Abstract
The present work investigates the potential use of metal hydroxides sludge (MHS) generated from hot dipping galvanizing plant for adsorption of Congo Red and Naphthol Green B dyes from aqueous solutions. Characterization of MHS included infrared and X-ray fluorescence analysis. The effect of shaking time, initial dye concentration, temperature, adsorbent dosage and pH has been investigated. The results of adsorption experiments indicate that the maximum capacity of Congo Red and Naphthol Green B dyes at equilibrium (q e) and percentage of removal at pH 6 are 40 mg/g, 93 %, and 10 mg/g, 52 %, respectively. Some kinetic models were used to illustrate the adsorption process of Congo Red and Naphthol Green B dyes using MHS waste. Thermodynamic parameters such as (ΔG, ΔS, and ΔH) were also determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large amounts of dyes are annually produced from textile, cosmetics, paper, leather, pharmaceuticals, food, and other industries. Fifteen percent of the total world production of dyes is lost during the dying process and released in the textile effluents (Zollinger 1991). Dye wastewater is characteristically high in organic content, salt content, and low in biodegradation. The release of dyes into the environment is of further concern due to their toxic, mutagenic, and carcinogenic characteristics of the dyes and their biotransformation products (Mittal et al. 2010a, 2012a). The trace amount of the dye present in the living systems may cause several health problems to mankind as well as animals (Mittal et al. 2012a). Congo Red and Naphthol Green B are a derivative of benzidine and napthoic acid and metabolizes to carcinogenic products. It may affect blood factors, such as clotting, and induce somnolence and respiratory problems (Mittal et al. 2009). Thus, removal of colored dyes from wastewaters is a major environmental issue.
Discharge of dyes is objectionable not only for aesthetic reasons but also as many dyes and their degradation products are carcinogenic toward aquatic life and mutagenic for humans (McMullan et al. 2001; Papić et al. 2004; Mittal and Gupta 1996; Lee et al. 2006). Direct discharge of dye effluents from these industries may cause serious problems to the environment because they contribute with the organic load and toxicity to the environment. Several techniques like coagulation (Lee et al. 2006; Ozcan et al. 2004), chemical oxidation (Swaminathan et al. 2003), aerobic/anaerobic digestion (O’Neill et al. 2000), ion exchange (Raghu and Ahmed Basha 2007), layered double hydroxides (LDHs) and their calcined products were usually used (Legrouri et al. 2005; You et al. 2002; Géraud et al. 2007; Ni et al. 2007). However, each of these methods has its limitations and disadvantages in application. In recent years, electrochemical treatment using an iron cell for removal of the hazardous dye from industrial wastewater effluents have been reported by (Gupta et al. 2007a).
Among various water purification and recycling technologies, adsorption is a fast, inexpensive, and universal method. Adsorption has been found to be an effective removal method, due to its efficiency, simplicity, and applicability (Ali and Gupta 2007; Mittal et al. 2010a; Gupta et al. 2009, 2012; Saleh and Gupta 2012). Various researchers have studied the adsorption of dyes on various types of materials. The development of low-cost adsorbents has led to the rapid growth of research interest in this field. Therefore, a number of low-cost adsorbents have been examined for the treatment of wastewaters, such as bottom ash (a power plant waste) de-oiled soya (an agricultural waste) (Gupta et al. 2006a; Mittal and Gupta 2010; Mittal et al. 2009, 2010a,b, 2012a,b), hen feathers (Gupta et al. 2006b; Mittal 2006), activated charcoal and wheat husk (Gupta et al. 2007b; Mittal et al. 2010c), algal Oedogonium (Gupta and Rastogi 2008, 2009), carbon slurry as fertilizer industry waste material (Gupta et al. 2007c,d, 2010), photocatalytic degradation using TiO2 as catalyst (Gupta et al. 2007e), and activated carbon and activated rice husks (Gupta et al. 2006c).
Removal of Congo Red using different techniques have been reported by a number of authors (Gharbani et al. 2008; Chatterjee et al. 2007; Sharma and Janveja 2008; Sawhney and Kumar 2011; Hu et al. 2010; Namasivayam et al. 1994), while the literature on Naphthol Green B is limited. Metal hydroxide sludge resulting from electroplating industry was used for dye removal of Congo Red (Namasivayam et al. 1994; Netpradit et al. 2003, 2004; Golder et al. 2006). Santos et al. (2008), investigated a metal hydroxide sludge generated from zinc electroplating industry when used as adsorbent for removing a reactive textile dye (Remazol Brilliant Blue) in wastewater solution. They found that the maximum adsorption capacity obtained varied between 275 mg/g (at 25 °C and pH 4) and 21.9 mg/g (at 25 °C and pH 10). Furthermore, when a simulated textile effluent was used as adsorbate, a decrease in the adsorption capacity from 91.0 to 31.0 mg/g was observed.
In the present work, metal hydroxides sludge (MHS) is used for removal of Congo Red and Naphthol Green B dyes from aqueous solutions for an experimental and modeling study on the adsorption equilibrium and kinetics. Application on a simulated real effluent is also tested in the perspective of an industrial application of the process.
Materials and method
Materials
The dyes used in this study, Congo Red and Naphthol Green B dyes having respective formulae C32H22N6Na2O6S2 and C30H15FeN3Na3O15S3 as shown in Fig. 1 were supplied from Aldrich. A 1,000 mg l−1 dyestuff stock solution was prepared by dissolving appropriate quantity of dyes. All experimental solutions of dyestuff were prepared by proper dilution of this stock solution. All reagents used in this study were of analytical reagent grade. The initial pH was adjusted to the required value using NaOH or HCl solutions prior to addition of the adsorbent. The sludge used for the adsorption tests was obtained from the sludge resulting from hot dipping galvanizing bath plant, and it was collected in Egypt.
Adsorbent preparation
The residue sludge under investigation was filtered and then dried at 105 °C for ∼2 h then ground to powder in a grinder. The powder sludge was calcinated at 400 °C for 2 h for preparation of the adsorbent used for all the experiments in this work.
Instrumentation
The microstructural characterization of MHS was carried out using a transmission electron microscope (200CX model, JEOL). The IR spectrum of the adsorbent under study was measured using a Fourier transform infrared spectroscopy spectrophotometer (Perkin-Elmer, USA) for characterization of MHS. A high precision, double-beam spectrophotometer (Shimadzu UV-160A, Japan) was used to measure the absorbance of dyes.
Batch adsorption experiments
Batch adsorption experiments were carried out for an adsorption period of 30 min in a 250-ml Erlenmeyer flask, filled with 100 ml water containing the desired dye concentration at initial pH of 2.0–11.5. Different amounts of the adsorbent were added and were shaken for 30 min in flasks. The adsorbent and adsorbate were separated through filtration, and dye content of the filtrate solution was spectrophotometrically determined. The equivalent amount of adsorbed dyes was calculated from the difference in their initial and final concentrations.
The effect of adsorbent dosage on the percent removal was studied with adsorbent dosage varying between 0.05 and 0.25 g/100 ml and from 0.1 to 0.8 g/100 ml for Congo Red and Naphthol Green B dyes, respectively. Adsorption kinetic studies were carried out with different initial concentrations of dye in the range 50–200 mg/l, while maintaining the adsorbent dosage at 0.25 and 0.5 g/100 ml for Congo Red and Naphthol Green B dyes, respectively and at pH 6.
The amounts of dyes adsorbed were calculated from these concentrations in solutions before and after adsorption. The amount of dyes adsorbed (mg g−1), (q e), and efficient removal (R, %) onto MHS was calculated from the mass balance equation.
Results and discussion
The infrared spectrum of MHS (Fig. 2) shows the characteristic absorption bands of a hydroxyl group, particularly a broad band at 3,437 cm−1 (due to the interlayer water molecules) with a shoulder near 2,927 cm−1 (due to the H-bonded stretching vibration). Weak bands at 2,290 and 1,617 cm−1 can be assigned to the H2O bending vibration of interlayer water (Staurt 1996; Nakamoto 1978). The bands at 1,457, 1,359, 1,156, and 1,111 cm−1 can be assigned to the vibration of carbonate species, which may arise from the presence of calcium carbonate as impurities in the lime. The values found here for the positions of these carbonate bands are very close to those reported by Saiah et. al. (2009) for nickel–iron layered double hydroxide materials. Bands in the range of 450–600 cm−1 are attributed to metal–oxygen–metal stretching (Staurt 1996; Nakamoto 1978; Saiah et al. 2009). The peak of 465 cm−1 is attributed to the vibration of the chemical bond (Fe3+–O2−), and the band from 547 cm−1 is attributed to the vibration of the chemical bond (Zn+2–O−2). These bands confirm the presence of zinc ferrite in the studied samples (Pascuta et al. 2011).
MHS was also characterized by energy-dispersive X-ray spectroscopy in order to know its chemical composition. The constituents of the MHS were found to be Ca (1.68 %), Fe (56.12 %), O (22.76 %), Al (2.65 %(, Si (3.42 %), Cl (6.12 %), Mo (2.22 %), and Zn (5.0 %), as given in Table 1. This shows that the MHS mainly contains iron oxide, which was produced by treatment of pickling solution with lime. The other physicochemical properties of MHS adsorbent are listed in Table 2. The MHS was also characterized with respect to its pore structure and surface area using nitrogen adsorption/desorption at −196 °C, which was conducted using a gas sorption analyzer (Quantachrome, NOVA 1000e series, USA). The BET equation was used to determine the BET surface area S BET = 14.02 m²/g; the total pore volume calculated from the amount of nitrogen held at P/0 P = 0.95 is V P = 0.0178 cc/g.
Effect of hydrogen ion concentration
Since the surface charge of a solid phase (MHS) could be modified by changing the pH of the solution, pH plays an important role in the removal of ions by the adsorption process. Figure 3 shows that the effect of pH on the adsorption of Congo Red and Naphthol Green B dyes. It can be seen that the removal of Naphthol Green B gradually increases with the increase in pH of solution up to pH 6, followed by a slight decrease with further increase in pH of solution. In contrast, the Congo Red removal showed a maximum dye removal at pH 2–7 followed by a decrease in the higher pH ranges. The broad pH range 2–7 using MHS makes it a promising adsorbent material to remove dyes from wastewater. The MHS was charged positively from pH 2 to 7 by the adsorption of H+ and, in turn, became negative charge from pH 8 to 12 by the adsorption of OH−. Typical metal hydroxides [M(OH) n ], which are amphoteric, form metal hydroxo complex ions, and are soluble in acidic and strongly alkaline solutions, while the form in neutral charge is insoluble, as presented in the following equation (Namasivayam et al. 1994; Packter and Dhillon 1981).

The results show that the MHS affinity increases in the order to Congo Red > Naphthol Green B. This indicates that the greater charged dyes had a greater tendency to be adsorbed by the sludge, due to electrical attraction of dyes to sites of positive charge on the sludge surface. The structures of Congo Red and Naphthol Green B (Fig. 1a–b) carry 2 sulfonic, 2 amine, 2 azo groups, and 3 sulfonic groups, respectively. Thus, Congo Red with a larger negative charge, had a greater force to be attracted by the positive charge on the sludge, and hence had a larger exchange capacity than Naphthol Green B.
It was reported that (Stumm 1992) adsorption of anions on surface hydroxyl groups is coupled with a release of OH− ions, and the main mechanism is ligand exchange. It can be assumed that OH− ions were replaced by SO−3 ions because the sulfonic group is a strongly acidic cation exchanger (Out and Westland 1991), resulting in charge balance of the precipitate to be settled, as illustrated in the following equations:
where n = 2 or 3, in addition, more valence or amount of charge means more exchange capacity between adsorbate and adsorbent.
Generally, a positive charge develops on the surface of oxides of adsorbent in an acid medium, resulting in a higher adsorption of dyes than in a basic solution, as reported (Namasivayam et al. 1994; Lee et al. 1996). Many sulfonated dyes can be made insoluble by precipitating them as the salts of heavy metals or can be precipitated from solutions as dye metal complexes with soluble salts of heavy metal ions (Giles and Greczek 1962; Hou and Baughman 1992).
The removal of dyes at pH 2–6 may result from the complexation between dyes and heavy metal ions, which yielded precipitate. In addition, the trend of dyes removal decreased at a pH above 6.5, according to the presence of hydroxo complex anions by the adsorption of OH− (see above equation).
As the pH decreased, the surface charge of the metal hydroxides became increasingly positive. Congo Red has a higher number of negative sites available for adsorption or exchange than Naphthol Green B. Therefore, it appears that the adsorption Congo Red was still effective in the system pH range of 2–7, while that of Naphthol Green B was maximum at system pH ≈ 6. Appreciable amount of adsorption in this pH range suggests a strong involvement of physical forces such as hydrogen bonding, van der Waals force, etc. in the adsorption process (Chatterjee et al. 2007; Wong et al. 2003). Due to both dyes have N, S, O atoms and benzene ring. Therefore, we assume Congo Red to be representative of both dyes under study. Figure 4 represents all possible interactions between MHS and dyes.
Effect of shaking time and initial dye concentration
The removal of Congo Red and Naphthol Green B dyes onto MHS at different dye concentrations at 25 ± 1 °C is shown in Fig. 5. It was found that the removal of Congo Red and Naphthol Green B dyes gradually increases with the increase in the shaking time and reaches equilibrium after ∼60 and 30 min, respectively. At lower initial concentration of the dye, the removal is very fast, and higher removal of Congo Red and Naphthol Green B dye is obtained if we increase the contact time.
At 200 mg/l of initial dye concentration, 40 and 10 mg of Congo Red and Naphthol Green B were removed by 1 g of MHS adsorbent at pH 6.0. As listed in Table 3, in comparison with other materials, the maximum adsorption capacity of Congo Red onto MHS is lower than that of chitosan, but higher than that of wheat bran, waste orange peel, and sodium bentonite. Surprisingly, both granulated (13.80 mg/g) and powdered activated carbon (16.81 mg/g) (Fu and Viraraghavan 2002) are less efficient than MHS materials.
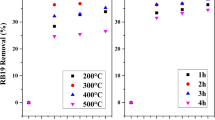
Effect of temperature
In this phase of study, a series of experiments was conducted at 15, 25, 35, and 50 °C to study the effect of temperature on the removal of Congo Red and Naphthol Green B dyes on MHS adsorbent. As shown in Fig. 6, the removal of Congo Red dye and Naphthol Green B dye has increased with increasing temperature in the range between 15 and 50 °C. This may be a result of an increase in the rate of diffusion of the adsorbate molecules across the external boundary layer to the internal pores of the adsorbent particle, owing to the decrease in the viscosity of the solution, and change of the equilibrium capacity of the adsorbent for a particular adsorbate. An increasing number of molecules may also acquire sufficient energy to undergo an interaction with active sites at the surface. Furthermore, increasing temperature may produce a swelling effect within the internal structure of the adsorbent enabling large dyes to penetrate further as found by (Asfour et al. 1985; Ozdemir et al. 2006).
The temperature dependence of the adsorption process is associated with changes in several thermodynamic parameters such as standard free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) changes of adsorption (Li et al. 2009).
The values of ΔH and ΔS were respectively evaluated from the slope and intercept of the Van’t Hoff plot by regression method as shown in Fig. 7 and given in Table 4. The positive value of ΔH confirms the endothermic nature of adsorption; the negative values of ΔG at different temperatures (288–323 K) indicate the spontaneous nature of Congo Red and Naphthol Green B adsorption. The positive value of ΔS suggests the increased randomness at the solid/solution interface during the adsorption of dyes on MHS adsorbent.
Effect of adsorbent mass
The effect of MHS dosage on Congo Red and Naphthol Green B dyes removal is shown in Fig. 8. The removal efficiency of Congo Red and Naphthol Green B dyes has increased with increasing dosage of MHS up to a maximum efficiency then remained constant, implying that the amount of adsorption sites also increases with increasing dosage of MHS during the adsorption process. The maximum dye removal efficiency reaches 93 and 46 % for Congo Red and Naphthol Green B dyes, respectively, after which an increase in MHS dosage does not further improve the dye removal, implying that a complete dye removal could not be achieved even though using a large amount of the MHS.
Kinetic adsorption modeling
The mechanism of adsorption often involves chemical reaction between functional groups present on the adsorbent surface and the adsorbate. The order of adsorbate–adsorbent interactions has been described using various kinetic models. In the present work, we have used the pseudo-first-order model derived by (Lagergren 1898). In the case of adsorption preceded by diffusion through a boundary, the kinetics in most cases follow the pseudo-first-order equation of Lagergren
where q t and q e are the amounts adsorbed (mg/g) at time t (min) and at equilibrium, respectively, and k 1 is the rate constant of the pseudo-first-order adsorption process (min−1). Plotting log (q e − q t ) versus t gives a straight line for pseudo-first-order kinetics, as shown in Fig. 9, which allows computation of the adsorption rate constant, k 1. The calculated parameters of the pseudo-first-order kinetic models are listed in Table 5. The calculated q e (mg/g) values deviate from the experimental q e (mg/g) for dyes under investigation. This indicates that it is not appropriate to use the pseudo-first-order kinetic model to predict the adsorption kinetics of Congo Red and Naphthol Green B dyes onto MHS materials.
Several authors report that the pseudo-second-order kinetic model can also be applied to these interactions in certain specific cases. The pseudo-second-order kinetic equation (Hu et al. 2010) is expressed as
where k 2 is the pseudo-second-order rate constant (g mg−1 min−1), and h is the initial adsorption rate at times approaching 0 (mg g−1 min−1).
To test the pseudo-second-order kinetic, graphs also plotted between t/q t and t are presented in Fig. 10, which allows computation of q e and k 2 parameters. The relationship is linear, and the correlation coefficient (R 2) suggests a strong relationship between the parameters and as well shows that the process of sorption of Congo Red and Naphthol Green B dyes follows the pseudo-second-order kinetics. As shown from Table 5, the correlation coefficient (R 2) has an extremely high value (0.999) and is closer to unity for the pseudo-second-order kinetic model than for a pseudo-first-orders kinetic model. The calculated equilibrium sorption capacity (q e ) is consistent with the experimental data. These results explain that the pseudo-second-order sorption mechanism is predominant and that the overall rate constant of a sorption process appears to be controlled by a chemisorption process (Ho and McKay 1999).
The effect of intraparticle diffusion resistance on adsorption can be evaluated by the following equation:
where k id is the rate constant of intraparticle diffusion (mg g−1 min−0.5). Values of C give the information regarding the thickness of boundary layer. According to this model, the plot of q t versus the square root of time (t 1/2) should be linear if intraparticle diffusion is involved in the adsorption process, and if these lines pass through the origin, then intraparticle diffusion is the rate controlling step. When the plots do not pass through the origin as shown in Fig. 11, this is indicative of some degree of boundary layer control, and this further shows that the intraparticle diffusion is not the only rate-controlling step, but also other kinetic models may control the rate of adsorption of the dyes (Congo Red and Naphthol Green B) onto MHS material, all of which may be operating simultaneously. The values of intercept listed in Table 6 and give an idea about the boundary layer thickness, such as the large is the intercept, the greater is the boundary layer effect (Vimonses et al. 2009; Chiou and Li 2003; Lorenc-Grabowska and Gryglewicz 2007). Similar types of results have been reported by other authors on different adsorbents (Hu et al. 2010; Hameed 2008).
Conclusion
The present work reveals that the MHS, which is an abundantly available industrial waste, can be easily converted into good adsorbent for removal of dyes from industrial wastewater. The adsorption of Congo Red dye using MHS material is more efficient than Naphthol Green B dye from aqueous solution. On applying both pseudo-first-order and pseudo-second-order kinetics to the adsorption of the dye on MHS, it was found that the interactions could be better explained by pseudo-second-order kinetics. Using MHS material as an adsorbent, the advantage is twofold: It not only acts as an effective and economic adsorbent as compared to other existing adsorbents for solving the problem of color pollution but also helps as an effective and useful disposal of industrial sludge waste.
References
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1(6):2661–2667
Asfour HM, Fadali OA, Nassar MM, El-Geundi MS (1985) Equilibrium studies on adsorption of basic dyes on hardwood. Chem Technol Biotechnol 35A:21
Chatterjee S, Chatterjee S, Chatterjee BP, Guha AK (2007) Adsorptive removal of Congo Red, a carcinogenic textile dye by chitosan hydrobeads: binding mechanism, equilibrium and kinetics. Colloids Surf A Physicochem Eng Asp 299:146–152
Chiou MS, Li HY (2003) Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 50:1095–1105
Fu Y, Viraraghavan T (2002) Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Adv Env Res 7:239
Géraud E, Bouhent M, Derriche Z, Leroux F, Prévot V, Forano C (2007) Texture effect of layered double hydroxides on chemisorption of Orange II. J Phys Chem Solids 68:818–823
Gharbani P, Tabatabaii SM, Mehrizad A (2008) Removal of Congo Red from textile wastewater by ozonation. Int J Environ Sci Tech 5(4):495–500
Giles CH, Greczek JJ (1962) A review of methods of purifying and analyzing water-soluble dyes. Text Res J 32:506
Golder AK, Samanta AN, Ray S (2006) Anionic reactive dye removal from aqueous solution using a new adsorbent—sludge generated in removal of heavy metals by electrocoagulation. Chem Eng J 122(1–2):107–115
Gupta VK, Ali I (2008) Removal of endosulfan and methoxychlor from water on carbon slurry. Environ Sci Technol 42:766–770
Gupta VK, Rastogi A (2008) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153(1–2):759–766
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Mittal A, Gajbe V, Mittal J (2006a) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2006b) Adsorption of Safranin-T from wastewater using waste materials- activated carbon and activated rice husks. J Colloid Interface Sci 303(1):80–86
Gupta VK, Mittal A, Kurup L, Mittal J (2006c) Adsorption of a hazardous dye, erythrosine, over hen feathers. J Colloid Interface Sci 304(1):52–57
Gupta VK, Ali I, Saini VK (2007a) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315(1):87–93
Gupta VK, Ali I, Saini VK (2007b) Defluoridation of wastewaters using waste carbon slurry. Water Res 41(15):3307–3316
Gupta VK, Jain R, Varshney S (2007c) Electrochemical removal of the hazardous dye Reactofix Red 3 BFN from industrial effluents. J Colloid Interface Sci 312:292–296
Gupta VK, Jain R, Varshney S (2007d) Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—an agricultural waste. J Hazard Mater 142(1–2):443–448
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007e) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309(2):464–469
Gupta VK, Mittal A, Gajbe V, Mittal J (2008) Adsorption of basic Fuchsin using waste materials—bottom ash and de-oiled soya as adsorbents. J Colloid Interface Sci 319:30–39
Gupta VK, Goyal RN, Sharma RA (2009) Novel PVC membrane based alizarin sensor and its application; determination of vanadium, zirconium and molybdenum. Int J Electrochem Sci 4(1):156–172
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342(1):135–141
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv. doi:10.1039/C2RA20340E
Hameed BH (2008) Equilibrium and kinetic studies of methyl violet sorption by agricultural waste. J Hazard Mater 154:204–212
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451
Hou M, Baughman GL (1992) Predicting the precipitation of acid and direct dyes in natural waters. Dyes Pigments 18:35
Hu Z, Chen H, Ji F, Yuan S (2010) Removal of Congo Red from aqueous solution by cattail root. J Hazard Mater 173:292–297
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 24(4):1–39
Lee CK, Low KS, Chow SW (1996) Chrome sludge as an adsorbent for colour removal. Environ Technol 17:1023
Lee J-W, Choi S-P, Thiruvenkatachari R, Shim W-G, Moon H (2006) Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigments 69:196–203
Legrouri A, Lakraimi M, Barroug A, De Roy A, Besse JP (2005) Removal of herbicide 2,4-dichlorophenoxyacetate from water to zinc–aluminium-chloride layered double hydroxides. Water Res 21:217–226
Li Y, Gao B, Wu T, Wang B, Li X (2009) Adsorption properties of aluminum magnesium mixed hydroxide for the model anionic dye Reactive Brilliant Red K-2BP. J Hazard Mater 164:1098–1110
Lorenc-Grabowska E, Gryglewicz G (2007) Adsorption characteristics of Congo Red on coal-based mesoporous activated carbon. Dyes Pigments 74:34–40
McMullan G, Meehan C, Conneely A, Kirby N, Robinson T, Nigam P, Banat IM, Marchant R, Smyth WF (2001) Microbial decolourisation and degradation of textile dyes. Appl Microbiol Biotechnol 56(1/2):81–87
Mittal A (2006) Removal of the dye. Amaranth from wastewater using hen feathers. Electron J Environ Agric Food Chem 5(2):1296–1305
Mittal AK, Gupta SK (1996) Biosorption of cationic dyes by dead macro fungus Fomitopsis carnea: batch studies. Water Sci Technol 34:81–87
Mittal A, Gupta VK (2010) Adsorptive removal and recovery of the azo dye Eriochrome Black T. Toxicol Environ Chem 92(10):1813–1823
Mittal A, Mittal J, Malviya A, Gupta VK (2009) Adsorptive removal of hazardous anionic dye “Congo Red” from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340(1):16–26
Mittal A, Jain R, Mittal J, Varshney S, Sikarwar S (2010a) Removal of Yellow ME 7 GL from industrial effluent using electrochemical and adsorption techniques. Int J Environ Pollut 43(4):308–323
Mittal A, Mittal J, Malviya A, Gupta VK (2010b) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344:497–507
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010c) Decoloration treatment of a hazardous triarylmethane dye, Light Green SF (Yellowish) by waste material adsorbents. J Colloid Interface Sci 342(2):518–527
Mittal A, Thakur V, Gajbe V (2012a) Adsorptive removal of toxic azo dye Amido Black 10B by hen feather. Environ Sci Pollut Res. doi:10.1007/s11356-012-0843-y
Mittal A, Thakur V, Gajbe V (2012b) Evaluation of adsorption characteristics of an anionic azo dye Brilliant Yellow onto hen feathers in aqueous solutions. Environ Sci Pollut Res. doi:10.1007/s11356-012-0756-9
Nakamoto K (1978) Infrared and raman spectroscopy of inorganic and coordination compounds. Wiley, New York
Namasivayam C, Jeyakumar R, Yamuna RT (1994) Dye removal from wastewater by adsorption on waste Fe(III)/Cr(III) hydroxide. Waste Manage 14(7):643–648
Netpradit S, Thiravetyan P, Towprayoon S (2003) Application of ‘waste’ metal hydroxide sludge for adsorption of azo reactive dyes. Water Res 37(4):763–772
Netpradit S, Thiravetyan P, Towprayoon S (2004) Adsorption of three azo reactive dyes by metal hydroxide sludge: effect of temperature, pH, and electrolytes. J Colloid Interface Sci 270(2):255–261
Ni ZM, Xia SJ, Wang LG, Xing FF, Pan GX (2007) Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: adsorption property and kinetic studies. J Colloid Interface Sci 316:284–291
O’Neill C, Hawkes FR, Hawkes DL, Esteves S, Wilcox SJ (2000) Anaerobic-aerobic biotreatment of simulated textile effluent containing varied ratios of starch and azo dye. Water Res 34:2355–2361
Out EO, Westland AD (1991) Solvent extraction with sulphonic acids. Solvent Extr Ion Exch 9:875
Ozcan S, Erdem B, Özcan A (2004) Adsorption of acid Blue 193 from aqueous solutions onto Na-bentonite and DTMA-bentonite. J Colloid Interface Sci 280:44–54
Ozdemir Y, Dogan M, Alkan M (2006) Adsorption of cationic dyes from aqueous solutions by sepiolite. Microporous Mesoporous Mater 96:419–427
Packter A, Dhillon HS (1981) The dissolution and crystallisation of amphoteric metal hydroxides from sodium hydroxide solutions: ionic equilibria, crystalline phases and crystallisation mechanisms. Cryst Res Technol 16:567
Papić S, Koprivanac N, Božič AL, Meteš A (2004) Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigments 62:291–298
Pascuta P, Vladescu A, Borodi G, Culea E, Tetean R (2011) Structural and magnetic properties of zinc ferrite incorporated in amorphous matrix. Ceram Int 37:3343–3349
Raghu S, Ahmed Basha C (2007) Chemical or electrochemical techniques, followed by ion exchange, for recycle of textile dye wastewater. J Hazard Mater 149:324–330
Saiah FBD, Su BL, Bettahar N (2009) Nickel–iron layered double hydroxide (LDH): textural properties upon hydrothermal treatments and application on dye sorption. J Hazard Mater 165:206–217
Saleh TA, Gupta VK (2012) Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ Sci Pollut Res. doi:10.1007/s11356-011-0670-6
Santos SCR, Vilar VJP, Boaventura RAR (2008) Waste metal hydroxide sludge as adsorbent for a reactive dye. J Hazard Mater 153:999–1008
Sawhney R, Kumar A (2011) Congo Red (Azo dye) decolourization by local isolate VTII inhabiting dye effluent exposed soil. Int J Environ Sci 1(6):1261–1267
Sharma J, Janveja B (2008) A study on removal of Congo Red dye from the effluents of textile industry using rice husk carbon activated by steam. Rasayan J Chem 1(4):936–942
Staurt B (1996) Modern infrared spectroscopy. Wiley, West Sussex
Stumm W (1992) Chemistry of the solid–water interface. Wiley, New York
Swaminathan K, Sandhya S, Sophia AC, Pachhade K, Subrahmanyam YV (2003) Decolorization and degradation of H-acid and other dyes using ferrous-hydrogen peroxide system. Chemosphere 50:619–625
Vimonses V, Lei SM, Jin B, Chowd CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem Eng J 148:354–364
Wong YC, Szeto YS, Cheung WH, McKay G (2003) Equilibrium studies for acid dye adsorption onto chitosan. Langmuir 19:7888
You Y, Zhao H, Vance GF (2002) Adsorption of dicamba (3,6-dichloro-2-methoxy benzoic acid) in aqueous solution by calcined-layered double hydroxide. Appl Clay Sci 21:217–226
Zollinger H (1991) Color chemistry. Synthesis, properties and applications of organic dyes and pigments, 2nd revised edn. VCH, New York
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Attallah, M.F., Ahmed, I.M. & Hamed, M.M. Treatment of industrial wastewater containing Congo Red and Naphthol Green B using low-cost adsorbent. Environ Sci Pollut Res 20, 1106–1116 (2013). https://doi.org/10.1007/s11356-012-0947-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0947-4