Abstract
Antibiotics are becoming ubiquitous emerging contaminants in the aquatic environments due to their large amount of production and extensive application, which have received increasing public concern. In this paper, the degradation and mineralization of sulfamethoxazole (SMX) by ionizing radiation in the presence of Fe3O4 as Fenton-like catalyst were evaluated, the influencing factors, such as the initial SMX concentration, initial pH, water matrix, and radical scavenger, etc. were examined. The results demonstrated that SMX could be efficiently degraded. The addition of Fe3O4 could improve the degradation efficiency of SMX and increased the dose constant at various SMX initial concentrations. More than 98% of SMX was degraded in Fe3O4/gamma radiation system at a wide range of pH (about 3.0–11.0). The mineralization of SMX in the presence of Fe3O4 was increased by 200%. Adding free radical scavenger (tert-butyl alcohol) inhibited the degradation of SMX. The addition of Fe3O4 enhanced the dose constant of ·OH, indicating that Fe3O4 promoted the formation of hydroxyl radicals (·OH) and then improved SMX degradation and mineralization. The degradation efficiency of SMX in secondary effluent of WWTP decreased from 100 to 84% in secondary effluent compared with that in deionized water. The intermediate products during the degradation of SMX by ionizing radiation were identified by high-performance liquid chromatography, and a possible pathway of SMX degradation in such a system was tentatively proposed.

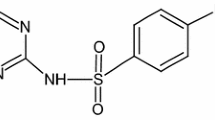
Schema illustration of SMX degradation by irradiation in the presence of Fe3O4
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are emerging contaminants in aquatic environments, which have received increasing attention in recent years. Antibiotics can enter into the environment through different sources, including pharmaceutical industry effluent, hospital wastewater, and some livestock farm wastewater (Wang et al. 2019).
Sulfonamides are used for preventing and curing bacterial infectious diseases. They can be added in fodder to prevent diseases and enhance the animal growth. They are widely used around the world due to their stable chemical properties, broad antibacterial spectrum, convenient use, and low price. Among them, sulfamethoxazole (SMX) is widespread used and detected in aquatic environments (Wang and Wang 2018b), such as city canal (Phan et al. 2011), river (Chen and Zhou 2014), sewage treatment plant secondary effluent (Karthikeyan and Meyer 2006), and activated sludge (Gobel et al. 2005). The traditional wastewater treatment processes are not effective for the removal of antibiotics (Gros et al. 2010; Wang and Wang 2016).
Advanced oxidation processes (AOPs) are powerful for the degradation of toxic organic pollutants in water and wastewater (Wang and Xu 2012), which have been gradually applied to degrade antibiotics, such as ozonation (Chen and Wang 2019; Wang and Bai 2017; Dantas et al. 2008), photocatalytic oxidation (Khan et al. 2019; Sayed et al. 2018, 2019), UV/chlorine (Sichel et al. 2011), persulfate oxidation (Rehman et al. 2018; Shah et al. 2018a, 2019; Wang and Wang 2017, 2018a, 2019), and Fenton-like oxidation (Liu et al. 2018; Shah et al. 2018b; Tang and Wang 2018a, b, 2019; Wan et al. 2016).
Among the stated advanced oxidation processes, ionizing radiation is a promising technique to decompose organic pollutants (Wang and Chu 2016). Decomposition of organic contaminants in aqueous solutions can be performed through direct action and indirect action. As for direct action, high energy rays can directly decompose the organic pollutants. As for the indirect action, water molecules are excited to generate plenty of reactive radicals, including hydroxyl radicals (·OH), hydrated electrons (eaq−), hydrogen radical (·H), and H2O2. The hydroxyl radicals (·OH) is a strong oxidant. The hydrated electrons (eaq−) is a powerful reductant with − 2.87 V reduction potential in neutral and alkaline condition. And the hydrogen radical (·H) is a strong reductant (Wardman 1989; Trojanowicz et al. 2019). Therefore, organic pollutants can be degraded by oxidation and reduction ways during ionizing radiation.
Ionizing radiation has been used to degrade antibiotics because of its no selectivity and high reactivity (Shen et al. 2019; Yu et al. 2010a, 2010b; Hu and Wang 2007). Shen et al. (2019) found that the removal efficiency of erythromycin A was 86% by ionizing radiation in antibiotic fermentation residues. Chu et al. (2018) demonstrated that with 2.5 kGy irradiation, 0.27 mmol/L penicillin G was completely removed. Liu and Wang (2013) found that the degradation efficiency of sulfamethazine reached 95% at 1 kGy. Sayed et al. (2016) reported that 4.6 mg/L ciprofloxacin was nearly completely decomposed with 870 Gy irradiation. However, the mineralization of antibiotics is difficult by ionizing radiation. Chu et al. (2018) found that with 10 kGy irradiation, TOC removal rate of penicillin G was only 21.7%. Liu and Wang (2013) also found that TOC removal rate of sulfamethazine was 5% with 5 kGy irradiation. Wang and Wang (2018c) reported the TOC removal rate of sulfamethoxazole was 12.8% with 1 kGy irradiation. The industrial application of radiation technology is promising. We have built a demonstration plant for the advanced treatment of textile wastewater using electron beam technology in China in 2017, with the treatment capacity of 2000 m3/d. The continuous operational experience of 2 years shows that this technology is effective and stable.
Fenton oxidation is a commonly used method of advanced oxidation processes (AOPs), which utilizes the oxidation of Fe2+ by H2O2 to generate hydroxyl radicals (·OH) at acidic pH condition. Our previous studies found the addition of Fe2+ during ionizing radiation could enhancing the antibiotics degradation (Chu et al. 2018; Liu et al. 2014), which may be due to that Fe2+ reacting with H2O2 which was generated from ionizing radiation to generate ·OH via Fenton reaction, thus improving antibiotics degradation. However, the traditional Fenton reaction is limited to narrow pH range (Mirzaei et al. 2017). Thus, the heterogeneous Fenton-like reaction is widely explored, aiming to expand the feasible operating pH range. Magnetite (Fe3O4) has been applied in environmental remediation (Liu and Wang 2019), such as in heterogeneous Fenton-like process for degrading chlorophenol (Xu and Wang 2011, 2012b), phenol and aniline (Zhang et al. 2009), di-azo-aminobenzene (DAB) (Gao et al. 2007), and bisphenol A (Huang et al. 2014). However, no research has been reported about using Fe3O4 as a Fenton-like catalyst during gamma radiation to enhance degradation and mineralization of sulfamethoxazole. It is needed to explore the degradation characteristic as well as the mechanism.
The objectives of this study were to explore the degradation and mineralization of sulfamethoxazole (SMX) using gamma radiation with the addition of Fe3O4 particles as a Fenton-like catalyst. The effects of initial SMX concentration and pH value on the SMX removal efficiency were studied. TOC removal efficiency at various initial SMX concentrations was also explored. In addition, the dose constant of hydroxyl radicals (·OH) with and without the addition of Fe3O4 were also determined. The intermediate products were identified, and the possible pathway for the degradation of SMX was proposed.
Materials and methods
Chemicals
Sulfamethoxazole (SMX, > 98% purity) was obtained from Aladdin Industrial Corporation (China). Fe3O4 particles were prepared as follows: FeSO4 (20 mmol/L, 250 mL), and Fe2(SO4)3 (20 mol/L, 250 mL) solutions were mixed in round-bottom flask under the protection of argon gas. Then 25%(v/v) ammonia was added dropwise until pH reached 10–11. After stirring for a while, the resulting precipitate was centrifugated for separation of Fe3O4 particles. After washing with water and ethanol for three times, Fe3O4 particles were freeze-dried for use.
Irradiation experiment
The SMX solution samples were irradiated using a 60Co radiation source, which is located in Tsinghua University. The radioactivity of 60Co gamma-ray source was about 3.6 × 1014 Bq. All SMX aqueous solution samples were preserved in 10-mL glass tubes and irradiated close to the irradiator at ambient temperature. The desired absorbed doses ranging were achieved according to the irradiation time. Fe3O4 (0.1 g/L) was added into SMX solution before irradiation to evaluate the influence of Fenton-like catalysts on SMX radiolytic degradation. Different initial concentration of SMX aqueous solution samples was prepared through diluting 200 mg/L SMX stock solution using deionized water, for investigating the impacts of initial concentration on the degradation of SMX. pH value was adjusted by H2SO4 and NaOH.
Analytical methods
SMX concentration was determined using high-performance liquid chromatography (1200 Series, Agilent, USA) at the wavelength of 275 ± 10 nm by diode array detector (DAD) (Zhuan and Wang 2019). Total organic carbon (TOC) was determined by a Multi N/C 2100 TOC/TN analyzer (Analytik Jena AG Corporation, Germany). The intermediate products of SMX degradation were determined by LC-MS (Q Exactive, Thermo Scientific).
Results and discussion
Sulfamethoxazole degradation in the presence of Fe3O4
Compared with control group which was absence of Fe3O4, the addition of Fe3O4 could enhance the SMX degradation at various SMX concentration (Fig. 1). During gamma irradiation of SMX aqueous solution, various reactive radical species were formed through water radiolysis, such as ·OH, eaq− and H2O2, as represented in Eq. (1).
The numbers in bracket express the numbers of the reactive species formed when absorbed 100 eV energy.
Fe3O4 magnetic particles can react with H2O2 to generate hydroxyl radicals (·OH), as shown in Eqs. (2) and (3) (Song et al. 2006). Fe (II) was oxidized by H2O2 and produce hydroxyl radicals (·OH) on the surface of Fe3O4 magnetic particles. These hydroxyl radicals (·OH) could attack and destroy SMX molecules, finally promoted the degradation efficiency. Hou et al. (2016) observed that in heterogeneous catalytic system of Fe3O4/ H2O2, 72.2% of tetracycline was removed, and the generation of ·OH from the heterogeneous active site (≡Fe (II)) through reaction with H2O2 played an important role. Huang et al. (2012) reported that the removal efficiency of bisphenol A was over 80% in Fe3O4/H2O2 system, and found that the Fe3O4 magnetic nanoparticles could catalyze H2O2 to form ·OH and then promote the degradation of bisphenol A. Xu and Wang (2012a) found that ·OH which formed on the surface of magnetic nanoparticles played a major role in 2,4-dichlorophenol degradation.
Figure 2 exhibited the results of SMX degradation at various initial SMX concentrations in the presence of Fe3O4 under ionizing radiation. The initial concentration of SMX had apparent influence on its degradation rate by gamma irradiation. SMX degradation efficiency decreased when SMX concentration increased. With 0.6 kGy irradiation, SMX removal efficiency was 100.0%, 97.0%, 85.4%, and 71.8%, respectively when the initial concentration was 5, 10, 20, and 30 mg/L. The degradation efficiency was 45.7%, 72.6%, 85.4%, 90.1%, 97.6%, 98.9%, and 100.0% at SMX initial concentration of 20 mg/L with 0.2, 0.4, 0.6, 1.0, 1.5, and 2 kGy irradiation, respectively. It was reported that SMX removal percentage decreased from 100 to 91.3% when its concentration increased from 1 to 5 mg/L (Wang et al. 2017). Kim et al. (2017) also observed that degradation of SMX decreased with increase of its initial concentration.
The pseudo first-order reaction kinetic model (Eqs. 4 and 5) was applied to fit the SMX degradation process.
where C0 is initial SMX concentration (mg/L); C is the residual SMX concentration after irradiation (mg/L); D is the absorbed dose (kGy); and k is the dose constant (kGy-1).
It is obvious that − ln(C/C0) increased linearly with the absorbed dose, with the correlation coefficient higher than 0.95, suggesting that the degradation of SMX followed pseudo-first-order kinetics model (Fig. 3). The dose constants were 8.972, 5.033, 3.188, and 2.183 kGy−1 at 5, 10, 20, and 30 mg/L, respectively. The relation between dose constant and the initial SMX concentration could be described by power function as Eq. (6) and illustrated in Fig. 4.
This power function (R2 > 0.99) revealed that dose constant was strongly related to initial SMX concentration. The dose constants (k) declined with increase of initial concentration of SMX. At higher initial concentration of SMX, more intermediate products were generated during ionizing irradiation, which inevitably consumed more reactive species. Thus, the effective collision between SMX molecules and these reactive radicals decreased (Wang et al. 2017; Shah et al. 2016). Based on the dose constant k (kGy-1) shown in Table 1, the addition of Fe3O4 magnetic particles slightly enhanced dose constant k of SMX degradation by gamma irradiation. This could be due to that Fe3O4 magnetic particles accelerated the decomposition of H2O2 to generate ·OH radicals.
The radiation chemical yield (G) represents the number of particles including molecules, ions, and radicals formed or decomposed when absorbing 100 eV energy. According to Eq. (7), G-value was calculated as the number of molecules in micromole (μmol) produced or consumed when receiving 1 J of irradiation energy (Shah et al. 2014).
where D is the absorbed dose (Gy), and R was the change of the reactant concentration (mol/L).
Figure 5 showed the change of G-values at various initial SMX concentrations after ionizing irradiation. The G-value increased with increasing initial concentration for a certain absorbed dose. When initial SMX concentration increased, it would allow more chances for the reaction between the reactive radicals and SMX molecules, leading to higher G-values.
Basfar et al. (2005) observed that G-values decreased as the concentration of methyl tert-butylether (MTBE) decreased during gamma irradiation. Sayed et al. (2016) observed the similar results in the research of radiation-induced degradation of ciprofloxacin. More active radicals and the intermediate products were produced with an increase of absorbed dose. First of all, the competition between SMX parent molecules and intermediate products for reactive radicals might result in the decline of G-value (Sayed et al. 2016). Besides, this trend can be caused by the recombination of reactive radicals, as given by Eqs. (8)–(11) (Yu et al. 2008; Zheng et al. 2011).
Moreover, the competition between reactive radicals for SMX molecules increased with the increase of absorbed dose, which led to decrease of G-values (Sayed et al. 2016; Yu et al. 2008). The similar result was reported for the decomposition of various pollutants by gamma irradiation, such as cefaclor (Yu et al. 2008), methyl tert-butyl ether (MTBE) (Basfar et al. 2005), and ciprofloxacin (Sayed et al. 2016).
Effect of pH on SMX degradation
For exploring the impact of pH on SMX degradation by irradiation, SMX solution at various initial pH values ranging from 3.01 to 10.96 was irradiated. Figure 6 illustrated the effect of pH on SMX degradation and the pseudo-first-order kinetic plots during SMX radiolytic degradation at different pH values.
SMX could be degraded in a wide range of pH values, and the degradation efficiency was more than 98%. The degradation rate constant was 3.432, 3.006, 2.428, 2.948, and 2.151 kGy−1 at pH 3.01, 4.96, 6.97, 9.03, and 10.96, respectively. It can be seen that acidic condition was more suitable for SMX degradation. The pH values determined the concentration of H+ and OH− in aqueous solution, thereby affecting the active radical composition formed during water radiolysis by Eqs. (12)–(14).
At alkaline condition, OH− can easily react with ·OH radicals, thus decreasing the reaction rate, which was in agreement with the previous studies in the degradation of ofloxacin (Changotra et al. 2019) and sulfadiazine (Guo et al. 2012).
Mineralization of sulfamethoxazole
Unlike the complete removal of SMX, ionizing radiation process cannot achieve complete mineralization of SMX, as shown in Fig. 7. When the absorbed dose was less than 1 kGy, the TOC removal efficiency for 5 mg/L SMX changed slowly and reached 20.2%, while SMX could be degraded completely, as illustrated in Fig. 7(a). In this stage, SMX degraded into small molecular weight compounds. When absorbed dose increased to 2.0 kGy, the TOC removal efficiency gradually improved and reached the highest (51.3%), which can be explained by small molecular weight compounds gradually degrading into CO2 and H2O.
The similar results were obtained in previous researches on the degradation of BPA by heterogeneous sono-Fenton (Huang et al. 2014), by the Fenton-like degradation of 2,4-DCP (Xu and Wang 2012b). The remaining TOC may relate to some small molecular intermediate products, such as refractory organic acids generated from SMX degradation (Yang et al. 2015; Niu et al. 2011). As shown in Fig. 7(b), with the addition of Fe3O4 during the radiation process, TOC removal efficiency enhanced from 23.8 to 51.3% in comparison with the control group which was absence of Fe3O4 when SMX initial concentration was 5 mg/L. Fe3O4 may react with H2O2 which was formed during water radiolysis process, which generated more ·OH radicals, finally resulting in the improvement of TOC removal.
Role of ·OH radicals in sulfamethoxazole degradation
In order to investigate the role of ·OH radical in the SMX degradation process by gamma radiation, 100 mM tert-butyl alcohol was added as ·OH radical scavenger in SMX solution before irradiation because tert-butyl alcohol can react with ·OH according to the following Eq. (15).
As illustrated in Fig. 8, the SMX degradation efficiency declined from 100 to 47% and 37% with and without the addition of Fe3O4 at the dose of 1 kGy.
The corresponding dose constants were calculated by pseudo-first-order kinetic plots as follows:
So, the dose constants for ·OH radical can be calculated as:
These calculated results revealed that with addition of 0.1 g/L Fe3O4, the dose constant of ·OH enhanced from 7.009 to 8.036 kGy−1 compared with the control group (Fig. 9). Thus, with the addition of Fe3O4, more ·OH radicals were formed, resulting in higher removal efficiency of SMX and TOC. Huang et al. (2012) also found that the reactive rate decreased remarkably from 7.96 × 10−3 min−1 (without t-BuOH) to 1.52 × 10−3 min−1 in the presence of 0.1 M t-BuOH during degradation of bisphenol A, indicating that Fe3O4 could excite H2O2 to form ·OH radicals. Zhang et al. (2009) applied Fe3O4 magnetic nanoparticles to degrade phenol and aniline in Fenton reaction, they found that the addition of tert-butyl alcohol inhibited the reactive rate and degradation efficiency, indicating that ·OH radicals played a responsible role in decomposition of phenol and aniline. Our previous study also indicated that Fe3O4-Mn3O4 could be used as Fenton-like catalyst for the degradation of sulfamethazine (Wan and Wang 2017)
Effect of water matrix on SMX degradation
Considering the practical application, the degradation of SMX in the secondary effluent of WWTP (wastewater treatment plant) by ionizing radiation was explored. Compared with SMX degradation in deionized water, SMX degradation efficiency decreased from 100 to 84% in secondary effluent (in Fig. 10). The substances such as anions and organic compounds in the secondary effluent could compete reactive species with antibiotic molecules, finally inhibiting the degradation efficiency (Peñalver et al. 2013).
Zhuan and Wang (2019) found that anions and organic matters in the solution had negative influence on degradation of SMX by gamma radiation. The presence of some common anions in real water such as CO32-, HCO3-, NO3-, SO42-, Cl-, and HPO42- could inhibit the degradation of SMX by radiation, because these anions could scavenge OH· and lead to a decrease of OH· concentration in the solution (Wang and Wang 2019). Adding organic matters also inhibited SMX degradation for their competition with SMX molecule to react with OH·.
Reusability of Fe3O4
The reusability of catalysts is important for their practical application. The reusability performance of Fe3O4 was illustrated in Fig. 11. Fe3O4 was used in the reaction solution for 5 cycles, and SMX was added repeatedly after each cycle under gamma irradiation. With 2 kGy radiation, SMX could be completely degraded after each cycle. Fe3O4 performed efficient reusability and stability for 5 cycles. Therefore, Fe3O4 was stable in gamma radiation process for the removal of antibiotics.
Degradation of intermediate products and pathway
Ionizing radiation is very effective for the degradation of organic pollutants; however, it is not efficient for their mineralization. Some degradation intermediate products formed during the radiation process, which were persistent after the total removal of the parent compounds (Fig. 12). Thus it is needed to identify the intermediate products generated during ionizing radiation. The intermediate products of SMX degradation by radiation treatment were identified by liquid chromatography (LC)–mass spectrometry (MS) (Table 2). The intermediate product at m/z of 283 corresponded to the oxidation of amino group at benzene ring, which was also identified in the research of ozone oxidation sulfamethoxazole (Gomez-Ramos et al. 2011), sulfamethoxazole removal by Fenton process (Wang and Wang 2017), radiolytic degradation of sulfamethoxazole (Kim et al. 2017).
As for the intermediate product at m/z of 283, the breakage of the bond between sulfur and benzene ring might result in the generation of the intermediate products at m/z 123 and 178, respectively. The intermediate product at m/z 299 may be the product of adding a ·OH to the aniline ring of nitro-sulfamethoxazole at m/z 283. The intermediate product at m/z 299 was also detected during the process of SMX degradation by Fenton oxidation (Wang and Wang 2017) and by ozone oxidation treatment (Abellan et al. 2008). The fragments at m/z 155 and m/z 99 may be due to the cleavage of the intermediate product at m/z of 299. The fragment at m/z of 189 could be the desulfurated product of SMX, as the loss of SO2 ions from SMX molecule under the oxidation of ·OH. This SO2 elimination and rearrangement pathway contained three steps: direct breakage of arylsulfone bond (C–S), direct breakage of sulfonamide bond (S–N), and SO2 extrusion (Kim et al. 2017) Desulfonated intermediate products were also reported previously (Kim et al. 2017; Garcia-Galan et al. 2012; Boreen et al. 2005). Inorganic product at m/z 96 was identified as sulfate ion (SO42- ) during the degradation of SMX, which could be formed due to the cleavage of arylsulfone bond (C–S), such as the oxidation of intermediate product at m/z 178 (Kim et al. 2017).
Conclusion
Sulfamethoxazole can be efficiently degraded by ionizing radiation combined with Fenton-like process. The addition of Fe3O4 could enhance SMX degradation as well as dose constant compared with the control group. SMX degradation followed pseudo-first-order kinetic model, and its efficiency decreased with the increase of initial concentration. SMX could be degraded in a wide pH range. Over 98% of SMX were degraded at pH ranging from 3.01 to 10.96. Moreover, the presence of Fe3O4 could promote the mineralization of SMX. TOC removal efficiency increased from 23.8 to 51.3% with the addition of Fe3O4. The addition of free radical scavenger (tert-butyl alcohol) obviously inhibited SMX degradation, indicating that ·OH radicals played the main role in the SMX degradation in the radiation/Fe3O4 system. Fe3O4 could enhance dose constants of ·OH, suggesting that Fe3O4 could promote the form of ·OH radicals and enhance the degradation efficiency of SMX and TOC. The degradation efficiency of SMX in secondary effluent of WWTP decreased from 100 to 84% compared with that in deionized water. The intermediate products of SMX degradation were identified, and the possible degradation pathway was tentatively proposed.
References
Abellan MN, Gebhardt W, Schroder HF (2008) Detection and identification of degradation products of sulfamethoxazole by means of LC/MS and -MSn after ozone treatment. Water Sci Technol 58:1803–1812
Basfar AA, Khan HM, Al-Shahrani AA, Cooper WJ (2005) Radiation induced decomposition of methyl tert-butyl ether in water in presence of chloroform: kinetic modelling. Water Res 39:2085–2095
Boreen AL, Arnold WA, McNeill K (2005) Triplet-sensitized photodegradation of sulfa drugs containing six-membered heterocyclic groups: Identification of an SO2 extrusion photoproduct. Environ Sci Technol 39:3630–3638
Changotra R, Guin JP, Khader SA, Varshney L, Dhir A (2019) Electron beam induced degradation of ofloxacin in aqueous solution: kinetics, removal mechanism and cytotoxicity assessment. Chem Eng J 356:973–984
Chen H, Wang JL (2019) Catalytic ozonation of sulfamethoxazole over Fe3O4/Co3O4 composites. Chemosphere 234:14–24
Chen K, Zhou JL (2014) Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 95:604–612
Chu LB, Zhuang ST, Wang JL (2018) Degradation kinetics and mechanism of penicillin G in aqueous matrices by ionizing radiation. Radiat Phys Chem 145:34–38
Dantas RF, Contreras S, Sans C, Esplugas S (2008) Sulfamethoxazole abatement by means of ozonation. J Hazard Mater 150:790–794
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583
Garcia-Galan MJ, Diaz-Cruz MS, Barcelo D (2012) Kinetic studies and characterization of photolytic products of sulfamethazine, sulfapyridine and their acetylated metabolites in water under simulated solar irradiation. Water Res 46:711–722
Gobel A, Thomsen A, McArdell CS, Joss A, Giger W (2005) Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ Sci Technol 39:3981–3989
Gomez-Ramos MD, Mezcua M, Aguera A, Fernandez-Alba AR, Gonzalo S, Rodriguez A, Rosal R (2011) Chemical and toxicological evolution of the antibiotic sulfamethoxazole under ozone treatment in water solution. J Hazard Mater 192:18–25
Gros M, Petrovic M, Ginebreda A, Barcelo D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36:15–26
Guo Z, Fei Z, Zhao Y, Zhang C, Liu F, Bao C, Lin M (2012) Gamma irradiation-induced sulfadiazine degradation and its removal mechanisms. Chem Eng J 191:256–262
Hou LW, Wang LG, Royer S, Zhang H (2016) Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J Hazard Mater 302:458–467
Hu J, Wang JL (2007) Degradation of chlorophenols in aqueous solution by gamma-radiation. Radiat Phys Chem 76:1489–1492
Huang RX, Fang ZQ, Yan XM, Cheng W (2012) Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem Eng J 197:242–249
Huang RX, Fang ZQ, Fang XB, Tsang EP (2014) Ultrasonic Fenton-like catalytic degradation of bisphenol A by ferroferric oxide (Fe3O4) nanoparticles prepared from steel pickling waste liquor. J Colloid Interface Sci 436:258–266
Karthikeyan KG, Meyer MT (2006) Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci Total Environ 361:196–207
Khan S, Han C, Sayed M, Sohail M, Jan S, Sultana S, Khan HM, Dionysiou DD (2019) Exhaustive photocatalytic Lindane degradation by combined simulated solar light-activated nanocrystalline TiO2 and inorganic oxidants. Catalysts 9:425
Kim HY, Kim TH, Cha SM, Yu S (2017) Degradation of sulfamethoxazole by ionizing radiation: Identification and characterization of radiolytic products. Chem Eng J 313:556–566
Liu YK, Wang JL (2013) Degradation of sulfamethazine by gamma irradiation in the presence of hydrogen peroxide. J Hazard Mater 250:99–105
Liu Y, Wang JL (2019) Reduction of nitrate by zero valent iron (ZVI)-based materials: a review. Sci Total Environ 671:388–403
Liu YK, Hu J, Wang JL (2014) Fe2+ enhancing sulfamethazine degradation in aqueous solution by gamma irradiation. Radiat Phys Chem 96:81–87
Liu Y, Fan Q, Wang JL (2018) Zn-Fe-CNTs catalytic in situ generation of H2O2 for Fenton-like degradation of sulfamethoxazole. J Hazard Mater 342:166–176
Mirzaei A, Chen Z, Haghighat F, Yerushalmi L (2017) Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—a review. Chemosphere 174:665–688
Niu HY, Zhang D, Zhang SX, Zhang XL, Meng ZF, Cai YQ (2011) Humic acid coated Fe3O4 magnetic nanoparticles as highly efficient Fenton-like catalyst for complete mineralization of sulfathiazole. J Hazard Mater 190:559–565
Peñalver JJL, Pacheco CVG, Polo MS, Utrilla JR (2013) Degradation of tetracyclines in different water matrices by advanced oxidation/reduction processes based on gamma radiation. J Chem Technol Biotechnol 88:1096–1108
Phan TPH, Managaki S, Nakada N, Takada H, Shimizu A, Anh DH, Viet PH, Suzuki S (2011) Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci Total Environ 409:2894–2901
Rehman F, Sayed M, Khan JA, Shah NS, Khan HM, Dionysiou DD (2018) Oxidative removal of brilliant green by UV/S2O8 2–, UV/HSO5 –and UV/H2O2 processes in aqueous media: a comparative study. J Hazard Mater 357:506–514
Sayed M, Ismail M, Khan S, Tabassum S, Khan HM (2016) Degradation of ciprofloxacin in water by advanced oxidation process: kinetics study, influencing parameters and degradation pathways. Environ Technol 37(5):590–602
Sayed M, Arooj A, Shah NS, Khan JA, Shah LA, Rehman F, Arandiyan H, Khan AM, Khan AR (2018) Narrowing the band gap of TiO2 by co-doping with Mn2+ and Co2+ for efficient photocatalytic degradation of enoxacin and its additional peroxidase like activity: a mechanistic approach. J Mol Liq 272:403–412
Sayed M, Gul M, Shah NS, Khan JA, Khan ZUH, Rehman F, Khan AR, Rauf S, Arandiyan H, Yang CP (2019) In-situ dual applications of ionic liquid coated Co2+ and Fe3+ co-doped TiO2: superior photocatalytic degradation of ofloxacin at pilot scale level and enhanced peroxidase like activity for calorimetric biosensing. J Mol Liq 282:275–285
Shah NS, Khan JA, Nawaz S, Khan, HM (2014) Role of aqueous electron and hydroxyl radical in the removal of endosulfan from aqueous solution using gamma irradiation. J Hazard Mater 278:40-48
Shah NS, Khan JA, Al-Muhtaseb AH, Sayed M, Khan HM (2016) Gamma radiolytic decomposition of endosulfan in aerated solution: the role of carbonate radical. Environ Sci Pollut Res 23(12):12362–12371
Shah NS, Khan JA, Sayed M, Khan ZUH, Rizwan AD, Muhammad N, Boczkaj G, Murtaza B, Imran M, Khan HM, Zaman G (2018a) Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ co-doped ZnO with the addition of HSO5 −: toxicities and degradation pathways investigation. Chem Eng J 351:841–855
Shah NS, Rizwan AD, Khan JA, Sayed M, Khan ZUH, Murtaza B, Lqbal J, Din SU, Imran M, Nadeem M, Al-Muhtaseb AH, Muhammad N, Khan HM, Ghauri M, Zaman G (2018b) Toxicities, kinetics and degradation pathways investigation of ciprofloxacin degradation using iron-mediated H2O2 based advanced oxidation processes. Process Saf Environ Prot 117:473–482
Shah NS, Khan JA, Sayed M, Khan ZUH, Ali HS, Murtaza B, Khan HM, Imran M, Muhammad N (2019) Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O8 2−. Chem Eng J 356:199–209
Shen YP, Zhuan R, Chu LB, Xiang X, Sun H, Wang JL (2019) Inactivation of antibiotic resistance genes in antibiotic fermentation residues by ionizing radiation: exploring the development of recycling economy in antibiotic pharmaceutical factory. Waste Manag 84:141–146
Sichel C, Garcia C, Andre K (2011) Feasibility studies: UV/chlorine advanced oxidation treatment for the removal of emerging contaminants. Water Res 45(19):6371–6380
Song WJ, Cheng MM, Ma JH, Ma WH, Chen CC, Zhao JC (2006) Decomposition of hydrogen peroxide driven by photochemical cycling of iron species in clay. Environ Sci Technol 40(15):4782–4787
Tang JT, Wang JL (2018a) Fenton-like degradation of sulfamethoxazole using Fe-based magnetic nanoparticles embedded into mesoporous carbon hybrid as an efficient catalyst. Chem Eng J 351:1085–1094
Tang JT, Wang JL (2018b) Metal organic framework with coordinatively unsaturated sites as efficient Fenton-like catalyst for enhanced degradation of sulfamethazine. Environ Sci Technol 52:5367–5377
Tang JT, Wang JL (2019) MOF-derived three-dimensional flower-like FeCu@C composite as an efficient Fenton-like catalyst for sulfamethazine degradation. Chem Eng J 375:122007
Trojanowicz M, Bartosiewicz I, Bojanowska-Czajka A, Kulisa K, Szreder T, Bobrowski K, Nichipor H, Garcia-Reyes JF, Nalecz-Jawecki G, Meczynska-Wielgosz S, Kisala J (2019) Application of ionizing radiation in decomposition of perfluorooctanoate (PFOA) in waters. Chem Eng J 357:698–714
Wan Z, Wang JL (2017) Degradation of sulfamethazine using Fe3O4-Mn3O4 /reduced graphene oxide hybrid as Fenton-like catalyst. J Hazard Mater 324:653–664
Wan Z, Hu J, Wang JL (2016) Removal of sulfamethazine antibiotics using Ce-Fe-graphene nanocomposite as catalyst by Fenton-like process. J Environ Manag 182:284–291
Wang JL, Bai ZY (2017) Fe-Based Catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem Eng J 312:79–98
Wang JL, Chu LB (2016) Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: an overview. Radiat Phys Chem 125:56–64
Wang JL, Wang SZ (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J Environ Manag 182:620–640
Wang SZ, Wang JL (2017) Comparative study on sulfamethoxazole degradation by Fenton and Fe (II)-activated persulfate process. RSC Adv 7(77):48670–48677
Wang JL, Wang SZ (2018a) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J 334:1502–1517
Wang JL, Wang SZ (2018b) Microbial degradation of sulfamethoxazole in the environment. Appl Microbiol Biotechnol 102:3573–3582
Wang SZ, Wang JL (2018c) Radiation-induced degradation of sulfamethoxazole in the presence of various inorganic anions. Chem Eng J 351:688–696
Wang JL, Wang SZ (2019) Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem Eng J 356:350–358
Wang JL, Xu LJ (2012) Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Technol 42:251–325
Wang JJ, Guo ZY, Shen XY, Guo QJ, Zhao YF, Zhu SN, Guo ZB (2017) Gamma irradiation-induced decomposition of sulfamethoxazole in aqueous solution: the influence of additives, biological inhibitory, and degradation mechanisms. Environ Sci Pollut Res 24:23658–23665
Wang JL, Zhuan R, Chu LB (2019) The occurrence, distribution and degradation of antibiotics by ionizing radiation: an overview. Sci Total Environ 646:1385–1397
Wardman P (1989) Reduction potentials of one-electron couples involving free-radicals in aqueous-solution. J Phys Chem Ref Data 18:1637–1755
Xu LJ, Wang JL (2011) A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol. J Hazard Mater 186:256–264
Xu LJ, Wang JL (2012a) Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ Sci Technol 46:10145–10153
Xu LJ, Wang JL (2012b) Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl Catal B Environ 123:117–126
Yang B, Tian Z, Zhang L, Guo YP, Yan SQ (2015) Enhanced heterogeneous Fenton degradation of methylene blue by nanoscale zero valent iron (nZVI) assembled on magnetic Fe3O4/reduced graphene oxide. J Water Process Eng 5:101–111
Yu S, Lee B, Lee M, Cho IH, Chang SW (2008) Decomposition and mineralization of cefaclor by ionizing radiation: kinetics and effects of the radical scavengers. Chemosphere 71(11):2106–2112
Yu SQ, Hu J, Wang JL (2010a) Gamma radiation-induced degradation of p-nitrophenol (PNP) in the presence of hydrogen peroxide (H2O2) in aqueous solution. J Hazard Mater 177:1061–1067
Yu SQ, Hu J, Wang JL (2010b) Radiation-induced catalytic degradation of p-nitrophenol (PNP) in the presence of TiO2 nanoparticles. Radiat Phys Chem 79:1039–1046
Zhang SX, Zhao XL, Niu HY, Shi YL, Cai YQ, Jiang GB (2009) Superparamagnetic Fe3O4 nanoparticles as catalysts for the catalytic oxidation of phenolic and aniline compounds. J Hazard Mater 167(1-3):560–566
Zheng BG, Zheng Z, Zhang JB, Luo XZ, Wang JQ, Liu Q, Wang LH (2011) Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation. Desalination 276(1-3):379–385
Zhuan R, Wang JL (2019) Degradation of sulfamethoxazole by ionizing radiation: Kinetics and implications of additives. Sci Total Environ 668:67–73
Funding
We are thankful for the financial support from the National Natural Science Foundation of China (51338005) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhuan, R., Wang, J. Enhanced mineralization of sulfamethoxazole by gamma radiation in the presence of Fe3O4 as Fenton-like catalyst. Environ Sci Pollut Res 26, 27712–27725 (2019). https://doi.org/10.1007/s11356-019-05925-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05925-1