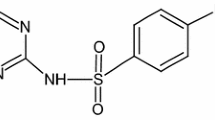
Abstract
Sulfamethoxazole (SMX) was decomposed by using gamma irradiation in the presence of different additives such as NO3 −, NO2 −, Cr(VI), 2-propanol, and tert-butanol. The results demonstrated that NO3 −, NO2 −, 2-propanol, and tert-butanol inhibited SMX radiolytic removal. However, there existed a synergetic effect for radiolytic removal of the mixture containing SMX and Cr(VI). At an absorbed dose of 150 Gy, the removal percentages of SMX and Cr(VI) in the mixture were 73.5 and 84.6%, respectively, which was higher than the removal percentages of 70.6 and 4.1% for the single component of SMX and Cr(VI). This provides us an insight into treating the combined pollution in micro-polluted water. The SMX radiolytic removal followed a pseudo first-order reaction kinetic model, and the rate constant ratios of ·OH, eaq −, and H· towards SMX radiolysis were 10.4:1:2.9. In addition, 24-h bio-inhibitory to the macroalgae of SMX solution during gamma irradiation reached the maximum of 0.85 at an adsorbed dose of 100 Gy, then gradually decreased with the increasing adsorbed dose. Based on LC-MS analysis and quantum chemical calculation, the degradation intermediates were determined and concluded that SMX radiolytic removal was mainly via ·OH radical attack and direct decomposition of SMX molecule by gamma ray.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increase of antibiotics usage, more and more residual antibiotics have been determined in the water body and sediments, which pose an adverse effect on the environment and human health (Wegst-Uhrich et al. 2014). As a member of sulfonamides antibiotics, sulfamethoxazole (SMX) is widely present in the ground water, surface water (Yang et al. 2011), ocean (Zheng et al. 2012), and sediment (Zhang et al. 2013). The concentration of SMX in Beibu Gulf Rim and Yellow river ranged from 0.51 to 6.30 ng L−1 and 25 to 152 ng L−1, respectively (Zheng et al. 2012). Especially, the concentration of SMX can increase to 4330 ng L−1 in the river of North Vietnam (Le and Munekage 2005).
The discharge of aquaculture wastewater is an important source of SMX. Generally, SMX cannot be removed completely with conventional sewerage treatments due to the strong inhibition of SMX on the microorganisms (Hoa et al. 2011). As a result, it is very common to contain SMX in the effluent of sewage plant. The occurrence of SMX in water may threaten the aquatic organisms and further endangers human health for the accumulation of SMX in the body due to its long half-life (Watkinson et al. 2009). Therefore, the presence of SMX in the aquatic environment is an emerging environmental problem. It is desirable to effectively remove SMX in water body using the alternative methods.
At present, SMX removals from aqueous solution are mainly via oxidation techniques. Garoma et al. (2010) used O3 to oxidize SMX and studied the influence of CO3 2− and pH on SMX removal. Li et al. (2011) adopted H2O2/UV to treat SMX solution and obtained a 95% degradation percentage at 254 nm. Trovó et al. (2009) investigated the effect of Fenton reagent on SMX degradation and found the mineralization degree of SMX in distilled water was more significant than that in sea water. Ayoub and Ghauch (2014) transformed SO4 2− into SO4 − radical by using different irons such as Fe2+, Fe0, Au-Fe, and Co-Fe. Meanwhile, SO4 − radical was applied to remove SMX under the aerobic condition. Abellán et al. (2007) found that TiO2 could facilitate SMX degradation under UV irradiation and SMX removal was mainly controlled by ·OH radical. However, it is necessary to introduce the extra chemicals to the solution during these removal processes and there is presence of the risk of secondary pollution.
Gamma ray not only excites and ionizes water molecule to produce the reactive species of ·OH, eaq −, and H·, but also exerts a direct action to organic molecules. Therefore, gamma irradiation has been proven to be a promising method to remove the stable organic pollutants from aqueous solution due to high efficiency of the pollutants removal without adding the chemicals (Garoma et al. 2010; Singh and Kremers 2002; Zhu et al. 2014). However, to our knowledge, SMX radiolytic degradation and its removal pathways are seldom studied. Consequently, we systemically studied SMX radiolytic removal efficiencies with different additives, explored the reaction mechanisms between SMX and ·OH, eaq −, as well as ·H·. In addition, LC-MS analysis and quantum chemical calculation were carried out to clarify the degradation intermediates and removal pathways during SMX radiolysis. Meanwhile, 24-h bio-inhibitory to the macroalgae of SMX and degradation intermediates was determined to study the change of biological toxicity during gamma irradiation.
Materials and methods
Materials
SMX (> 98%) and chromatography-grade HCOOH were purchased from SIGMA-ALDRICH, chromatography-grade CH3CN was from TEDIA (USA). HCl, NaOH, NaNO3, NaNO2, K2Cr2O7, 2-propanol, and tert-butanol were obtained from Shanghai Chemicals Factory and used without further purification. All these chemicals used were of analytical grade, and the solutions in the experiment were prepared in twice-distilled water.
Irradiation treatment
Gamma irradiation of SMX was carried out by using a 60Co source at the Application Institute of Atomic Energy, Jiangsu Academy of Agricultural Sciences, China. The radioactivity of 60Co radiation source was about 1.85 × 1016 Bq. SMX samples (20 mL each) were preserved in 25-mL airtight glass vessels, which were placed at the same distance from source center. The desired absorbed dose was achieved according to the continuous irradiation time. The absorbed dose was determined by a method of silver dichromate dosimeter with the deviation within ± 3% (Zhang et al. 2008).
Experimental procedures
SMX solutions were prepared with initial concentrations varying from 1 to 20 mg L−1, and the solutions were irradiated at absorbed doses from 0 to 400 Gy. In addition, 2 mmol L−1 NaNO2 and NaNO3 as well as 0.1 mmol L−1 K2Cr2O7 was separately added to 5 mg L−1 SMX solutions to study the effect of different additives on SMX radiolytic removal. Besides, 0.1 mol L−1 2-propanol and tert-butanol was, respectively, introduced to 5 mg L−1 SMX solution in order to explore the reaction mechanisms between SMX and ∙OH, eaq −, as well as ·H· during gamma irradiation.
In addition, 24-h bio-inhibitory to the macroalgae of SMX solution was measured according to the method of GB/T13266-91 to evaluate the biochemical toxicity of SMX and the degradation intermediates during the process of SMX radiolytic removal. The macroalgae were acquired by field collection, further cultivated, and purified in the laboratory.
Analytical methods
SMX concentration was determined by using HPLC (Waters e2695, USA) equipped with an Agilent ZORBAX SB-C18 column (4.6 × 250 mm, 5 μm) and 2998 PDA detector (Waters, USA). The mobile phase was 30% acetonitrile and 70% water with a flow rate at 1.0 mL min−1, and the injection volume was 20 μL. The column temperature was maintained at 35 °C, and the detection wavelength was set at 265 nm.
SMX degradation intermediates during gamma irradiation were determined by LC-MS including UltiMate 3000 HPLC (Diones, USA) with a Hypersil GOLD column (100 × 2.1 mm, 3 μm) and TSQ Quantum Access Max (Thermo Fisher, USA). The mobile phase was 30% acetonitrile and 70% water with flow rate of 0.3 mL min−1 and injection volume of 20 μL. The column temperature was at 25 °C. The ESI-MS analysis was performed in the positive mode and full scan acquisition (80–400) with spray voltage of 3500 V, vaporizer temperature of 380 °C, sheath gas pressure of 35 L min−1(N2), ion sweep gas of 0 L min−1 (N2), aux gas pressure of 5 L min−1 (N2), capillary temperature of 350 °C, tube lens offset of 110 V, and skimmer offset of 0 V.
Cr(VI) concentration was measured with a 1,5-diphenylcarbazide spectrophotometer method by CARY50 UV-vis spectrophotometer (Varian, USA) at the detection wavelength of 540 nm.
All the experiments in this study were carried out in duplicate; the average was calculated to study SMX removal efficiency during gamma radiation under different conditions.
Results and discussion
Effect of the initial concentration on SMX radiolytic degradation
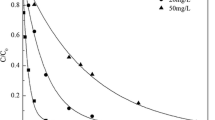
SMX solutions with different initial concentrations were degraded by using gamma irradiation at solution pH of 4.8. The corresponding SMX removal efficiencies under different absorbed doses are shown in Fig. 1. It is obvious that the initial concentration of SMX effectively affected the removal efficiency. A low initial concentration was favorable for SMX removal at a given absorbed dose. At an absorbed dose of 300 Gy, SMX removal percentages were 100, 96.2, and 91.3% at the initial concentration of 1, 2, and 5 mg L−1, respectively. This provides us a new insight into removing low-concentration organic matters in the micro-polluted water. High SMX removal during gamma irradiation is mainly ascribed to the reactive species of ·OH, eaq −, and H· generated from gamma irradiation towards water molecule.
In addition, the pseudo first-order reaction kinetic model was applied to study SMX radiolysis. It is noteworthy that the relationship between ln(C 0/C) and absorbed dose is found to be linear (see inset of Fig. 1) with the correlation coefficients higher than 0.98, indicating SMX radiolytic removal fitted the pseudo first-order reaction kinetics. The corresponding pseudo first-order reaction rate constants during SMX radiolysis are compared in Table 1. It can be observed that the rate constants were 1.70 × 10−2, 1.29 × 10−2, and 9.82 × 10−3 Gy−1 at the initial concentrations of 1, 2, and 5 mg L−1, respectively. At high SMX concentration, a great amount of the intermediates were produced during DEX radiolysis. This inevitably consumed the more reactive species such as OH·, eaq −, and as ·H·, and thereby decreasing the effective amounts of the reactive species for reactions with SMX. Besides, in order to achieve 95% SMX removal percentage, the required absorbed dose was 1.76 × 102, 2.32 × 102, and 3.06 × 102 Gy at the initial concentrations of 1, 2, and 5 mg L−1. This showed that low-concentration SMX presented high removal efficiency and degradation rate during gamma irradiation. At high SMX concentration, there is a presence of large quantities of degradation intermediates, which consumed the reactive species from water radiolysis and thereby decreasing the chance of effective collision between SMX and these reactive species.
Effect of NO2 − and NO3 − on SMX radiolytic degradation
NO2 − and NO3 − are widely present in natural water body, which causes some adverse impacts on human health. In addition, NO2 − and NO3 − may affect SMX radiolytic degradation due to their prompt reactions with the reactive species. Therefore, 2 mmol L−1 NO2 − and 2 mmol L−1 NO3 − were individually added to 5 mg L−1 SMX solution with pH at 4.8 to investigate the effect of NO2 − and NO3 − on SMX radiolysis, and the corresponding results are shown in Fig. 2. It is found that the presence of NO2 − and NO3 − restrained SMX removal during gamma irradiation. Moreover, the inhibiting effect of NO3 − was more obvious compared to that of NO2 −. At an absorbed dose of 100 Gy, the SMX removal percentage was 56.89% in the solution without inorganic anion. While in the solutions with NO2 − and NO3 −, SMX radiolytic removal percentages were 44.84 and 34.83%, respectively. Besides, we note that SMX the radiolytic removal percentage increased to 99% at an absorbed dose of 400 Gy regardless of the presence of inorganic anions, which indicated that SMX radiolytic removal could be achieved at a relatively lower absorbed dose than those of dexamethasone and ciprofloxacin (Guo et al. 2017; Guo et al. 2015).
The inhibiting effect of NO2 − and NO3 − on SMX radiolytic degradation is mainly attributed to the consumption of the reactive species such as ·OH and eaq −. It is reported that NO2 − can easily react with ·OH to produce NO2· and OH− (Singh and Kremers 2002) with a high rate constant of 1.0 × 1010 L (mol s)−1. The decrease of ·OH amounts inhibited SMX radiolytic degradation, which suggested that SMX radiolysis was controlled by the oxidation of ·OH radicals. NO3 − is an efficient scavenger for \( {\mathrm{e}}_{\mathrm{aq}}^{-} \) with a rate constant of 9.7 × 109 L (mol s)−1. The intermediate of NO3 2− is unstable and can be transformed into NO2 − and OH−. Thus, the generated NO2 − can significantly decrease the ∙OH concentration (Singh and Kremers 2002). The further increase of SMX degradation percentage in the presence of NO3 − demonstrated that eaq − played a certain role in SMX radiolytic removal.
Effect of 2-propanol and tert-butanol on SMX radiolytic degradation
It is noteworthy that there is presence of reductive group (−NH2) and oxidative group (−SO2 −) in SMX molecule. Thus, SMX can react with the reactive species from water radiolysis. In order to explore the reaction mechanisms between SMX and ·OH·, eaq −, as well as ·H· , 0.1 mol L−1 2-propanol and 0.1 mol L−1 tert-butanol were, respectively, added to 5 mg L−1 SMX solution during gamma irradiation. It can be observed from Fig. 3 that SMX removal reached 98.3% at the adsorbed dose of 400 Gy without the additive, while SMX removal percentages were only 27.5 and 76.5% in the solution containing 2-propanol and tert-butanol. The distinct decrease of SMX removal percentages in the presence of 2-propanol and tert-butanol is mainly attributed to the rapid reactions between 2-propanol and ·OH (k = 1.9 × 1010 L (mol s)−1), 2-propanol and ·H (k = 7.4 × 107 L (mol s)−1), as well as tert-butanol and ·OH (k = 6.0 × 108 L (mol s)−1). This inevitably consumed large number of reactive species, thereby decreasing SMX radiolytic degradation efficiency. Compared to that between tert-butanol and ·OH, the higher rate constant between 2-propanol and OH resulted in the less ·OH radical to react with SMX molecules. The significantly lower SMX degradation efficiency with 2-propanol indicates that ·OH radical played an important role in removing SMX from the solution. Besides, it should be pointed out that the reaction between 2-propanol and ·H might also exert a certain influence on SMX removal.
As shown in the inset of Fig. 3, SMX radiolytic degradation in the presence of 2-propanol and tert-butanol well followed the pseudo first-order reaction kinetic model. According to Table 1, the rate constants of SMX radiolytic removal were 6.87 × 10−4 and 2.69 × 10−3 Gy−1 with the addition of 2-propanol and tert-butanol, respectively. Combining with the rate constant of 9.82 × 10−3 Gy−1 without the additive, we may obtain SMX degradation rate constant ratios of ·OH, eaq −, and H· according to the following calculation:
It is obvious that the relative contribution of the reactive species to SMX radiolysis obeyed a decreasing order of ·OH, H·, and eaq −, which is different from a decreasing order of ·OH, eaq −, and H· during dexamethasone radiolytic removal (Guo et al. 2017).
Radiolytic degradation of combined pollution containing SMX and Cr(VI)
Cr(VI) is widely present in water body which possesses a strong carcinogenic effect (Chen et al. 2003). Generally, Cr(VI) is removed from water firstly by converting it into Cr(III) with the reductive agents (Blowes et al. 1997). However, this inevitably adds the chemicals to the water, thereby producing the secondary pollution. It is known that there exists oxidative radical of ·OH and reductive species of eaq − and H· during SMX radiolysis, and SMX radiolytic degradation was mainly ascribed to ·OH oxidation; we tried to change Cr(VI) into Cr(III) with the extra eaq − and H· species during SMX radiolytic process. Therefore, the mixture of 0.01 mmol L−1 K2Cr2O7 and 5 mg L−1 SMX solution was prepared for gamma irradiation to clarify the removal efficiencies of Cr(VI) and SMX in this study.
As described in Fig. 4, the radiolytic removal of both SMX and Cr(VI) in the mixture was enhanced compared to that of the single pollutants. At an absorbed dose of 150 Gy, the removal percentages of SMX and Cr(VI) in the mixture were 73.5 and 84.6%, while the corresponding removals for the single component of SMX and Cr(VI) were 70.6 and 4.1%, respectively. It is observed that there is presence of an synergistic effect during radiolytic removal between SMX and Cr(VI), which demonstrated that gamma irradiation was a feasible method for treating combined pollution containing SMX and Cr(VI). Especially, the removal percentage of Cr(VI) was significantly improved when mixing with SMX, which provides us an efficient and green technique to convert Cr(VI) into Cr(III) in the solution. The reduction of Cr(VI) during gamma irradiation is due to the prompt reactions with eaq − and H· to form Cr(III) with a rate constant at 2.1 × 1010 L (mol s)−1 (Yuan et al. 2005), which resulted in an increase of ·OH radicals, thereby synchronously promoting the oxidation of SMX.
The change of bio-inhibitory to the macroalgae during SMX radiolytic degradation
Due to the presence of some degradation intermediates during SMX radiolysis, it is necessary to investigate the change of biological toxicity of SMX solution before and after gamma irradiation. It can be observed from Fig. 5 that the biological toxicity of SMX solution without gamma irradiation was different from that with SMX radiolysis. The 24-h bio-inhibitory to the macroalgae of SMX solution was 0.26 before gamma irradiation, which increased to the maximum of 0.85 at the adsorbed dose of 100 Gy, then decreased to 0.51, 0.35, 0.27, and 0.21 at the adsorbed dose of 150, 200, 300, and 400 Gy, respectively. The change of 24-h bio-inhibitory to the macroalgae during SMX radiolytic degradation is related to the generated intermediates. There are diverse degradation intermediates at different adsorbed doses, which may result in various biological toxicities. It is noted that 24-h bio-inhibitory to the macroalgae at the adsorbed dose of 400 Gy was lower than that without gamma irradiation, indicating relatively high adsorbed dose was suitable for decreasing the potential danger of the solution during SMX radiolytic degradation.
Analysis of degradation products and degradation mechanism of SMX
Results of quantum chemical calculations
The geometry structure of SMX molecule was optimized with Gaussian 09 software at density functional theory in the level of B3LYP/6-31G(d), which can be used to study the charge distribution and bond length of SMX molecule. As shown in Fig. 6, 4O (− 0.52034) and 5O (− 0.51628) present the most negative charges, which results in the increase of bond polarity between 1N (− 0.370081) and 3S (1.145051) due to the connection of 3S with 4O and 5O. As a result, the bond of 1N and 3S becomes unstable. In addition, 1N (− 0.37008) possesses the strong negative charge, which can be easily attacked by ·OH radical (Xu 2012). It is noted that there exists a relatively strong bond polarity between 1N (− 0.370081) and 19C (0.400781), which is easily damaged by the external force. According to the optimized calculation for bond length of SMX molecule, we find 1N–3S bond is broken after losing four electronics from SMX and the bond lengths of 22N–19C and 21C–23O become longer than those in the original SMX molecule. These bonds are easily destroyed to open isoxazole ring.
Results of LC-MS
The degradation intermediates of 5 mg L−1 SMX at an adsorbed dose of 400 Gy were determined by using the LC-MS method, which are listed in Table 2. On the basis of the degradation intermediates and the results of quantum chemical calculation, the degradation pathways of SMX during gamma irradiation are summarized in Fig. 7. It can be observed that SMX degradation was mainly via ·OH radical attack and direct decomposition of SMX molecules by gamma ray.
Due to the negative charge of 22N (− 0.159629), ·OH radical was liable to attack the isoxazole ring and generated the intermediate (D). In addition, the 1N–3S bond was unstable, which could be directly damaged by gamma ray and formed the intermediate (H), which might be inferred from quantum chemical calculation. The ·OH radical attack on the intermediate (H) and the further cleavage of 21C–23O bond formed the new intermediates (B) and (G), respectively. This conclusion was in agreement with that from quantum chemical calculation. Besides, 21C–23O bond in SMX molecule was directly damaged and produced the intermediate (A) due to the increase of 21C–23O bond length. Then, 19C–20C bond was destroyed and created the intermediate (E). Due to that there existed the relatively strong bond polarity between 1N and 19C, the bond of 1N–19C was easily damaged to generate the intermediates (C) and (F). Therefore, quantum chemical calculation was very favorable for studying SMX degradation processes as an auxiliary method.
Conclusions
Gamma irradiation is proven to be a very effective method to remove low-concentration SMX from aqueous solution. In addition, it provides us a new insight into treating the combined pollution containing SMX and Cr(VI) in micro-polluted water. SMX radiolytic removal was related to the reactive species of ·OH, eaq −, and H·, and the relative contribution of these species to SMX radiolysis followed a decreasing order of ·OH, H·, and eaq −. According to LC-MS analysis and quantum chemical calculation, we attributed SMX radiolytic removal mainly to ·OH radical attack and direct decomposition of SMX molecule. The change of 24-h bio-inhibitory to the macroalgae during SMX radiolytic degradation indicated biological toxicity of SMX and the degradation intermediates, which is favorable for evaluating the feasibility of using gamma irradiation to remove SMX from aqueous solution.
References
Abellán M, Bayarri B, Giménez J, Costa J (2007) Photocatalytic degradation of sulfamethoxazole in aqueous suspension of TiO2. Appl Catal B Environ 74:233–241
Ayoub G, Ghauch A (2014) Assessment of bimetallic and trimetallic iron-based systems for persulfate activation: application to sulfamethoxazole degradation. Chem Eng J 256:280–292
Blowes D, Ptacek C, Jambor J (1997) In-situ remediation of Cr(VI)-contaminated groundwater using permeable reactive walls: laboratory studies. Environ Sci Technol 31:3348–3357
Chen H, Pan G, Yan H, Qin Y (2003) Toxic effects of hexavalent chromium on the growth of blue-green microalgae. J Environ Sci 24:13–18
Garoma T, Umamaheshwar SK, Mumper A (2010) Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 79:814–820
Guo Z, Zhu S, Zhao Y, Cao H, Liu F (2015) Radiolytic decomposition of ciprofloxacin using γ irradiation in aqueous solution. Environ Sci Pollut Res 22:15772–15780
Guo Z, Guo A, Guo Q, Rui M, Zhao Y, Zhang H, Zhu S (2017) Decomposition of dexamethasone by gamma irradiation: kinetics, degradation mechanisms and impact on algae growth. Chem Eng J 307:722–728
Hoa P, Managaki S, Nakada N, Takada H, Shimizu A, Anh D, Viet P, Suzuki S (2011) Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci Total Environ 409:2894–2901
Le T, Munekage Y (2005) Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Mar Pollut Bull 49:922–929
Li W, Lan M, Peng X (2011) Removal of antibiotic from swine wastewater by UV/H2O2 combined oxidation. Environ Pollut Control 33:25–28 (in Chinese)
Singh A, Kremers W (2002) Radiolytic dechlorination of polychlorinated biphenyls using alkaline 2-propanol solutions. Radiat Phys Chem 65:467–472
Trovó A, Nogueira R, Agüera A, Fernandez-Alba A, Sirtori C, Malato S (2009) Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res 43:3922–3931
Watkinson A, Murby E, Kolpin D, Costanzo S (2009) The occurrence of antibiotics in an urban watershed: from wastewater to drinking water. Sci Total Environ 407:2711–2723
Wegst-Uhrich S, Navarro D, Zimmerman L, Aga D (2014) Assessing antibiotic sorption in soil: a literature review and new case studies on sulfonamides and macrolides. Chem Central J 8:5
Xu X (2012) Theoretical study on degradation mechanism and QSAR of organic pollutants in marine environment. Ocean University of China (in Chinese)
Yang J, Ying G, Zhao J, Tao R, Su H, Liu Y (2011) Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China. J Environ Sci Health B 46:272–280
Yuan S, Zheng Z, Mou Y, Yu X, Zhao Y (2005) The removal of chromium (VI) in water by gamma-irradiation. China Environ Sci 25:655–659
Zhang J, Zheng Z, Zhao T, Zhao Y, Wang L, Zhong Y, Xu Y (2008) Radiation-induced reduction of diuron by gamma-ray irradiation. J Hazard Mater 151:465–472
Zhang Y, Xu J, Zhong Z, Guo C, Li L, He Y, Fan W, Chen Y (2013) Degradation of sulfonamides antibiotics in lake and sediment. Environ Sci Pollut Res 20:2372–2380
Zheng Q, Zhang R, Wang Y, Pan X, Tang J, Zhang G (2012) Occurrence and distribution of antibiotics in the Beibu Gulf, China: impacts of river discharge and aquaculture activities. Mar Environ Res 78:26–33
Zhu D, Jiang L, Liu R, Chen P, Lang L, Feng J, Yuan S, Zhao D (2014) Wire-cylinder dielectric barrier discharge induced degradation of aqueous atrazine. Chemosphere 117:506–514
Acknowledgments
We gratefully acknowledge supports from the National Natural Science Foundation of China (41373023, 91544229/002, and 41625006) and Jiangsu Province “333 Talent Project”; Sponsored by Jiangsu Province “Qing Lan Project”; Jiangsu Student Innovation Training Program “DMS formation of typical HAB species in the Yangtze estuary adjacent waters under polluted conditions”; A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, J., Guo, Z., Shen, X. et al. Gamma irradiation-induced decomposition of sulfamethoxazole in aqueous solution: the influence of additives, biological inhibitory, and degradation mechanisms. Environ Sci Pollut Res 24, 23658–23665 (2017). https://doi.org/10.1007/s11356-017-0006-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0006-2