Abstract
Environmental heterogeneity contributes to various habitats and may influence the diversity and activity patterns of wildlife among habitats. We used camera traps to assess wildlife habitat use in Guanyinshan Nature Reserve from 2009 to 2012. We focused on four types of habitat including open areas with gentle slope (<15°) (Type1), low elevation areas (about 1500–1700 m) with high bamboo coverage (Type2), high elevation areas (about 2100–2300 m) with high canopy coverage (Type3), and wildlife migration passages (Type4). We analyzed the differences in species richness, relative abundance index (RAI), species diversity, and animals’ activity pattern among habitats. Total six species were analyzed on activity pattern, which are Takin (Budorcas taxicolor), tufted deer (Elaphodus cephalophus), Himalayan goral (Naemorhedus goral), wild boar (Sus scrofa), golden pheasant (Chrysolophus pictus), and porcupine (Hystrix hodgsoni). The results are (1) that there were significant differences in richness and RAIt among habitats; (2) Type4 habitat had the highest richness and RAIt while Type2 had the highest species diversity; giant pandas were found in these two habitats; (3) there were significant differences in species’ activity during daytime and nighttime; and (4) differences appeared in habitat preference of the most abundant species. Takin and tufted deer preferred Type1, Himalayan goral preferred Type2, and golden pheasant preferred Type3. Type4 habitat was used by most animals. All these revealed that habitat heterogeneity plays an important role in species diversity and the importance for conservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Habitat is composed of different variables that would influence the allocation of time and energy by an individual (Morris 1987; Jorgensen 2004; Liu et al. 2015; McCall et al. 2016). Habitat heterogeneity supports variation (Kotler and Brown 1988) and may influence differences in diversity and activity patterns of wildlife among these habitats. Leviten and Kohn (1980) proved that habitat heterogeneity is an important determinant of abundance and number of species. The availability of suitable habitats might determine abundance of species on a local scale (Yahner 1982). Further, it is clear that habitat partition and preference can explain the coexistence of sympatric species (Manson and Stiles 1998; Palmer 2003; Jorgensen 2004).
Many studies have investigated differences in habitat use in a range of taxa, for example, on small mammals (Jorgensen et al. 1995; Liang and Li 2004; Rocha et al. 2011), birds (Hansen et al. 1995; Estrada et al. 2000), bats (Estrada and Coates-Estrada 2002; Medina et al. 2007), beetles (Harvey et al. 2006), and some larger animals (Wei et al. 2000; Zhang et al. 2004). Jorgensen (2004) explored the difficulties in researching habitat use of small mammals by reviewing and tabulating data from 70 studies, and he pointed out that the limitations such as species, plots, and trapping efforts should be taken into account. Christian et al. (1997) assessed bird and mammal abundance, species richness, and species composition on plantations and four other types of habitats in the north central USA. Harvey et al. (2006) analyzed animal diversity associated with different forms of tree cover for birds, bats, butterflies, and dung beetles in a pastoral landscape in Nicaragua. Medina et al. (2007) continued this study and discussed movement patterns of bats in Nicaragua. Viewed from these studies, the characteristics of habitat could contribute to difference in diversity and analyzing diversity among habitats is an important way to study habitat use.
However, studying habitat use of various animals is often a difficult task, and people have been trying trapping methods for various species. Trapping provides a classic means to obtain capture data on small animals and has been used for decades (Rendigs et al. 2003; Borchers 2012). For example, Yahner (1986) used Tomahawk live traps to analyze habitat partitioning by small mammals and explored relationships with habitat variables through stepwise multiple regression analyses. Douglass (1989) discussed habitat selection by deer mice (Peromyscus maniculatus) using live trapping and radio-telemetry in southwestern Montana. McShea and Gilles (1992) used live trapping and fluorescent powder tracking in Virginia for comparing the ability to quantify the foraging movements of white-footed mice (Peromyscus leucopus). Nevertheless, live-trapping, radio-telemetry, and powder tracking are highly time and labor costing and weather conditions can make it difficult to sustain a year-round effort. Especially for medium-to-large animals, it is much more difficult. Camera trapping, which is a non-invasive method for detecting wildlife activity, can provide photographic data for a broad range of species and has the advantage of long-term, non-stop data collection. It has become a mainstream tool in conservation and ecology (Rowcliffe and Carbone 2008) and is extremely useful when conditions preclude direct observation or efficient indirect surveys (Treves et al. 2010). Due to these advantages, camera trapping has been widely used to monitor wildlife diversity and activity patterns (Azlan and Sharma 2006; Wang et al. 2006; Liu et al. 2013).
Our study site, Guanyinshan Nature Reserve (GNR), was previously a timber logging bureau, and converted into nature reserve in 2002 primarily for conserving giant pandas. It harbors abundant species and high species diversity (Wu et al. 2012; Liu et al. 2013; Jia et al. 2014). However, little is known about the difference of wildlife diversity in various habitats in GNR. Based on camera trapping data from August 2009 to July 2012, we categorized four types of habitat and we hypothesized that the frequently occurred species, the degree of using habitat, and the activity pattern of animals are different in these four types of habitat. Therefore, the aims of our study are (1) to identify the dominant species in four types of habitats, (2) to assess the degree of difference on using four types of habitats, and (3) to analyze activity patterns of the most abundant species in four types of habitats.
Methods
Research area
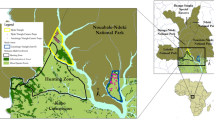
GNR was founded in 2002 with an area of 13,534 ha and is located in the central portion of the Qinling Mountains (107° 51′–108° 01′ E, 33° 35′–33° 45′ N) in the core of its nature reserve network (Fig. 1). The average annual temperature is 11.5 °C (ranging from −14.3 to 36.4 °C) and annual rainfall of 922.8 mm. The area is covered with three major forest types: conifer forest, mixed conifer-broadleaf forest, and deciduous broadleaf forest. The three major bamboo species are Bashania fargesii, Fargesia spathacea, and Fargesia dracocephala. The elevation ranges from 1150 to 2574 m.
According to the Third National Survey of giant pandas in China (SFA 2006), giant pandas were found in two small areas of GNR in recent years, namely the Liangfengya area and the Xigou area (shown in Fig. 1). Based on this survey, we selected these two areas (with a horizontal distance of about 6 km) as our study regions. Liangfengya area is at higher elevation (2100–2300 m) mainly characterized by mixed conifer-broadleaf forest, and Xigou area is at lower elevation (about 1500–1700 m) with deciduous broadleaf forest. GNR is adjacent to Foping Nature Reserve (FNR), which has 76 giant pandas (the highest population in Shaanxi) estimated from the Third National Survey on giant pandas in China (SFA 2006) and also a highest density in China (Wang et al. 2010). As GNR recovered from logging operations (1971–1998) and the population of giant panda in neighbor FNR increased, we assumed that giant pandas would migrate from neighbor FNR to GNR (Liu et al. 2009). The habitat quality of GNR could therefore impact the survival of giant pandas as well as other wildlife. According to the Fourth National Survey on giant pandas in China, a total population of about ten giant pandas was estimated in GNR according to the local government’s document (unpublished).
Camera trapping and habitat identifying
In this research, we discussed sympatric animal diversity and activity with camera traps with two branches of Quddaback and Reconyx. Camera trapping, which can minimize human disturbance on animals, is one of the non-invasive methods in wildlife monitoring. In August 2009 through July 2012, we set up eight camera traps to detect wildlife in eight different sites in two sensitive areas of GNR (Liangfengya and Xigou) for long-term wildlife monitoring (Fig. 2). In order to make two-type cameras working in an efficient way, we downloaded camera trapping data every 3 or 4 months. These eight sites contained four types of habitat based on four environmental variables, including canopy coverage, elevation, slope, and bamboo coverage, which are Type1 (open areas with gentle slope), Type2 (low elevation areas with high bamboo coverage), Type3 (high elevation areas with high canopy coverage), and Type4 (wildlife migration passages) (Table 1). We mounted cameras on the trees at approximately 80 cm above the ground and aimed the sensor parallel to the ground to cover the maximum area possible (Jenks et al. 2011).
After installation, camera data was collected at approximately 3-month intervals, depending on the status of battery and the weather conditions. We carefully examined each photo to identify species and record date and time of capture into an Excel spreadsheet. According to Kawanishi et al. (1999) and O’Brien et al. (2003), we removed repeating photos which can be clearly recognized as the same individual for further analysis. Moreover, we added a variable of season, with May to October representing the summer-autumn period and November to April representing the winter-spring period. We counted the number of species in each summer-autumn and winter-spring period and calculated the relative abundance index (shown in Table 2) for each species in each period.
Analysis of animal diversity and activity patterns
We used nine indices to reflect three aspects of animal using habitats: species diversity (Ahumada et al. 2011; Liu et al. 2014a), relative abundance index (Chen et al. 2016), and activity patterns (Liu et al. 2013; Liu et al. 2014b) shown in Table 2.
Species diversity analysis
In ecology, diversity is an index which combined species richness and evenness. So, we chose species richness (R), Shannon-Wiener diversity index (D), and Pilou evenness index (E) for analyzing species diversity and evenness. We also tried to find the dominant wildlife species in four types of habitat.
Relative abundance analysis
We used relative abundance index (RAI) to show the abundance of species in each type of habitat. Liu et al. (2013) had successfully put this method into practice. Here we introduced three types of RAI (namely, RAIs, RAIt, RAIw), and the meaning and calculation of them are shown in Table 2. RAIs and RAIw were calculated for each species while RAIt was for all species in each habitat. The difference of RAIs and RAIw was that the former was obtained when dividing the data with the variable season and the later was obtained for whole research years.
Activity pattern analysis
We made activity pattern analysis to look at how wildlife response to four types of habitat. We developed daytime relative abundance index (DRAI) and nighttime relative abundance index (NRAI) to compare the percentage of occurrence of species at daytime and nighttime. We considered 6:00–18:00 as the day period and the rest as the night period. Based on RAIw, we selected the three most abundant species for each habitat. Then we developed the time-period relative abundance index (TRAI) to show the daily activity patterns for the top 3 species in order to understand coexistence pattern of species in each type of habitat. We divided 24 h of a day into 12 periods (i.e., 0:00–2:00, 2:00–4:00, 4:00–6:00).
Significance test for R, RAIt, DRAI, and NRAI
For R, RAIt, DRAI, and NRAI, we adopted the Kruskal-Wallis method to test the differences among four habitat types and used the Mann-Whitney method to pair and test the variation between each of the two habitat pairs. Statistical analysis was performed in SPSS software, and the level of significance was set at α = 0.05 or α = 0.01.
Results
Difference in species diversity among four types of habitat
During 3 years, we collected a total of 1693 photos and identified 24 species (Table 3). Species richness in each type of habitat (R) was 15, 17, 14, and 22 respectively (Table 4). Low elevation areas with high bamboo coverage (Type2) had the highest value of diversity (D) at 2.21, and open areas with gentle slope (Type1) had the lowest diversity at 1.74. The peak evenness (E) was also obtained in low elevation areas with high bamboo coverage (Type2) while wildlife migration passages (Type4) had the lowest evenness.
Species richness was also calculated seasonally to show the seasonal influence on animal diversity across habitats. We found the richness changed with median values of 9.00 ± 0.89 (mean ± SE), 10.00 ± 1.32, 8.00 ± 0.58, and 12.67 ± 1.38 for four types of habitat, respectively (Fig. 3a). There was a significant difference on species richness among four types of habitat (Kruskal-Wallis: H = 8.508, df = 3, p = 0.037). The significant differences existed between Type1 (open areas with gentle slope) and Type4 (wildlife migration passages) habitats (p = 0.037) and also between Type3 (high elevation areas with high canopy coverage) and Type4 (wildlife migration passages) habitats (p = 0.006). Regardless of seasonal period, species richness in Type4 was always higher than other three types of habitat. Species richness for Type1, Type2, and Type4 was higher during the summer-autumn period (Fig. 3b) than the one during the winter-spring period (Fig. 3c).
Differences in species richness (R) in each type of habitat in Guanyinshan Nature Reserve. a Difference in species richness during all 3 years. b R of the summer-autumn period for each habitat type. c R of the winter-spring period for each habitat type. Type1 open areas with gentle slope, Type2 low elevation areas with high bamboo coverage, Type3 high elevation areas with high canopy coverage, Type4 wildlife migration passages. Single asterisk and double asterisks represent significance levels of 0.05 and 0.01, respectively
Difference in RAI among four types of habitat
While data divided by season, the relative abundance indices (RAIt) in each type of habitat for all 3 years were 4.21 ± 1.15, 3.40 ± 0.83, 2.57 ± 0.25, and 6.50 ± 0.78 respectively (Fig. 4a). There was a significant difference in seasonal RAI among four types of habitat (Kruskal-Wallis: H = 9.289, df = 3, p = 0.026). Significant differences were also found between Type2 (low elevation areas with high bamboo coverage) and Type4 (wildlife migration passages) (p = 0.025) and also between Type3 (high elevation areas with high canopy coverage) and Type4 (p = 0.004). For both periods, RAIt in Type4 was higher than that in other three types of habitat, and Type1, Type2, and Type4 all have higher RAIt in summer-autumn period (Fig. 4b) compared with the one in winter-spring period (Fig. 4c).
Difference in relative abundance index in each type of habitat in Guanyinshan Nature Reserve. a Difference in relative abundance index during all 3 years (RAIt). b RAIt of the summer-autumn period. c RAIt of the winter-spring period. Type1 open areas with gentle slope, Type2 low elevation areas with high bamboo coverage, Type3 high elevation areas with high canopy coverage, Type4 wildlife migration passages. Single asterisk and double asterisks represent significance levels of 0.05 and 0.01, respectively
Difference of species occurrence at daytime and nighttime among habitats
The daytime relative abundance index (DRAI) in each habitat was 3.29 ± 0.86, 1.94 ± 0.44, 1.72 ± 0.23, and 4.20 ± 0.51, respectively (Fig. 5a). There was a significant difference in DRAI among four habitats (Kruskal-Wallis: H = 10.384, df = 3, p = 0.016). The difference of DRAI was significant between Type2 (low elevation areas with high bamboo coverage) and Type4 (wildlife migration passages) (p = 0.016) and highly significant between Type3 (high elevation areas with high canopy coverage) and Type4 (p = 0.004).
Difference in species occurrences during daytime and nighttime in Guanyinshan Nature Reserve. a Difference in daytime relative abundance index (DRAI) during all 3 years. b Difference in nighttime relative abundance index (NRAI) during all 3 years. c Difference in species occurrence between DRAI and NRAI. Type1 open areas with gentle slope, Type2 low elevation areas with high bamboo coverage, Type3 high elevation areas with high canopy coverage, Type4 wildlife migration passages. Single asterisk and double asterisks represent significance levels of 0.05 and 0.01, respectively
The nighttime relative abundance index (NRAI) in each habitat was 0.92 ± 0.39, 1.46 ± 0.44, 0.85 ± 0.14, and 2.29 ± 0.34 respectively (Fig. 5b). There was a significant difference in NRAI among four habitats (Kruskal-Wallis: H = 8.069, df = 3, p = 0.045). The difference of NRAI was significant between Type1 (open areas with gentle slope) and Type4 (p = 0.045) and extremely significant between Type3 and Type4 (p = 0.006).
There also are highly significant differences in species occurrence between daytime and nighttime (p = 0.001) (Fig. 5c).
Habitat preference of high RAI species
The three species with the highest RAIw values in each habitat were different (Table 5). Ungulates were the most abundant species in three habitats except Type2 habitat (low elevation areas with high bamboo coverage). Giant panda, a key species of concern, was found only in Type2 and Type4 (wildlife migration passages) habitats. So totally, the six species with the highest RAIw values were analyzed further.
Figure 6 shows the habitat preference of these six species with the highest RAIw in four types of habitat. Takin preferred Type1 habitat (open areas with gentle slope) and Type4 habitat, and the relative abundance in Type4 was above 15%. Tufted deer accounted for the most in Type1 habitat. Himalayan goral and wild boar were abundant in Type2 and Type4 habitats. Golden pheasant preferred Type3 habitat (high elevation areas with high canopy coverage) while porcupine mostly used Type2.
Habitat preference of six species with the highest RAIw in four types of habitat in Guanyinshan Nature Reserve, Shaanxi, China. Type1 open areas with gentle slope, Type2 low elevation areas with high bamboo coverage, Type3 high elevation areas with high canopy coverage, Type4 wildlife migration passages
The three most abundant species in each of the four habitats all showed higher activity patterns during the day, except porcupine which had higher activity patterns at night (Fig. 7). Tufted deer and Himalayan goral had two activity peaks (6:00–10:00 and 16:00–20:00) while the peak TRAI of tufted deer was four times higher than that of Himalayan goral. Pheasant had the peak value at 12:00–14:00. For species that were the most abundant in more than one type, we found their activity peaks to be consistent across habitats.
Discussion
The relationship between species richness, evenness, and diversity
Diversity is a function of species richness and evenness and provides a means of combining the measures rather than just relying on richness for comparison (Magurran 1988; Wilsey and Potvin 2000). Cotgreave and Harvey (1994) pointed out that the evenness index shows the proportion of species within communities. It is possible that the diversity of habitat with fewer species and higher evenness was similar to that of habitat with more species and lower evenness (Fu et al. 2005). According to Magurran (2004), diversity index can be used to extract information from a species abundance distribution into a single statistic. Shannon-Wiener index is a popular diversity index used often in conservation field which integrates both species richness and evenness. In this research, we applied Shannon-Wiener diversity index, and our results gave evidence on incompletion of using only richness index. Type4 habitat (wildlife migration passages) showed the highest species richness and relative abundance index (RAIt), while Type2 habitat (low elevation areas with high bamboo coverage) had the highest diversity index as well as evenness index (see Table 4). Takin dominated Type4 habitat with a proportion of 48.79% of the captures and resulting in low evenness index value (0.62). Type2 habitat owed a middle-level richness but highest evenness which resulted in a highest diversity in this research. We could see the role played by species evenness.
Difference between evenness and our RAI
Generally, relative abundance means the proportion of species and ought to correspond to meaning of evenness (James and Rathbun 1981). Kirwan et al. (2007) also thought that evenness was a measure of the distribution of the relative abundance of species in a habitat.
It should be mentioned that the relative abundance index (RAI) in this paper was not the same as the one described by James and Rathbun (1981) and Kirwan et al. (2007). All kinds of RAI in this research were calculated using the number of species’ photo records in each habitat divided by the number of total photos records in all habitats. It revealed the difference of animal species occurring among the various types of habitat while the evenness index explained the distribution of species within each habitat. We have applied RAI in our previous camera tracking studies successfully, and the findings got published (Wu et al. 2012; Liu et al. 2013; Jia et al. 2014; Wang et al. 2015). RAI has also been widely accepted in China and applied by many wildlife researchers in their studies (Yu et al. 2013; Liu et al. 2014b; Zhang et al. 2015; Chen et al. 2016).
Indication of different species in each habitat
Our results support the ecological assumption that heterogeneity would form various types of habitat which are preferred by different species (Rodríguez-Pérez et al. 2012) and that relative abundance is a reflection of preference of species to different habitat (Price and Kramer 1984). Here we noted that Type4 habitat (wildlife migration passages) in our study represented a special habitat for wildlife to move for foraging and other activities. Takin seemed to prefer Type1 habitat (open areas with gentle slope) where they had the highest RAIw and RAIs. Especially the RAIw value was about three times higher compared to that in Type3 habitat (high elevation areas with high canopy coverage). We also observed that more tufted deer was detected in Type1 habitat, probably because open habitat might facilitate vigilance behavior and threat detection for deer (Zhang et al. 2004).
Many ecological factors can influence the wildlife activities, while food may be one of the most important factors influencing animals’ distribution and occurring. Wild boar preferred Type2 (low elevation areas with high bamboo coverage) and Type4 habitats. Wild boar is predominately an herbivore, and therefore, its distribution and occurring are likely related to the amount of plant material (Leaper et al. 1999). We assumed that these two types of habitat provided abundant food for wild boar. In our another paper published by Wang et al. (2015), we found that feeding was the dominant behavior of wild boar for the whole year, taking a proportion of over 40% of the total behavior. Giant pandas as a key species in GNR were only found in these Type2 and Type4 habitats. We attribute this primarily to two reasons: bamboo limits pandas’ distribution (Liu et al. 2005), and canopy conditions affect pandas’ activity. Giant pandas are preferable to habitat with moderate canopy (Hu 2001; Kang et al. 2011) as well as gentle slope. Yahner (1982) also stated that the availability of suitable habitats has a strong influence on abundance of various species, which was agreed by our results too.
Variance of activity pattern of high RAI species in each habitat
Our results showed that each habitat was used by many animal species which can be called sympatric species. However, we have not found two species occurring in the same photo among collected data by the paper writing time. Different activity patterns indicate coexistence and partitioning of species in different habitats (Fig. 7). The activity patterns in our study were in agreement with previous studies. We knew that takin, Himalayan goral, and tufted deer all demonstrated the similar activity patterns and in which activities were more frequent at dawn and dusk based on our dataset of 2-year detecting (Wu et al. 2012). Zeng and Song (2001) drew similar conclusion through studying daily active rhythm of four radio-collared takin in FNR. Takin usually avoided acting at the highest temperature in the afternoon (Chen et al. 2007). Fakhar-i-Abbas et al. (2011) indicated that Himalayan goral spent major part of the night sleeping and were active during the day in Pakistan, Azad Jammu and Kashmir. Porcupine is a typical nocturnal animal (Saltz and Alkon 1989; Brown and Alkon 1990), and we found a great proportion of their activity occurring in the night between 18:00 and 6:00.
Both geographical region and definition of research parameters may influence the animals’ activity pattern. Russo et al. (1997) analyzed 24-h activity of 17 radio-tracked wild boars between May and September in the Maremma Natural Park in Central Italy and concluded that wild boar was more active at night than at daytime. In our study, we obtained the different result on the activity pattern of wild boars which were more active in daytime than at night (see Fig. 7). There are two reasons possibly to explain this difference. One could be the geographical region’s impact on animal activity, such as Russo et al.’s study in central Italy and our study in central China. The other could be the definition on activity pattern given by researchers. For instance, Russo et al.’s study defined “active” as moving state and “inactive” as motionless state. However, in our research, we defined active by using the RAI during each time period (RAIt), which indicated the proportion of occurrences of wild boar at different time periods. For widely distributed species such as wild boar, variations of ecological facts (e.g., habitat quality, food resource, ambient temperature, and predator risk) and human activities in different distribution areas may result in different activity patterns too.
Furthermore, the activities of animals alternatively go up to peak value at different time periods, which is an effective way to avoid direct competition by sympatric animals to make good use of resources (Dai et al. 2001). Habitat partitioning is not only the means for coexistence of wildlife, but also is the result of competition and adaptability of various species (Dai et al. 1998). This can explain very well why we hardly obtained the camera tracking photos with two species occurring at the same time.
Conclusion and implication
There exists significant difference in the number of species and RAI among four types of habitat, and the characteristics of each habitat strongly influence species activity patterns. Type4 habitat (wildlife migration passages) had the most species while Type2 habitat (low elevation areas with high bamboo coverage) had the highest species diversity and evenness among the habitats. The top 3 species in each habitat with highest RAI were different, and activity pattern showed their preferences for different habitat. For the conservation management in the future, we should give more attention on those areas with higher diversity and enhance the habitat quality as possible, attempting to maintain more sympatric species and promote their coexistence.
References
Ahumada JA, Silva CEF, Gajapersad K, Hallam C, Hurtado J, Martin E, McWilliam A, Mugerwa B, O’Brien T, Rovero F, Sheil D, Spironello WR, Winarni N, Andelman SJ (2011) Community structure and diversity of tropical forest mammals: data from a global camera trap network. Phil Trans R Soc B 366:2703–2711
Azlan JM, Sharma DSK (2006) The diversity and activity patterns of wild felids in a secondary forest in Peninsular Malaysia. Oryx 40:36–41
Borchers D (2012) A non-technical overview of spatially explicit capture-recapture models. J Ornithol 152:435–444
Brown J, Alkon P (1990) Testing values of crested porcupine habitats by experimental food patches. Oecologia 83:512–518
Chen W, Shen Q, Ma QY, Pan GL, Lei CZ (2007) Diurnal activity rhythms and time budgets of captive Qinling golden takin (Budorcas taxicolor bedfordi) in the Qinling Mountains, Shaanxi, China. J For Res 18:149–152
Chen YX, Xiao ZS, Li M, Wang XW, He CX, He GP, Li HS, Shi SJ, Xiang ZF (2016) Preliminary survey for the biodiversity of mammal and bird using camera traps in the west slope of mid-section Mt. Gaoligong. Acta Theriologica Sinica 36:302–312 (in Chinese)
Christian DP, Collins PT, Hanowski JM, Niemi GJ (1997) Bird and small mammal use of short-rotation hybrid poplar plantations. J Wildlife Manage 61:171–182
Cotgreave P, Harvey PH (1994) Evenness of abundance in bird communities. J Anim Ecol 63:365–374
Dai K, Yao J, Hu DF (1998) Microhabitat preference of desert rodent in southern Dzungaria Basin. Arid Zone Research 15:34–37 (in Chinese)
Dai K, Pan WS, Zhong WQ (2001) The desert rodent community patterns. Arid Zone Research 18:1–7 (in Chinese)
Douglass RJ (1989) The use of radio-telemetry to evaluate microhabitat selection by deer mice. J Mammal 70:648–652
Estrada A, Coates-Estrada R (2002) Bats in continuous forest, forest fragments and in an agricultural mosaic habitat-island at Los Tuxtlas, Mexico. Biol Conserv 103:237–245
Estrada A, Cammarano P, Coates-Estrada R (2000) Bird species richness in vegetation fences and in strips of residual rain forest vegetation at Los Tuxtlas, Mexico. Biodivers Conserv 9:1399–1416
Fakhar-i-Abbas S, Akhtar T, Mian A (2011) Time budgets and ethological observations of wild and enclosed grey goral. Wildl Biol Pract 7:23–31
Fu HP, Wu XD, Yang ZL (2005) Diversity of small mammals communities at differenct habitats in Alashan region, Inner Mongolia. Acta Theriologica Sinica 25:32–38 (in Chinese)
Hansen AJ, McComb WC, Vega R, Raphael MG, Hunter M (1995) Bird habitat relationships in natural and managed forests in the west cascades of Oregon. Ecol Appl 5:555–569
Harvey CA, Medina A, Sánchez DM, Vílchez S, Hernández B, Saenz JC, Maes JM, Casanoves F, Sinclair FL (2006) Patterns of animal diversity in different forms of tree cover in agricultural landscapes. Ecol Appl 16:1986–1999
Hu JC (2001) The study of giant panda. Shanghai Scientific and Technological Education Press, Shanghai (in Chinese)
James FC, Rathbun S (1981) Rarefaction, relative abundance, and diversity of avian communities. Auk 98:785–800
Jenks KE, Chanteap P, Damrongchainarong K, Cutter P, Cutter P, Redford T, Lynam AJ, Howard J, Leimgruber P (2011) Using relative abundance indices from camera-trapping to test wildlife conservation hypotheses-an example from Khao Yai National Park, Thailand. Trop Conserv Sci 4:113–131
Jia XD, Liu XH, Yang XZ, Wu PF, Songer M, Cai Q, He X, Zhu Y (2014) Seasonal activity patterns of ungulates in Qinling Mountains based on camera-trap data. Bio Sci 22:737–745 (in Chinese)
Jorgensen EE (2004) Small mammal use of microhabitat reviewed. J Mammal 85:531–539
Jorgensen EE, Demarais S, Neff S (1995) Rodent use of microhabitat patches in desert arroyos. Am Midl Nat 134:193–199
Kang DW, Kang W, Tan LY, Li JQ (2011) The habitat selection of giant panda in Wanglang Nature Reserve, Sichuan Province, China. Acta Ecol Sin 31:401–409 (in Chinese)
Kawanishi K, Sahak AM, Sunquist M (1999) Preliminary analysis on abundance of large mammals at Sungai Relau, Taman Negara. Journal of Wildlife and Parks 17:62–82
Kirwan L, Lüscher A, Sebastia MT, Finn JA, Collins RP, Porqueddu C, Helgadottir A, Baadshaug OH, Brophy C, Coran C, Dalmannsdottir S, Delgado I, Elgersma A, Fothergill M, Frankow-Lindberg BE, Golinski P, Grieu P, Gustavsson AM, Hoglind M, Huguenin-Elie O, Iliadis C, Jorgensen M, Kadziuliene Z, Karyotis T, Lunnan T, Malengier M, Maltoni S, Meyer V, Nyfeler D, Nykanen-Kurki P, Parente J, Smit HJ, Thumm U, Connolly J (2007) Evenness drives consistent diversity effects in intensive grassland systems across 28 European sites. J Ecol 95:530–539
Kotler B, Brown J (1988) Environmental heterogeneity and the coexistence of desert rodents. Annu Rev Ecol Syst 19:281–307
Leaper R, Massei G, Gorman ML, Aspinall R (1999) The feasibility of reintroducing wild boar (Sus scrofa) to Scotland. Mammal Rev 29:239–259
Leviten PJ, Kohn AJ (1980) Microhabitat resource use, activity patterns, and episodic catastrophe: conus on tropical intertidal reef rock benches. Ecol Monogr 50:55–75
Liang J, Li JG (2004) Seasonal fluctuations of the small mammals community in plantation of Yan’an. Acta Theriologica Sinica 24:322–328 (in Chinese)
Liu XH, Toxopeus AG, Skidmore AK, Shao XM, Dang GD, Wang TJ, Prins HHT (2005) Giant panda habitat selection in Foping Nature Reserve, China. J Wildlife Manage 69:1623–1632
Liu XH, Shao XM, He XB, Tian RX, Zhu Y (2009) Giant panda habitat dynamics in a recovering nature reserve. In: Proceeding of the 23rd Annual Meeting & 2009 International Congress for Conservation Biology. Beijing, pp 299–300
Liu XH, Wu PF, Songer M, Cai Q, He XB, Zhu Y, Shao XM (2013) Monitoring wildlife abundance and diversity with infra-red camera traps in Guanyinshan Nature Reserve of Shaanxi Province, China. Ecol Indic 33:121–128
Liu F, Su XJ, Li DQ, Wang BZ, Zhang ZL (2014a) Using camera trap to investigate animal diversity in Hunan Gaowangjie National Nature Reserve. Biodivers Sci 22:779–784
Liu YC, Huang XW, Chu HJ, Liu DZ, Zhang F, Chen G, Qi YJ (2014b) Camera trap survey of wildlife in Buergen Beaver National Nature Reserve, Xinjiang. Biodivers Sci 22:800–803 (in Chinese)
Liu XH, Wang TJ, Wang T, Skidmore AK, Songer M (2015) How do two giant panda populations adapt to their habitats in the Qinling and Qionglai Mountains, China. Environ Sci Pollut Res 22:1175–1185
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Magurran AE (2004) Measuring biological diversity. Afr J Aquat Sci 29:285–286
Manson RH, Stiles EW (1998) Links between microhabitat preferences and seed predation by small mammals in old fields. Oikos 82:37–50
McCall AG, Pilfold NW, Derocher AE, Lunn NJ (2016) Seasonal habitat selection by adult female polar bears in western Hudson Bay. Popul Ecol 58:407–419
McShea WJ, Gilles AB (1992) A comparison of traps and fluorescent powder to describe foraging for mast by Peromyscus leucopus. J Mammal 73:218–222
Medina A, Harvey CA, Merlo DS, Vílchez S, Hernández B (2007) Bat diversity and movement in an agricultural landscape in Matiguás, Nicaragua. Biotropica 39:120–128
Morris DW (1987) Ecological scale and habitat use. Ecology 68:362–369
O’Brien TG, Kinnaird MF, Wibisono HT (2003) Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6:131–139
Palmer TM (2003) Spatial habitat heterogeneity influences competition and coexistence in an African acacia and guild. Ecology 84:2843–2855
Price MV, Kramer KA (1984) On measuring microhabitat affinities with special reference to small mammals. Oikos 42:349–354
Rendigs A, Radespiel U, Wrogemann D, Zimmermann E (2003) Relationship between microhabitat structure and distribution of mouse lemurs (Microcebus spp.) in northwestern Madagascar. Int J Primatol 24:47–64
Rocha MF, Passamani M, Louzada J (2011) A small mammal community in a forest fragment, vegetation corridor and coffee matrix system in the brazilian atlantic forest. PLoS One 6:1–8
Rodríguez-Pérez J, Larrinaga AR, Santamaria L (2012) Effects of frugivore preferences and habitat heterogeneity on seed rain: a multi-scale analysis. PLoS One 7:1–9
Rowcliffe JM, Carbone C (2008) Surveys using camera traps: are we looking to a brighter future? Anim Conserv 11:185–186
Russo L, Massei G, Genov PV (1997) Daily home range and activity of wild boar in a Mediterranean area free from hunting. Ethol Ecol Evol 9:287–294
Saltz D, Alkon P (1989) On the spatial behavior of Indian crested porcupines (Hystrix indica). J Zool 217:255–266
SFA (State Forest Administration) (2006) The third national survey report on the giant panda in China. Science Press Beijing, China
Treves A, Mwima P, Plumptre AJ, Isoke S (2010) Camera-trapping forest-woodland wildlife of western Uganda reveals how gregariousness biases estimates of relative abundance and distribution. Biol Conserv 143:521–528
Wang DJ, Li S, McShea WJ, Li MF (2006) Use of remote-trip cameras for wildlife surveys and evaluating the effectiveness of conservation activities at a nature reserve in Sichuan Province, China. Environ Manag 38:942–951
Wang TJ, Skidmore AK, Zeng ZG, Beck PSA, Si YL, Song YL, Liu XH, Prins HHT (2010) Migration pattern of two endangered sympatric species from a remote sensing perspective. Photogramm Eng Rem S 76:1343–1352
Wang CP, Liu XH, Wu PF, Cai Q, Shao XM, Zhu Y, Songer M (2015) Research on behavior and abundance of wild boar (Sus scrofa) via infra-red camera in Guanyinshan Nature Reserve in Qinling Mountains, China. Acta Theriologica Sinica 35:147–156 (in Chinese)
Wei FW, Feng ZJ, Wang ZW, Hu JC (2000) Habitat use and separation between the giant panda and the red panda. J Mammal 81:448–455
Wilsey BJ, Potvin C (2000) Biodiversity and ecosystem functioning: importance of species evenness in an old field. Ecology 81:887–892
Wu PF, Liu XH, Cai Q, He XB, Songer M, Zhu Y, Shao XM (2012) The application of infrared camera in mammal research in Guanyinshan Nature Reserve, Shaanxi. Acta Theriologica Sinica 32:67–71 (in Chinese)
Yahner RH (1982) Microhabitat use by small mammals in farmstead shelterbelts. J Mammal 63:440–445
Yahner RH (1986) Microhabitat use by small mammals in even-aged forest stands. Am Midl Nat 115:174–180
Yu LG, Chen MJ, Yang SJ, Li XY, Shi L (2013) Camera trapping survey of Nyticebus pygmaeus, Nyticebus coucang and other sympatric mammals at Dawei Mountain, Yunnan. Sichuan Journal of Zoology 32:814–818 (in Chinese)
Zeng ZG, Song YL (2001) Daily activity rhythm and time budget of golden takin in spring and summer. Acta Theriologica Sinica 21:7–13 (in Chinese)
Zhang ZJ, Wei FW, Li M, Zhang BW, Liu XH, Hu JC (2004) Microhabitat separation during winter among sympatric giant pandas, red pandas, and tufted deer: the effects of diet, body size, and energy metabolism. Can J Zool 82:1451–1458
Zhang JD, Li YJ, Li RG (2015) Application of infrared camera technology in studies of mammal activity patterns. Sichuan Journal of Zoology 34:671–676 (in Chinese)
Acknowledgements
This research received partial support from the project of National Natural Science Foundation of China: “Influences of Forest Landscape Pattern on Animal Diversity and Behavior Characteristics under Disturbance in the Qinling Mountains” (41271194). We acknowledge staff members from Guanyinshan Nature Reserve and Foping Nature Reserve for their help in the field work and other supports. And we would like to thank Smithsonian Conservation Biology Institute and Friends of the National Zoo (FONZ), USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Liu, X., Wu, P., Shao, X. et al. Diversity and activity patterns of sympatric animals among four types of forest habitat in Guanyinshan Nature Reserve in the Qinling Mountains, China. Environ Sci Pollut Res 24, 16465–16477 (2017). https://doi.org/10.1007/s11356-017-9232-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9232-x