from treated sewage by prepared activated carbon.
Effect of temperature
Temperature change is an effective parameter in studying any adsorption process. To know and determine the thermodynamic parameters of adsorption process, effect of temperature on adsorption capacity should be investigated. Figure 10 illustrates the relation between q e and temperature. It shows that the amount of COD and BOD adsorbed from treated sewage at pH 6 for 150 min using 0.1 g/100 ml with agitation rate of 400 rpm increases with increase temperature, this confirms the endothermic behavior of the investigated adsorption process.
Different thermodynamic parameters, enthalpy (ΔH 0), entropy changes (ΔS 0), and Gibbs free energy (ΔG 0), of the adsorption process of COD and BOD by activated carbon form treated sewage were calculated. These parameters can be calculated by use the following equations (Maiti et al. 2008)
where K d is the distribution coefficient (ml/g), T is the absolute temperature (in Kelvin), and R is the general gas constant (R = 8.314 J/mol K). The plots between log 1/T against K d are given in Fig. 11. The calculated values of (ΔH 0) and (ΔS 0) can be obtained from the slopes and intercepts of linear relations in the Fig. 11, respectively, as listed in Table 4.
From the data obtained in Table 4, it can be concluded that the calculated positive values of ΔH 0 indicating the endothermic nature of the adsorption system of COD and BOD by activated carbon from treated sewage. The negative values of (ΔG) confirm the spontaneous behavior and the feasibility of the adsorption process. The magnitude of ΔG increased as the temperature increase, this indicating that the degree of spontaneity of the system increased as the temperature increase. The calculated positive values of (ΔS) shows an increasing in the randomness and the disorderliness degree occur at the activated carbon/treated sewage interface in the adsorption process.
The chemical analysis of the final resulted treated sewage showed that the resulted solution treated under the optimum conditions with carbon dose of 0.1 g/100 ml aqueous solution of pH 6 for 150 min at 25 °C with agitation rate of 400 rpm contained 1.75 mg/l COD, 1.0 mg/l BOD, and 2.4, 2.8, 0.9, 0.6, and 0.2 mg/l for TSS, TKN, NH3, TB, and organic nitrogen, respectively. The concentration of impurities in the treated sewage sample before and after treatment process by prepared activated carbon under the investigated parameters is shown in Fig. 12.
Conclusion
The experimental results obtained showed that the activated carbon prepared form date palm shell waste by chemical activation method is a good adsorbent for the removal of COD and BOD. The removal percentage (%R) of 95.4 and 92.8% for COD and BOD, respectively, was obtained with carbon dose of 0.1 g/100 ml aqueous solution of pH 6 for 150 min at 25 °C with agitation rate of 400 rpm. It was also found that the adsorption kinetic of the investigated process followed more pseudo-second-order model than pseudo-first-order model. The adsorption isotherm results are fitted well to Langmuir and Freundlich models. Thermodynamic results obtained reveals that the positive value of (ΔH) confirming the endothermic behavior of the investigated adsorption process while the negative values of (ΔG) indicate the feasibility and spontaneous behaviors and revealing that the degree of spontaneity increased with temperature. The calculated positive values of (ΔS) shows an increasing in the randomness and the disorderliness degree occur at the activated carbon/treated wastewater interface in the adsorption process. The analysis of the resulted treated sewage showed that the resulted solution treated under the optimum conditions in this adsorption process contained 1.75 mg/l COD, 1.0 mg/l BOD, 2.4, 3.8, 0.9, 0.6, and 0.2 mg/l for TSS, TKN, NH3, TB, and organic nitrogen, respectively. The results obtained in this investigated work can be used for planning and designing an small and economically plant for final treatment of treated sewage.
References
Adeniji BA, Budimir-Hussey MT, Macfie SM (2010) Production of organic acids and adsorption of Cd on roots of durum wheat (Triticum turgidum L. var. durum). Acta Physiol Plant 32(6):1063–1072
Ahmed MJ, Theydan SK (2014) Adsorptive removal of p-nitro phenol on microporous activated carbon by FeCl3 activation: equilibrium and kinetics studies. Desalin Water Treat 55(2):522–531
Ali I, Aboul-Enein HY (2004) Chiral. Pollutants: Distribution, Toxicity and Analysis by Chromatography and Capillary Electrophoresis. John Wiley & Sons, Chichester
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183
Alslaibi TM, Abustan I, Ahmad MA, Foul AA (2013) A review: production of activated carbon from agricultural byproducts via conventional and microwave heating. J Chem Technol Biotechnol 88:1183–1190
Aluyor EO, Badmus OAM (2008) COD removal from industrial wastewater using activated carbon prepared from animal horns. African J Biotechnol 7(21):3887–3891
Amor HB, Ismail M (2015) Adsorption of chromium (VI) on activated carbon prepared by acid activation of date stones. Int J Sci Res 4:309–314
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Attallah MF, Ahmed IM, Hamed MM (2013) Treatment of industrial wastewater containing Congo Red and Naphthol Green B using low-cost adsorbent. Environ Sci Pollut Res 20(2):1106–1116
Badmus MAO, Audu TOK, Anyata BU (2007) Removal of copper from industrial wastewaters by activated carbon prepared from periwinkle shells. Korean J Chem Eng 24:246–252
Bansode RR, Losso JN, Marshall WE, Rao R, Portier MRJ (2004) Pecan shell-based granular activated carbon for treatment of chemical oxygen demand (COD) in municipal wastewater. Bioresour Technol 94:129–135
Bleiman N, Mishael YG (2010) Selenium removal from drinking water by adsorption to chitosan–clay composites and oxides: batch and columns tests. J Hazard Mater 183:590–595
Damià B (2005) Emerging organic pollutants in waste waters and sludge. Springer, Berlin
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167:1–9
Eckenfelder W Jr, (1999) Industrial water pollution control, third ed., McGraw-Hill Companies Inc.
Fiuza RA Jr, Neto RMDJ, Correia LB, Andrade HMC (2015) Preparation of granular activated carbons from yellow mombin fruit stones for CO2 adsorption. J Environ Manag 161:198–205
Foo KY, Hameed BH (2009) Utilization of biodiesel waste as a renewable resource for activated carbon. Application to environmental problems. Ren Sust Energy Rev 13:2495–2504
Ghodale MD, Kankal SB (2014) Investigation of optimum operating parameters for BOD & COD removal using activated carbon. Int J Recent Dev Eng Tech 2(4):38–43
Guo Y, Zhang H, Tao N, Liu Y, Qi J, Wang ZXH (2003) Adsorption of malachite green and iodine on rice husk based porous carbon. Mater Chem Phys 82:107–115
Hamed MM, Ali MI, Nayl AA (2016) Kinetics and thermodynamics studies of cobalt, strontium and caesium sorption on marble from aqueous solution. Chem Ecol 32(1):68–87
Hararah MA, Ibrahim KA, Al-Muhtaseb AH, Yousef RI, Abu-Surrah A, Qatatsheh A (2010) Removal of phenol from aqueous solution by adsorption onto polymeric adsorbents. J Appl Polym Sci 117(4):1908–1913
Hazzaa R, Hussein M (2015) Adsorption of cationic dye from aqueous solution onto activated carbon prepared from olive stones. Environ Technol Innovation 4:36–51
Hyung H, Kim JH (2008) Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters. Environ Sci Technol 42(11):4416–4421
Kavita D, Namacivayam C (2007) Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour Technol 98:14–21
Kavita D, Namacivayam C (2008) Capacity of the activated carbon on the removal acid brilliant blue, determination of equilibrium and kinetic model parameters. J Chem Eng 139:453–461
Ling PP, Liu FQ, Li LJ (2010) Adsorption of divalent heavy metal ions onto IDA-chelating resins: simulation of physicochemical structures and elucidation of interaction mechanisms. Talanta 81:424–432
Maiti S, Purakayastha S, Ghosh B (2008) Production of low-cost carbon adsorbents from agricultural wastes and their impact on dye adsorption. Chem Eng Commun 195:386–403
Mall ID, Mishra N, Mishra IM (1994) Removal of organic matters from sugar mill effluent using bagasse fly ash activated carbon. Res Ind 39:115–119
Matsuo T, Nishi T (2000) Activated carbon filter treatment of laundry waste water in nuclear power plants and filter recovery by heating in vacuum. Carbon 38:709–714
Mohammad-Khah A, Ansari R (2009) Activated charcoal: preparation, characterization and applications: a review article. Int J Chem Tech Res 1(4):859–864
Mohan S, Karthikeyan J (1997) Removal of lignin and tannin colour from aqueous solution by adsorption onto activated charcoal. Environ Pollut 97:183–187
Nor NM, Chung LL, Teong LK, Mohamed AR (2013) Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—a review. J Environ Chem Eng 1:658–666
Olafadehan OA, Aribike DS (2000) Treatment of industrial wastewater effluent. J Nigerian Soci Chem l Engs 19:50–53
Olafadehan OA, Jinadu OW, Salami L, Popoola OT (2012) Treatment of brewery wastewater effluent using activated carbon prepared from coconut Shell. International J Appl Sci Tech 2(1):166–178
Sadhasivam S, Savitha S, Swaminathan K (2007) Exploitation of Trichoderma harzianum mycelial waste for the removal of rhodamine 6G from aqueous solution. J Environ Manag 85:155–161
Tsai WY, Chang CY, Lee SL (1998) A low coast adsorbent from agricultural waste corn by zinc activation. Bioresour Technol 64:211–217
Yahya MA, Al-Qodah Z, Ngah CWZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Ren Sust Energy Rev 46:218–235
Zhang Y, Wei F, Xing J, Lv F, Meng X, Chu PK (2012) Adsorption behavior and removal of organic materials from TNT red water by lignite activated carbon. J Residuals Sci Technol 9(3):121–129
Acknowledgements
This work has been carried out within the framework of a project number (35/351) financed by the Al Jouf University during the year 1436 to 1437 H. The author would like to express their deep thanks to Mr. A. Hajiaj Alshammari, Chemistry Dept. College of Science, Al Jouf University, and Mr. Mohammed H. Abo Hashem, Wastewater Treatment Facility, Sakakah, Al Jouf, for their continuous help through this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Nayl, A.E.A., Elkhashab, R.A., El Malah, T. et al. Adsorption studies on the removal of COD and BOD from treated sewage using activated carbon prepared from date palm waste. Environ Sci Pollut Res 24, 22284–22293 (2017). https://doi.org/10.1007/s11356-017-9878-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9878-4