Abstract
Shortage of conventional power sources, ordinary energizes and the unrefined petroleum extracts increases the cost of processed fuel. It is necessary to find out fuel hotspots for viable use in automobile and industrial applications. Research in this area of using oil extracted using pyrolysis process from the waste tyre and mixed with biodiesel is attracting attention. In this survey, a single-cylinder four-stroke conventional diesel engine with waste tyre pyrolysis oil and biodiesel mixtures was performed. Testing on single-barrel four-stroke diesel motor by using biodiesel synthesized with palm stearin oil from waste tyre pyrolysis oil is taken up in this work. Analysis shows the rise in brake thermal efficiency (BTE) to the mix level. It is also noted that pollution such as CO, HC and NOx is limited compared to traditional diesel fuel.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Coal, petroleum products, natural gas and other fossil fuels are fast depleting due to increase in vehicular population and industrial usage. Fuels and energy produced using nuclear isotopes such as uranium, thorium and artificial radio-isotopes are good alternatives to fossil fuels but have some limitations. Energy can be produced using methanol, ethanol, butanol and other bio-alcohols and can be stored in electrical form in batteries and fuel cells. Another source of energy production is by hydrogen, non-fossil natural gas, biomass and non-fossil methane. Biodiesel is considered to be less harmful to the environment as it is biodegradable.
In India, every year million tyres are being thrown into garbage as scrap after utilizing in automobiles. Direct burning of these scrap tyres emits a lot of dangerous and dangerous gas like CO2, CO, smoke and NOx, etc. These waste tyres can be utilized to produce pyrolysis oil and this can be blended to produce biodiesel. Fuel produced by this method can be characterized by their physical and chemical parameters. Chemical and thermophysical properties of the blended diesel are to be evaluated and can be compared with that of raw oil after refining and distillation process. This blended diesel is considered to be useful for better engine performance and increased efficiency. Experimental investigations are needed to make this process still more viable and environmental friendly, which can bring significant economical improvement [1,2,3].
2 Literature Survey
Ali et al. [4] reviewed the process of pyrolosis and extracted alternative diesel fuels from vegetable oils and their blends in diesel and process of preparation, i.e. transesterification, dilution and micro-emulsion are reviewed. Murugan et al. [5] disclosed emission features and efficiency of a direct injection and single-cylinder diesel engine using 50, 30 and 10% of pyrolysis oil in diesel blends. It was noted that the engine’s thermal brake effectiveness improved with enhanced concentration of mixture. Emission gases and smoke content were discovered to be greater at greater loads due to longer ignition delay and elevated aromatic content. Aydin et al. [6] recorded feasibility process for the production of oil from waste tyres using pyrolysis and diesel fuel by multiple desulphurization techniques. Sulphur content was reduced by using CaO, Ca (OH) and NaOH catalysts. 34.25% lower sulphur content was reported using 5% Ca (OH) catalyst compared to diesel. Al-Lal et al. [7] studied about pyrolysis fuels and their desulphurization. Two desulphurization methods are tried in this study and hydrogen was not used in these methods in order to obtain reduced sulphur content. The desulphurization rate reported was 64%. De Marco Rodriguez et al. [8] carried out chemical analysis and studied the behaviour of oil blends of tyre pyrolysis in diesel. Higher portion of aromatics was found in the tyre oil bends and it was observed to be a complex organic mixture. Carbon compounds of five to twenty carbons are present in the mixture. Aromatics percentage varied between 34.7 and 75.6 with operating temperatures from 300 to 700 °C and aliphatics were about 19.8–59.2%.
3 Fuel Preparation and Characteristics
3.1 Preparation of Tyre Pyrolysis Oil
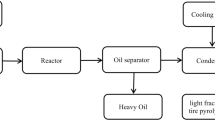
Fixed-bed type reactor was used to carry out pyrolysis process. Temperature was varied between 350 and 400 °C. Condenser and a fractionating column were used in this system to condensate and fractionate the fuel oils. Nitrogen gas was used in an inert environment. Waste tyre was cleaned and cut into small pieces after removing the unwanted iron wires. Figure 1 shows the flow chart for the development of pyrolysis oil
Pyrolytic oil extracted from waste tyre using pyrolysis process was taken and hydro-sulphuric acid of 8% by weight is added. The mixture was stirred using electrical stirrer for 3–4 h. Temperature was preserved at 50 °C. The combination has been permitted to settle for 40 h. By this time, the mixture was formed in two layers. Clear and higher viscous oil was formed on the top and acidic sludge with non-viscous was formed at the bottom. The viscous oil was then activated by using bentonite–calcium oxide (CaO) treatment.
Pyrolytic oil treated with activated bentonite is taken in the ratio of 10–1. Pyrolytic oil and CaO are taken in the ration of 20–1. These are added and mixed in an electrical stirrer for 4 h maintaining 70 °C. The blend can be settled for 24 h and then cleaned utilizing sample fabric. Healed pyrolytic oil was obtained and was distilled using vacuum distillation. Table 1 illustrates the proportion of oil acquired from TPO.
Table 1 shows that the TPO percentage obtained from raw pyrolytic oil after removing sludge initially is 66.9. After vacuum distillation, the percentage is 22.79. The raw TPO sludge carries almost 77.21% of oil.
3.2 Preparation of Palm Stearin Methyl Ester
Methyl esters obtained from vegetable oils with diesel mixtures are proven to be effective appropriate fuels. Ethyl and isopropyl acids of shallow or uncooked palm oil and palm stearin were synthesized utilizing synthetic transesterification responses. The fuel properties were evaluated using different instruments. Alkyl esters had higher viscosities in the range of 4.4 × 10−6m2/s–5.2 × 10−6 m2/s. Petroleum diesel had viscosity value of 4.0 × 10−6 m2/s. But the SO2 emission from alkyl esters was low compared to diesel. Figure 2 shows the flow chart of biodiesel production from palm stearin.
3.3 Properties of TPO
(See Table 2).
4 Experimentation
A vertical air-cooled 1C4S diesel engine is used for experimentation. Compression ratio of 17.5:1 was maintained and 4.4 kW power developed at 1500 rpm. The engine was combined with electric current dynamometer. Figure 3 shows the experimental set-up. Five levels of load were applied, i.e. at 0, 25, 50, 75 and 100%. At all the five load conditions, speed, airflow rate, fuel flow rate, exhaust gas temperature, combustion characteristics and exhaust emissions of HC, CO and smoke were measured. Horiba MEXA-584L model exhaust gas analyser with microprocessor control was used to measure emission levels of CO and HC. Blend percentages of 20, 40 and 50 are experimented in this work.
5 Results and Discussion
Combination of CO and NOx of pedal capacity and difference of smoke density, BTE and BSFC with BP are evaluated.
5.1 Carbon Monoxide Versus BP
The variation of CO emission with BP is revealed in Fig. 4. This can be seen in the CO pollution decreases with enhance in load. CO emission values obtained are 0.03, 0.03, 0.04 and 0.02 at full load conditions for fuels of diesel, TPO20, TPO40, TPO50 and pure biodiesel, respectively. At full load, CO emission using tyre oil blend TPO20 is decreased compared to diesel fuel.
5.2 NOx Versus BP
NOx emission values variation with brake power is exhibited in Fig. 5. NOx pollution increases with augment in load. NOx emission values at full freight are 1568, 1692, 1860 and 1701 ppm for fuels of diesel, TPO20, TPO40, TPO50 and pure biodiesel, respectively. The NOx emission, at full load, using tyre oil blend TPO20 is increased compared to the other blends.
5.3 Smoke Density Versus BP
It shows smoke density variety with brake strength. As load increases, smoke density reduces in relation to diesel. The emissions of smoke at complete load are 26, 45, 48 and 31 mg/m3 for diesel, TPO20, TPO40, TPO50 and pure biodiesel fuels, respectively. The smoke density emission using tyre oil blend TPO20 is decreased when compared to the other blends at full load conditions (Fig. 6).
5.4 Brake Thermal Efficiency Versus BP
Brake thermal efficiency variation with brake power is shown in Fig. 7. Brake thermal efficiencies obtained at full load are 33.69, 28.1, 26.73, 26.58 and 26.56% for fuels of diesel, TPO20, TPO40, TPO50 and pure biodiesel, respectively. The brake thermal efficiency using tyre oil blend TPO20 is decreased compared to the diesel at full load conditions.
5.5 BSFC Versus BP
Figure 8 displays variations in specific fuel consumption with brake energy. The emissions are decreasing as the load rises. The SFC levels at steaming pile are 0.33, 0.53, 0.30, 0.31, 0.30, 0.31 and 0.31 kg/kw-hr for diesel, TPO20, TPO40, TPO50 and pure biofuel successively. At max power environments, the SFC using tyre oil blend TPO20 is decreased compared to the diesel.
6 Conclusion
Experimentation is conducted to examine and evaluate the effect of palm stearin methyl ester (PSME) fuel injection pressure, exhaust gas quality and stricter emission levels that used a single-cylinder constant CI engine compressor ratio. The by-product of palm derived oils (palm stearin) could be useful alternate for producing biodiesel with less harm to environment. PSME of about 98.10% yield was obtained from refined, bleached and deodourized (RBD) palm stearin. Brake thermal efficiency and rise in specific fuel efficiency are decreased across the engine installation using PSME relative to diesel fuels. Decline in CO, HC emissions and smoke density is noted for PSME throughout engine operation relative to baseline operation. It is observed that better performance and emission characteristics of the engine can be obtained with TPO addition to PSME. It can be shown that direct injection pressure plays a crucial role in emission reduction and improving engine performance PSME-TPO fuel is best suited for CI engine.
References
Reisman JI (1997) Air emissions from scrap tyre combustion. Tech Rep EPA-600/R-97-115
Williams PT, Besler S (1995) Pyrolysis-thermogravimetric analysis of tyres and tyre components. Fuel 74(9):1277–1283
Mastral AM, Murillo R, Callen MS, Garcia T (2000) Optimisation of scrap automotive tyres recycling into valuable liquid fuels. Resour Conserv Rcycling 29(4):263–272
Ali Y (1994) Alternative diesel fuels from vegetable oils. Bioresour Technol 50:153–163
Murgan S, Ramaswamy M, Nagaranja C (2008) The use of tyre pyrolysis oil in dieselengines. Waste Manag 28(12):2743–2749
Aydin H, Ilkilic C (2012) Optimization of fuel production from waste vehicle tyres by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel 102:605–612
Al-Lal AM, Bolonio D, Llamas A, Lapuerta M, Canoira L (2015) Desulfurization of pyrolysis fuels obtained from water: lube oils, tyres and plastics. Fuel 150:208–216
de Marco Rodriguez I, Laresgoiti MF, Cabrero MA, Torres A, Chomon MJ, Caballero B (2001) Pyrolysis of scrap tyres. Fuel Process Technol 72(1):9–22
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Nagaraju, P., Ravichandra, M., Lahari, M.L.R.C., Sai, P.H.V.S.T. (2021). Experimental Investigation on the Performance of Tyre Pyrolysis Oil Blended with Palm Stearin Methyl Ester as a Fuel for Single-Cylinder C.I Engine. In: Arockiarajan, A., Duraiselvam, M., Raju, R. (eds) Advances in Industrial Automation and Smart Manufacturing. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-4739-3_82
Download citation
DOI: https://doi.org/10.1007/978-981-15-4739-3_82
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4738-6
Online ISBN: 978-981-15-4739-3
eBook Packages: EngineeringEngineering (R0)