Abstract
The waste tyre and waste cooking oils have a great potential to be used as alternative fuels for diesel engines. The aim of this study was to convert light fractions of pyrolysis oil derived from Pakistani waste vehicle tyres and waste soybean oil methyl esters into valuable fuel and to reduce waste disposal-associated environmental problems. In this study, the waste tyre pyrolysis liquid (light fraction) was collected from commercial tyre pyrolysis plant and biodiesel was prepared from waste soybean oil. The fuel blends (FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50) were prepared from a 30:70 mixture of waste tyre pyrolysis liquid and waste soybean oil methyl esters with different proportions of mineral diesel. The mixture was named as the fuel mixture of waste oils (FMWO). FT-IR analysis of the fuel mixture was carried out using ALPHA FT-IR spectrometer. Experimental investigations on a diesel engine were carried out with various FMWO blends. It was observed that the engine fuel consumption was marginally increased and brake thermal efficiency was marginally decreased with FMWO fuel blends. FMWO10 has shown lowest NOx emissions among all the fuel blends tested. In addition, HC, CO and smoke emissions were noticeably decreased by 3.1–15.6%, 16.5–33.2%, and 1.8–4.5%, respectively, in comparison to diesel fuel, thereby qualifying the blends to be used as alternative fuel for diesel engines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since last two decades, the world is facing shortage of crude oil supply because its price has been increased to many folds. Fast population growth, prompt industrialization, global warming threats due to pollutant emissions, high costs and rapid depletion of fossil fuels have compelled the researchers to explore cheap and feasible alternative fuel from various resources (Yoon et al. 2010; Qasim et al. 2017; Li et al. 2015; Danesan 2007).
Globally, about 1.5 billion tyres are being produced every year which would consequently become waste tyres (USEPA 2014). To manage these wastes in order to get healthy and clean environment is a hot issue nowadays (Islam et al. 2008). The stockpiles of waste tyres are very difficult to ignite but once ignited, tyres burn too hot to extinguish the fire resulting in sufficient release of air pollutant emissions which bitterly harm the public health by contaminating surrounding atmosphere (Alsaleh and Sattler 2014).
Decomposition products of the tyre pyrolysis feedstock are pyrolysis oil, char and gas (Martínez et al. 2013). The produced pyrolysis oil may be upgraded to a higher quality oil and may be used as fuel. The produced gases comprise hydrogen and carbons (C1–C4) having high heating value and the gases might be recycled as a fuel in the pyrolysis process. The solid char is generally black carbon used as filler (Williams 2013).
In developing countries, from environmental perspectives, there is a disposal problem of solid tyre waste because of its nondegradability and complex structure containing steel cord, carbon black, and other complicated chemical compounds. Commonly, waste tyres are disposed in land filling but this is not an environmentally allowable option (Rodriguez et al. 2001). The waste disposal is a worldwide unsolved issue. By adopting appropriate methods, a major part of the wastes can be converted into useful energy. Since last three decades, efforts have been made by many researchers by developing various methods of pyrolysis such as flash pyrolysis (Edwin Raj et al. 2013), vacuum pyrolysis (Lopez et al. 2010; Zhang et al. 2008; Benallal et al. 1995; pakdel et al. 2001), steam pyrolysis (Kaminsky and Mennerich 2001), fluidised bed pyrolysis (Kaminsky et al. 2009; Kalitko, 2010) and catalytic pyrolysis (Dung et al. 2009a, 2009b, 2009c and 2010; Witpathomwong et al. 2011). Literature reports various operating parameters like pyrolysis temperature (Williams et al. 1990; Mastral et al. 2000; Laresgoiti et al. 2004; Murillo et al. 2006), heating rate (Rombaldo et al. 2008; Unapumnuk et al. 2006 and 2008) and characterisation of pyrolysis oil (Islam et al. 2003). Various scientists have studied diesel engine behavior for the sake of alternative, renewable and sustainable energy (Murugan et al. 2008; Dogan et al. 2012; Hariharan et al. 2013; Bhatt and Patel 2012a, b; Wongkhorsub and Chindaprasert 2013; Naima and Liazid 2013).
Waste cooking oil (WCO) is a vegetable oil obtained after frying or cooking food items. The edible vegetable oil becomes unsuitable for consumption after repeated frying for preparation of food (Nantha et al. 2014). WCO has also many disposal problems like water and soil pollution, human health concern and disturbance to the aquatic ecosystem. The WCO may be effectively used for biodiesel production instead of its harmful environmental disposal options (Wanodya and Arief 2013; Anildo et al. 2013). The collected WCO might be used to prepare soap and additives for engine oils (Maurizio et al. 2014). Successful conversion of WCO into biodiesel has been documented by many researchers throughout the world (Nabanita et al. 2014). Conversion of WCO into biodiesel contributes to lower greenhouse-gas emissions in comparison to conventional diesel (Chang et al. 1996). Many scientists have recommended the suitability of veggie oil and its derivatives to be utilized as fuel or as effective fuel additive (Lapuerta et al. 2005 and 2003; Ramadhas et al. 2004 and 2005).
The aim of this study was to carry out experimental investigations on the fuel blends obtained from waste tyre oil and waste soybean oil biodiesel-diesel mixtures in a diesel engine to assess the suitability of the fuel blends to be utilized as conventional diesel fuel substitute for diesel engines. Various physicochemical characteristics were measured and compared with diesel and biodiesel fuel standard specifications. FT-IR analysis was carried out using ALPHA FT-IR spectrometer. Different performance and emission parameters of the engine were studied.
Experimental
Pyrolysis process of waste tyres
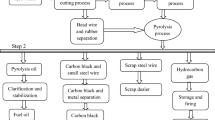
Thermal degradation of a substance into smaller and less complex molecules in the absence of oxygen is known as pyrolysis process. In pyrolysis process, three major products are produced such as gas, char and pyrolytic oil. In this study, light fraction of waste tyre pyrolysis oil was collected from a commercial pyrolysis plant located at Muzaffargarh industrial area, near Multan, Pakistan. The schematic flow diagram of the plant setup is given in Fig. 1.
After removal of steel wires and beads, the waste tyre chips are fed into the reactor unit to decompose hydrocarbon compounds at high temperature (460–660 °C) under oxygen-free atmosphere. The process makes the restructuring of rubber and conversts it into vapours and gases which pass through the separator where heavy oil is separated out from gases and the vapours of the light oil fractions are condensed into liquid after passing through the condenser yielding 42–46% tyre pyrolysis oil with 9–10% noncondensable gases which are recycled again and used for heating the reactor.
FT-IR analysis
The FT-IR analysis was carried out for fuel mixture of waste oils (FMWO) and diesel samples using ALPHA FT-IR spectrometer, Bruker Optics, Germany, having a resolution of 1.0 cm−1and scan range of 450–4000 cm−1. FTIR analysis confirms that the functional groups present in FMWO sample are almost alkanes (hydrocarbons) and halides. Comparable infrared absorption spectrums were observed for FMWO and diesel as shown in Figs. 2 and 3. The frequencies of the absorption spectrums are given in Table 1. Similar type of work has been reported by Kapura et al. (2014) and Bhatt and Patel (2012a, b).
Engine setup
The experiments were conducted in a stationary 5.5-kW, water-cooled, single-cylinder, four-stroke, naturally aspirated (NA) compression-ignition engine. The schematic diagram of the engine is given in Fig. 4. The engine had a stroke and bore of 110 and 87.5 mm, respectively. The compression ratio of the engine was 17.5:1.The fuel injection timing set by the manufacturer was 23° bTDC. An electronic weighing scale was used to measure the fuel flow rates. The outer temperature of the exhaust gas was measured directly from the thermocouples. AVL DiGas 444 exhaust gas analyzer was used to measure exhaust gas emissions like nitric oxide (NO), carbon monoxide (CO) and hydrocarbon (HC). An AVL 437 smoke meter was used to measure smoke opacity.
Calibration of each analyzer was performed before each test. In order to obtain the reference data, the engine was run initially with diesel for no load, 20%, 40%, 60%, 80%, and full load. Similarly, the engine was again run with diesel after conducting all tests with blends so as to clean the traces of different blends. The technical specifications of the engine are listed in Table 2.
Samples description
Two liters of waste cooking oil were collected from M/S Lasani Food Restaurant, Multan, Pakistan. The waste cooking oil description was enquired from the restaurant managers and was known to be waste soybean oil. The waste soybean oil was converted into biodiesel as explained in the “Production of soybean oil methyl esters (biodiesel)” section. The light fraction of waste tyre pyrolysis oil (WTPO) was collected from a commercial pyrolysis plant located at Muzzaffar Garh near Multan, Pakistan, and was mixed with biodiesel (waste soybean oil methyl ester) in a ratio of 30:70 to form a fuel blend named as fuel mixture of waste oils (FMWO). The FMWO was further mixed with petroleum diesel in different proportions as shown in Table 3.
Production of soybean oil methyl esters (biodiesel)
The waste cooking oil was subjected to base-catalysed transesterification reaction using potassium hydroxide (KOH) as catalyst. The waste cooking oil was pretreated and filtered so as to remove coagulation, moisture contents and suspended particles. This pretreated waste cooking oil was stored for further reaction. Sodium methoxide was prepared by dissolving 1% sodium hydroxide (NaOH) in methyl alcohol (CH3OH). The sodium methoxide solution was added to the pretreated waste cooking oil and the solution mixture was stirred for 1 h at 60 °C and then cooled and left overnight to settle under gravity. Two layers were formed; the upper biodiesel layer was separated out from lower glycerol layer and washed with water for purification. The biodiesel so produced was heated at 110 °C to remove moisture contents. After cooling, the biodiesel was blended with WTPO and petroleum diesel for further characterization.
Results and discussions
Physicochemical properties
The fuel blends FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 were analyzed for physicochemical properties as shown in Table 4. The obtained results of the fuel blends were compared with international fuel standards of diesel (ASTM 975) and biodiesel (ASTM 6751 and EN14214). It was observed that all the measured values of the fuel blends were found within the specified limits of above-mentioned standard fuel specifications.
Performance
Brake specific fuel consumption (BSFC) is a very important factor to compare the fuels having different densities and heating values (Agrawal 2007). Figure 5 shows the variations of the BSFC for diesel and FMWO blends. The BSFC for diesel was 343.25 g/kWh at 100% load and it was 344.63, 350.12, 362.30, 367.64 and 370.25 g/kWh for the FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50, respectively, at full-load conditions. BSFC was found to decrease as the engine load was increased. FMWO10 has shown lowest BSFC among all the blends measured. FMWO10 has shown comparable BSFC value to that of diesel due to better combustion and higher heating values in comparison to other fuel blends. The FMWO40 and FMWO50 have shown higher fuel consumption values due to lower heating values.
Brake thermal efficiency (BTE) of FMWO blends was compared with diesel fuel as shown in Fig. 6. BTE of the engine at full load was found at 29.61% for diesel and 29.24, 29.12, 28.67.16, 28.90 and 28.92% for FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 correspondingly at full load. The BTE of the fuel blends was found to be lower than diesel. This may be due to the lower calorific value of the fuel blends. The FMWO10 has shown better performance in comparison to all other fuels analyzed. The increase in BTE value for FMWO10 in comparison to other fuel blends night be attributed to its lower viscosity and better fuel atomisation.
The exhaust gas temperature (EGT) is a good indicator for amount of heat lost with exhaust gases (Ganesan 2007). Variations of EGT with respect to engine load percent are shown in Fig. 7. The EGT was found at 352, 377, 385, 376, 368 and 379 °C for diesel, and FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 correspondingly at full load. The figure shows that the EGT for all fuel blends was increased as the engine load was increased; however, the EGT of diesel was found lower than FMWO blends. This indicates that the combustion of FMWO blends might be delayed due to higher densities and viscosities.
Emissions
The hydrocarbons (HC) emissions are created as a result of incomplete combustion inside the combustion chamber, variations in the equivalence ratio and deposits on the internal combustion chamber walls (Ganesan 2007). The variations of HC emissions with engine load percent for diesel and FMWO blends are plotted in Fig. 8. It was found that the HC emissions were found to decrease with increase in engine load. The HC emissions for diesel, FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 were observed at 0.032, 0.030, 0.028, 0.029, 0.031, 0.027 g/kWh, respectively, at full load. The FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 blends have shown 6.25, 12.5, 9.37, 3.12 and 15.62% lower emissions than diesel. This indicates more complete combustions of the studied fuel blends.
Figure 9 shows carbon monoxide (CO) emissions for diesel and FMWO blends. The CO emission for diesel, FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 was found at 0.012, 0.010, 0.0080, 0.0092, 0.0086 and 0.0084, respectively, at full-load conditions. The CO emissions were found to decrease from no load to 100% load. All the FMWO blends have shown 16.5–33.2% lower CO emissions in comparison to diesel. This indicates complete mixing and complete combustion of the fuel blends.
The availability of oxygen, combustion duration, temperature, pressure and higher compression ratio are the factors which affect nitric oxide emissions (Murugan et al. 2008). The variations of NOx emissions with engine load for diesel and FMWO are plotted in Fig. 10. The CO emissions for diesel, FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 were observed at 5.41, 5.62, 5.72, 5.70, 5.71 and 5.72 g/kWh, respectively, at maximum load conditions. NOx emissions were found to increase as the engine load was increased. All the FMWO fuel blends have shown marginally higher NOx emissions as compared to diesel might be due to higher oxygen contents in the biodiesel portion of the blends.
Figure 11 depicts the smoke emissions of FMWO fuel blends and diesel. Generally, smoke opacity occurs due to the incomplete combustion inside the combustion chamber of the engine (Murugan et al. 2008). The smoke emissions for diesel, FMWO10, FMWO20, FMWO30, FMWO40 and FMWO50 were found at 58.21, 55.56, 57.14%, 55.23, 56.02 and 55.33%, respectively, at full load. Smoke emissions were found to increase as the engine load was increased. Lower smoke emissions were noticed for FMWO blends as compared to diesel. This indicates the better combustion of the fuel blends in the combustion chamber of the engine.
Conclusion
In present study, FMWO fuel blends obtained from a mixture of waste tyre oil and waste soybean oil biodiesel with various proportions of petroleum diesel were investigated in a diesel engine for emission and performance parameters. The physicochemical properties were measured by using ASTM standards. On the basis of analysis results, the important conclusions are as follows;
-
All the physicochemical properties of the FMWO fuel blends were found within the international fuel standards of diesel (ASTM 975) and biodiesel (ASTM 6751 and EN14214).
-
The BSFC of FMWO fuel blends was decreased with increase in the engine load. In comparison to diesel fuel, the BSFC of all the fuel blends was marginally increased. FMWO10 has shown lowest BSFC (344.63 g/kWh) and FMWO50 has shown highest BSFC (367.64 g/kWh) among all the fuel blends analyzed.
-
All the tested fuel blends exhibited lower BTE value than diesel; however, FMWO10 has shown higher BTE (29.24%) than other FMWO fuel blends.
-
3.12–15.62% and 16.5–33.2% lower CO and HC emissions were observed for overall FMWO fuel blends in comparison to diesel fuel. Decrease in smoke emissions was noticed to be 1.83–4.5% for FMWO blends in comparison to that of diesel.
-
The overall NOx emissions were increased as the engine load was increased; however, FMWO10 has shown less NOx emissions among all the FMWO fuel blends.
-
The engine was successfully operated with all FMWO blends without engine failure but the performance of FMWO10 was found outclass among all the fuel blends.
Abbreviations
- WTPO:
-
Waste tyre pyrolysis oil
- WSOME:
-
Waste soybean oil methyl esters
- FMWO:
-
Fuel mixture of waste oils (30% WTPO plus 70% WSOME)
- FMWO10:
-
10% FMWO and 90 petroleum diesel
- FMWO20:
-
20% FMWO and 80% petroleum diesel
- FMWO30:
-
30% FMWO and 70% petroleum diesel
- FMWO40:
-
40% FMWO and 60% petroleum diesel
- FMWO50:
-
50% FMWO and 50% petroleum diesel
- BTE:
-
Brake thermal efficiency
- BSFC:
-
Brake specific fuel consumption
- EGT:
-
Exhaust gas temperature
- CO:
-
Carbon monoxide
- NOx:
-
Oxides of nitrogen
- HC:
-
Hydrocarbons
- SD:
-
Smoke density
References
Agrawal AK (2007) Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Energy Combust Sci 33:233–271
Alsaleh A, Sattler ML (2014) Waste tire pyrolysis: influential parameters and product properties. Curr Sustainable Renewable Energy Rep 1:129–135
Cunha A Jr, Feddern V, De Prá MC, Higarashi MM, De Abreu PG, Coldebella A (2013) Fuel 105:228–234
Benallal B, Roy C, Pakdel H, Chabot S, Poirier MA (1995) Characterization of pyrolytic light naphtha from vacuum pyrolysis of used tyres comparison with petroleum naphtha. Fuel 74(11):1589–1594
Bhatt PM, Patel PD (2012a) Suitability of tyre pyrolysis oil (TPO) as an alternative fuel for internal combustion engine. Int J Adv Eng Res Stud 4:61–65
Bhatt PM, Patel PD (2012b) Suitability of tyre pyrolysis oil (TPO) as an alternative fuel for internal combustion engine. IJAERS 4:61–65
Chang DYZ, Gerpen JH, Lee I, Johnson LA, Hammond EG, Marley SJ (1996) Fuel properties and emissions of soybean oil esters as diesel fuel. J Am Oil Chem Soc 73:1549–1555
Danesan V. 2007. International combustion engine, 3rd ed. McGraw-Hill
Dogan Q, Celik MB, Ozdalyan B (2012) The effect of tyre derived fuel/diesel fuel blends utilization on diesel engine performance and emissions. Fuel 95:340–346
Dung NA, Klaewkla R, Wongkasemjit S, Jitkarnka S (2009a) Light olefins and light oil production from catalytic pyrolysis of waste tyre. J Anal Appl Pyrolysis 86:281–286
Dung NA, Mhodmonthin A, Wongkasemjit S, Jitkarnka S (2009b) Effects of ITQ-21 and ITQ-24 as zeolite additives on the oil products obtained from the catalytic pyrolysis of waste tyre. J Anal Appl Pyrolysis 85:338–344
Dung NA, Tanglumlert W, Wongkasemjit S, Jitkarnka S (2010) Roles of ruthenium on catalytic pyrolysis of waste tyre and the changes of its activity upon the rate of calcinations. J Anal Appl Pyrolysis 87:256–262
Dung NA, Wongkasemjit S, Jitkarnka S (2009c) Effects of pyrolysis temperature and Pt-loaded catalysts on polar-aromatic content in tyrederived oil. Appl Catal B Environ 91:300–307
Edwin Raj R, Robert Kennedy Z, Pillai BC (2013) Optimization of process parameters in flash pyrolysis of waste tyres to liquid and gaseous fuel in a fluidized bed reactor. Energy Convers Manag 67:145–151
Ganesan, V. (2007). An analysis of working capital management efficiency in telecommunication equipment. Industryrivier Academic Journal, 3:2, Fall
Hariharan S, Murugan S, Nagarajan G (2013) Effect of diethyl ether on tyre pyrolysis oil fueled diesel engine. Fuel 104:109–115
Islam MR, Haniu H, Beg Alam MR (2008) Liquid fuels and chemicals from pyrolysis of motorcycle tyre waste product yields, compositions and related properties. Fuel 87:3112–3122
Kalitko VA (2010) Steam thermolysis of tyre shreds: modernization in afterburning of accompanying gas with waste steam. J Eng Phys Thermo Phys 83:179–187
Kaminsky W, Mennerich C (2001) Pyrolysis of synthetic tyre rubber in a fluidised-bed reactor to yield 1,3-butadiene, styrene and carbon black. J Anal Appl Pyrolysis 59:803–811
Kaminsky W, Mennerich C, Zhang Z (2009) Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. J Anal Appl Pyrolysis l85:334–337
Kapura T, Muruganb S, Patelc SK (2014) Experimental study of diethyl ether addition on the performance and emissions of a diesel engine. Energy Procedia. 54:615–626
Lapuerta M, Armas O, Ballesteros R, Fernández J (2005) Diesel emissions from biofuels derived from Spanish potential vegetable oils. Fuel 84(6):773–780
Lapuerta M, Hernández JJ, Ballesteros R, Durán A (2003) Composition and size of diesel particulate emissions from a commercial European engine tested with present and future fuels. Proc Inst Mech Eng, Part D: J Automobile Eng 217:907–919
Laresgoiti MF, Caballero BM, de Marco I, Torres A, Cabrero MA, Chomón MJ (2004) Characterization of the liquid products obtained in tyre pyrolysis. J Anal Appl Pyrolysis 71:917–934
Li L, Wang J, Wang Z, Xiao J (2015) Combustion and emission characteristics of diesel engine fueled with diesel/biodiesel/pentanol fuel blends. Fuel 15(6):211–218
Lopez G, Olazar M, Aguado R, Elordi G, Amutio M, Artetxe M, Bilbao J (2010) Vacuum pyrolysis of waste tyres by continuously feeding into a conical spouted bed reactor. Ind Eng Chem Res 49:8990–8997
Islam M, Khan N.M.F.R, Alam MZ. 2003. Production and characterization of scrap tyre pyrolysis oil and its blend. International conference on mechanical engineering 26–28 December 2003, Dhaka, Bangladesh
Martínez JD, Puy N, Murillo R, García T, Navarro MV, Mastral AM (2013) Waste tire pyrolysis—a review. Renew Sust Energ Rev 23:179–213
Mastral AM, Murillo R, Callén MS, García T, Snape CE (2000) Influence of process variables on oils from tire pyrolysis and hydropyrolysis in a swept fixed bed reactor. Energy Fuel 14:739–744
Maurizio C, Sonia C, Silvia C (2014) A pilot-scale study of waste vegetable oil transesterification with alkaline and acidic catalysts. Energy Procedia 45:198–206
Murillo R, Aylón E, Navarro MV, Callén MS, Aranda A, Mastral AM (2006) The application of thermal processes to valorise waste tyre. Fuel Process Technol 87:143–147
Murugan S, Ramaswamy MC, Nagarajan G (2008) Performance, emission and combustion studies of a DI diesel engine using distilled tyre pyrolysis oil-diesel blends. Fuel Process Technol 89:152–159
Naima K, Liazid A (2013) Waste oils as alternative fuel for diesel engine: a review. J PetroleumTechnol Altern Fuels 4(3):30–43
Nabanita B, Ritica R, Tushar J (2014) Biodiesel production from used vegetable oil collected from shops selling fritters in Kolkata. Energy Procedia 54:161–165
Nantha GK, Arindam P, Sharma S, Charan Samanchi K, Elango ST (2014) Investigation of emissions and combustion characteristics of a CI engine fueled with waste cooking oil methyl ester and diesel blends. Alexandria Eng J 53:281–287
Pakdel H, Pantea DM, Roy C (2001) Production of dl-limonene by vacuum pyrolysis of used tyres. J Anal Appl Pyrolysis 57:91–107
Qasim M., Ansari T. M., and Mazhar Hussain. 2017. Preparation, characterization and engine performance of biodiesel fuel derived from waste cooking oil and its blends. Inter J Sci, 6(4): 113–118. http://www.ijsciences.com/pub/issue/2017-04/
Rodriguez IM, Laresgoiti MF, Cabrero MA, Torres A, Chomon MJ, Caballero B (2001) Pyrolysis of scrap tyres. Fuel Process Technol 72:9–22
Ramadhas AS, Jayaraj S, Muraleedharan C (2005) Characterization and effect of using rubber seed oil as fuel in compression ignition engines. Renew Energ. 30:795–803
Ramadhas AS, Jayaraj S, Muraleedharan C (2004) Use of vegetable oils as I.C. engine fuels—a review. Renew Energ 29:727–742
Rombaldo CFS, Lisbôa ACL, Méndez MOA, Reis Coutinho A (2008) Effect of operating conditions on scrap tyre pyrolysis. Mater Res 11:359–363
Unapumnuk K, Keener TC, Lu M, Liang F (2008) Investigation into the removal of sulfur from tyre derived fuel by pyrolysis. Fuel 87:951–956
Unapumnuk K, Lu M, Keener TC (2006) Carbon distribution from the pyrolysis of tyre-derived fuels. Ind Eng Chem Res 45:8757–8764
US Environmental Protection Agency 2014. Wastes–resource conservation—common wastes and materials—scrap tires: basic introduction. http://www.epa.gov/wastes/conserve/materials/tires/basic.htm. Retrieved on January 8, 2017
Wanodya AK, Arief B (2013) Synthesis of biodiesel from second-used cooking oil International conference on Sustainable Energy Engineering and Application. Energy Procedia 32:190–199
Williams PT, Besler S, Taylor DT (1990) The pyrolysis of scrap automotive tyres: the influence of temperature and heating rate on product composition. Fuel 69:1474–1482
Williams PT (2013) Pyrolysis of waste tyres: a review. Waste Manag 33(8):1714–1728
Witpathomwong C, Longloilert R, Wongkasemjit S, Jitkarnka S (2011) Improving light olefins and light oil production using Ru/MCM-48 in catalytic pyrolysis of waste Tyre. Energy Procedia 9:245–251
Wongkhorsub C, Chindaprasert N (2013) A comparison of the use of pyrolysis oils in diesel engine. Energy Power Eng 5(04):350–357
Yoon SH, Cha PJ, Lee CS (2010) An investigation of the effects of spray angle and injection strategy on dimethyl ether (DME) combustion and exhaust emission characteristics in a common-rail diesel engine. Fuel Process Technol 91(11):1364–1372
Zhang X, Wang T, Ma TL, Chang J (2008) Vacuum pyrolysis of waste tyres with basic additive. Waste Manag 28:2301–2310
Acknowledgements
The authors are grateful to the institute of chemical sciences, Bahauddin Zakariya University, Multan, Pakistan and M/S Chicago Metal Works, Multan, Pakistan for providing facilities to carry out this Ph.D research work of Muhammad Qasim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Qasim, M., Ansari, T.M. & Hussain, M. Experimental investigations on a diesel engine operated with fuel blends derived from a mixture of Pakistani waste tyre oil and waste soybean oil biodiesel. Environ Sci Pollut Res 25, 23657–23666 (2018). https://doi.org/10.1007/s11356-017-0380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0380-9