Abstract
Water pollution has been a major concern for agrarian societies like Pakistan. Pharmaceutical industries are amongst the foremost contributor to industrial waste. Present study addresses the generation of oxidative stress caused by 2 months exposure to pharmaceutical wastewater in rats and their response to oral treatment with vitamin E, a potent antioxidant. The rats were randomized into five groups (n = 5) named as negative control, pharmaceutical wastewater (PEW) 100 %, PEW 10 %, PEW 1 %, and PEW 100 % + vitamin E. Oxidative damage in rats was evaluated by estimation of the activities of total superoxide dismutase (T-SOD), catalase (CAT), and the concentration of hydrogen peroxide (H2O2) in the liver, kidney, and blood/plasma. Exposure to pharmaceutical wastewater significantly decreased the activities of T-SOD and CAT and concentration of H2O2 in the liver and kidney and blood/plasma. Exposure to 100 % pharmaceutical wastewater exhibited a maximum decline in T-SOD activity, and activity was reduced to only 63.57 U/mL, 32.65, and 43.57 U/mg of protein in the plasma, kidney, and liver, respectively. Exposure to wastewater minimized activity CAT to 89.25 U/g of hemoglobin, 54.36, and 62.95 U/mg of protein in the blood, kidney, and liver, respectively. Treatment with vitamin E significantly increased the activity of T-SOD and CAT. However, increase in concentration of H2O2 was also observed in vitamin E exposed rats. Histopathology of the kidney revealed coagulative necrosis of renal epithelial cells and peritubular congestion. Endocardium showed infiltration of inflammatory cells and cellular breakdown in some areas. Lung sections exhibited atelectasis and emphysema of alveoli suggesting decline in lung function. The anatomy of the liver was also compromised due to severe degeneration and cellular swelling. The present study concluded that pharmaceutical wastewater induced severe oxidative stress in Wistar rats and ensued in histopathological lesions in several vital organs suggesting its high toxicity. Non-enzymatic antioxidant vitamin E may ameliorate oxidative stress induced by pharmaceutical wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hundreds of tons of pharmaceuticals are released into the environment either as such or as their metabolites. Most of pharmaceuticals have been reported at trace levels (hundreds of nanograms per liter) in the rivers and lake water. It indirectly substantiates capability to persist in surface waters. The xenobiotic nature of pharmaceuticals would suggest to impede their release to the environment by confining the sources of pollution (Andreozzi et al. 2004) (Andreozzi et al. 2002).
Toxic heavy metals such as cadmium, arsenic, mercury, and lead have been reported to occur in environment. Humans are exposed to these metals through different sources such as contaminated air, food, water, and soil. Transition metals play a role of catalyst in oxidative reactions of biological macromolecules so toxicity induced by heavy metals might be due to oxidative damage. Iron, copper, and chromium undergoes redox recycling known as redox active metals whereas lead, cadmium, and mercury depletes major antioxidants especially thiol-containing antioxidants and enzymes (Ercal et al. 2001). It has been found that redox metals like iron (Fe), copper (Cu), and chromium (Cr) including some other metals have the property to produce reactive radicals such as super oxide anion radical and nitric oxide in biological systems (Jomova and Valko 2011).
Disturbance of metal ion homeostasis can lead to oxidative stress, which is characterized by generation of reactive oxygen species (ROS) which can overcome body antioxidant potential and results in damage to DNA, lipid peroxidation, protein modifications, and other effects which are indicative to certain diseases which include cardiovascular disease, diabetes, atherosclerosis, neurological disorders (Alzheimer’s disease, Parkinson’s disease), and chronic inflammation. The mechanism behind all these diseases is formation of superoxide radical, hydroxyl radical, and other ROS leading to mutagenicity and carcinogenicity due to malondialdehyde (MDA), 4-hydroxynoneal (HNE) and exocyclic DNA adducts (Jomova and Valko 2011). It is also found that metals owing to their toxicity interact with DNA and proteins leading to oxidative worsening of biological macromolecules. This process of breakdown of metal ions leads to a number of diseases (Mates 2000; Valko et al. 2006). A number of low molecular weight antioxidants, ascorbic acid (vitamin C), alpha tocopherol (vitamin E), glutathione (GSH), carotenoids, and flavonoids have the capacity of metal ions chelation reducing their catalytic activity to form ROS (Jomova and Valko 2011). It was suggested that biochemical markers used for hazard identification is the most suitable procedure. These biochemical markers have the potential of characterizing the hazardous substance under study and are considered a flexible approach in terms of using other tools (Jemec et al. 2010).
It has been determined earlier in our study that pharmaceutical wastewater (PEW) is a complex mixture of heavy metals and environmental contaminants (Sharif et al. 2016). In continuation of the study, oxidative stress and histopathological studies were conducted to validate that DNA damage caused by heavy metals, and environmental contaminants had also been associated with oxidative stress induction and toxicity to various organs. Furthermore, ameliorating effect of vitamin E on oxidative stress induction has also been investigated in the present study.
Material and method
Water sampling and characterization
PEW samples were collected from different pharmaceutical industries of Lahore in sterile glass bottles. Metal contents of the samples were determined by atomic absorption spectrophotometer (AAS), and gas chromatogram mass spectrophotometer (GC-MS) was used for screening of the organic environmental pollutants (Sharif et al. 2016).
Animals grouping
Experimental animals, 90-day-old male Wistar rats (130–150 g), were purchased from Department of Theriogenology, University of Veterinary and animal sciences (UVAS) Lahore. The rats were housed independently in stainless steel wire netting cages, air-conditioned room (temperature 21–25 °C and relative humidity was 50–60 %). All the animals were acclimatized for 10 days before the experiment start and were kept in 12 h day/light cycle.
Vitamin E (D-α-Tocopherol-polyethylene glycol 1000 succinate, water-soluble form) was obtained from Sigma-Aldrich. It was given orally as 100 mg/kg of body weight daily for 60 days. (El-Demerdash et al. 2004). The rats were randomized into five groups with five rats in each group and named as negative control, PEW 100 %, PEW 10 %, PEW 1 %, and PEW 100 % + vitamin E, respectively. The negative control group was provided with tap water, PEW 100 % group received pure pharmaceutical wastewater, PEW 10 % received 10 % pharmaceutical water, and PEW 1 % group was given 1 % of pharmaceutical wastewater. All the dilutions were made with the same water used for negative control. PEW 100 % + vitamin E group received pure pharmaceutical wastewater and vitamin E as an antioxidant. All the rats received treatment for 60 days. Rats were provided with same rat chow ad libtium (Banos et al. 2005).
Ethical consideration
All the experimental protocols were performed in compliance with Institutional Guidelines for the Care and Use of Laboratory Animals, UVAS, Lahore, Pakistan.
In vivo collection of samples
Wistar rats were fasted for 12 h, anesthetized (with diethyl ether), and were fixed on the experimental desk. Two to four milliliter blood was collected with the help of a catheter which was inserted into a celiac artery. The blood was centrifuged at 1000 rpm for 10 min at 4 °C. The plasma as top yellow layer was carefully removed and store on an ice pack for further analysis.
Removal of the liver and kidney
One gram of the kidney and liver tissues were taken, washed with cold normal saline, and dried with filter paper. These tissues were further minced and ground to prepare 10 % tissue homogenates according to the previous method (Akhtar et al. 2016a). One milliliter of supernatant was diluted with 9 mL normal saline to prepare 1 % tissue homogenate.
Measurement of protein content
Concentration of proteins in tissue was measured by Lowry’s method of protein determination (Lowry et al. 1951). Bovine serum albumin was used as standard.
Measurement of SOD activity
Total SOD (T-SOD) activity was determined using SOD assay kit A001 (Institute of Biological Engineering of Nanjing Jianchen, Nanjing, China) (Ji et al. 1991). Superoxide anion free radical was generated in reaction system which comprised of xanthine and xanthine oxidase, and the scavenging activity of superoxide anion free radical was measured at 550 nm with glass cuvette. T-SOD activity of the plasma and tissues (liver and kidney) were expressed in units per milliliter and units per milligram protein, respectively (Luo et al. 2014).
Estimation of catalase activity
The activity of CAT was measured using a commercial kit A007–2 (Institute of Biological Engineering of Nanjing Jianchen, Nanjing, China). CAT activity was estimated by directly measuring the substrate H2O2 which decreased directly as the CAT level decreases in solution mixture. It was expressed as units per milliliter of blood and units per milligram of protein for the blood and tissue, respectively (Zhang et al. 2008).
Concentration of H2O2
The concentration of H2O2 was determined using a commercial kit A064 (Institute of Biological Engineering of Nanjing Jincheng, Nanjing, China). H2O2 forms a complex with molybdate, and OD value at 405 nm is directly associated with hydrogen peroxide in test sample. It was expressed as millimole per liter.
Histopathology
The rats were starved for 12 h and then sacrificed by decapitation by following the experimental protocol in compliance with the Institutional Guidelines for Care and Use of Laboratory Animals of University of Veterinary and Animal Sciences, Lahore, Pakistan DR/459. The liver and kidney were carefully removed and weighed. Any gross lesions observed were recorded. The tissues were fixed in 10 % PFA (formaldehyde solution) and processed for histological investigation. The tissues were embedded in paraffin wax, sectioned for 2 μm thickness, mounted on slides, and were stained with hematoxylin and eosin (H&E) for routine light microscopy. The slides were investigated for any deleterious effects or appearance of lesions appeared after treatment with PEW (Ejaz et al. 2009).
Results
Chemical analysis
The results of chemical analysis of pharmaceutical wastewater revealed higher levels of Fe 4.96 mg L−1. Similarly, Cr concentration was found to be 0.43 mg L−1. Lead (Pb) was also present in higher than the normal permissible limits 0.21 mg L−1. Arsenic (As) concentration of wastewater was found to be 0.83 mg L−1. Cadmium (Cd) was also present in the sample, and its concentration was 0.02 mg L−1. All these metals were present in concentrations higher than normal permissible limits of Environmental Protection Agency. Organic pollutants were also detected in the sample pharmaceutical wastewater using GC-MS. It was found that the wastewater was polluted with a number of organic pollutants including prednisolone, phenolic compounds, trimethoprim, and tolune (Sharif et al. 2016).
Estimation of T-SOD activity
T-SOD activity in plasma of control group was 92.54 ± 0.19 U/mL. The activity of T-SOD in PEW 100 % group was 63.7 ± 0.19 U/mL. It was found that T-SOD activity decreased significantly when rats were exposed to PEW 100 % orally (p ˂ 0.05). Administration of vitamin E helped in alleviating the decreased activity of T-SOD. It was found that the group PEW 100 % + vitamin E exhibited marked improvement in T-SOD activity (70.65 ± 2.44 U/mL) when compared to group PEW 100 % group (Table 1).
The activity of T-SOD in the control group of kidney homogenate was 63.75 ± 0.15 U/mg of protein. T-SOD exhibited a decreased activity in PEW 100 % group (32.65 ± 0.77 U/mg) of protein. The results were significantly different from control group (p ˂ 0.05). The group receiving PEW 100 % + vitamin E exhibited a significant increase in activity (43.07 ± 0.29 U/mg) of protein. These results were significantly difference (p ˂ 0.0.5) when compared with 100 %PEW group (Table 2).
Liver homogenates of group PEW 100 % exhibited T-SOD activity (43.57 ± 0.23 U/mg) of protein which is less than control group (57.91 ± 0.29 U/mg) of protein. The results were significantly different (p ˂ 0.05). However, treatment with vitamin E moderately improved the activity of T-SOD (47.36 ± 0.29 U/mg) of protein (p ˂ 0.05) as compared to PEW 100 % group (Table 3).
Estimation of catalase activity
The activity of CAT in blood of control group was 146.5 ± 1.32 U/g of hemoglobin. PEW 100 % group exhibited activity 89.25 ± 2.03 U/mg of hemoglobin which is statically different from control group (p ˂ 0.05). PEW 100 % + vitamin E increased the activity of catalase in blood (100.1 ± 0.77) which was found to be statically significant (p ˂ 0.05) when compared with PEW 100 % (Table 1).
Activity of catalase in control and PEW 100 % groups of the kidney were 130.7 ± 1.75 and 54.36 ± 1.71 U/mg of protein, respectively. Treatment with vitamin E increased the activity of catalase in the kidney (68.11 ± 4.55 U/mg) of protein. The results were found to be significantly different (p ˂ 0.05) when compared with PEW 100 % group (Table 2).
Liver homogenate of control and PEW 100 % group showed catalase activity of 135 ± 2.95 and 62.95 ± 2.82 U/mg of protein respectively. Catalase activity in PEW 100 % + vitamin E group was significantly different 72.75 ± 3.26 U/mg of protein (p ˂ 0.05) when compared with both groups mentioned above (Table 3).
Concentration of H2O2
It was found that the plasma concentration of H2O2 in control group rats was found to be 220.6 ± 2.67 mmol/L, in group PEW 100 % was 68.80 ± 2.67 mmol/L, and in group PEW 100 % + vitamin E was 129.5 ± 2.67 mmol/L. H2O2 concentration in PEW 100 % + vitamin E group differed significantly (p ˂ 0.05) when compared with all other groups (Table 1).
Results of kidney homogenate showed that group which received PEW 100 % + vitamin E exhibited higher concentration of H2O2 (279.3 ± 1.75 mmol/L) when compared with group which received PEW 100 %. Results also exhibited that concentration of H2O2 differed significantly (p ˂ 0.05) when compared with all other groups (Table 2).
H2O2 concentration was also measured in liver homogenates of all groups. PEW 100 % group exhibited 162.9 ± 2.67 mmol/L which was increased to 267.1 ± 1.75 mmol/L in group PEW 100 % which also received vitamin E. The results were significantly different when compared with control group (Table 3).
Elevated concentrations of H2O2 significantly tapered by vitamin E in the plasma, liver, and kidney. Changes induced by PEW were ameliorated by vitamin E (p ˂ 0.05).
Histopathology
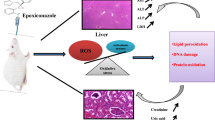
Histopathological lesions were observed in Wistar rats after 60 days exposure to PEW 100 %. Coagulative necrosis of renal epithelial cells and widespread disintegration of tubular cells were observed. Some tubular cells exhibited cellular swelling, and peritubular congestion was also observed. The anatomy of the liver was also compromised due to severe degeneration and cellular swelling. The hepatocytes of hepatic cord also indicated coagulative necrosis. The histopathological evaluation of lung sections exhibited atelectasis and emphysema of alveoli suggesting decline in lung function. Pharmaceutical wastewater adversely affected the intestinal epithelial cells, leading to their degeneration and sloughing off. Exposure to pharmaceutical wastewater also adversely affected histology of heart. Endocardium showed infiltration of inflammatory cells and cellular breakdown in some areas (Fig. 1). No significant changes were found in the brain (cerebellum and cerebrum). There were no changes in cell bodies. Microglial cells were intact, and no obvious pathological changes were observed.
a–e Representative histological images of tissue sections followed oral exposure of PEW for 60 days. a Kidney section of rat highlighting necrosis of cells along with control group showing intact glomerulus. b Liver section of rat highlighting degeneration and swelling of hepatocytes along with control c Lungs section of rat representing atekation and emphysema of alveoli d Intestine section of rat highlighting mild degeneration and sloughing of intestinal epithelial cells e Heart section of rat highlighting break down of cells in endocardium and presence of some inflammatory cells
Discussion
In the present study, role of pharmaceutical wastewater was investigated for induction of oxidative stress in Wistar rats. Role of vitamin E role in ameliorating oxidative stress in the kidney, liver, and blood/plasma was investigated after an exposure for 60 days. The liver and kidneys were selected because these are considered to be the major target of toxicity. The liver-containing metabolizing enzymes have the ability to biotransform the xenobiotics into less toxic or active metabolite. The liver has been a site for lipid peroxidation so considered a hall mark for the oxidative stress tests. The kidneys do perform same activities along with filtration (Patlolla et al. 2009).
Heavy metals whether redox active or redox inactive might have the potential of increasing the production of reactive oxygen species (ROS) e.g., hydroxyl (OH.), superoxide (O2 −) radicals, or H2O2. Generation of ROS in a large quantity overwhelms the intrinsic antioxidant defenses resulting in condition known as oxidative stress. Cells under oxidative stress display various anomalies which might be caused by interaction of ROS with lipids, proteins, and DNA (Ercal et al. 2001).
It was reported that K2Cr2O7 depressed the activities of SOD, CAT, and GSH-Px in rat’s epithelial intestinal cell (Sengupta et al. 1990). It was later confirmed that chromium (VI) decreased the enzymatic antioxidant levels which included SOD and CAT (Susa et al. 1996). Our study is in accordance with a previous investigation which reported that a 60 days exposure of textile wastewater ensued induction of oxidative stress in Wistar rats. However, treatment with vitamin C exhibited a decline in wastewater induced oxidative damage (Akhtar et al. 2016a). Normally enzymatic and non-enzymatic antioxidant systems exist in body. Overproduction of free radicals or diminished scavenging capacity of enzymes or both mechanisms has been involved in lipid peroxidation which is a chain process, initial oxidation of few molecules leads to massive tissue destruction (Zaidi and Banu 2004). Several diseases of the central nervous system and cardiovascular disorders have been linked to reactive oxygen species and the inhibition of antioxidant defense mechanism (Smythies 1999).
Pb has also been associated with oxidative stress. Lead nitrate decreased the activities of SOD, CAT, and GSH and resultant tissues (liver and kidneys) were exposed to per oxidative damage. Both of these enzymes are metalloproteins which perform their function by detoxifying peroxides (−OOH), H2O2, and O2 * (Lakshmi et al. 2013). Pb toxicity has also been found to be multifactorial. Possible mechanisms associated with Pb induced oxidative stress include enzyme inhibition and a decline in mineral absorption, and it reduced binding with sulfhydryl groups. As has also been linked with oxidative damage in tissues through generation of ROS e.g., superoxide, hydroxyl, and peroxyl radicals. Decreased activity of SOD and CAT in the liver of rats and increased MDA levels were suggested to be the possible mechanisms of arsenic induced oxidative injury (Santra et al. 1999), (Xu et al. 2013). Present study suggests that the occurrence of excess amounts of metals such as Pb, As, and Cr in pharmaceutical wastewater contributed to the oxidative potential of this wastewater.
Oxidative stress has been associated with redox imbalance in various cancer cells when compared with normal cells. This redox imbalance might be attributed to oncogenic stimulation. Mutation of DNA is linked with lesions associated with DNA which have been appeared in different tumors showing the involvement of oxidative stress in etiology of cancer (Akhtar et al. 2016b).
Pharmaceutical residues, sex steroid hormones, and personal care products have been categorized as new emerging pollutants. Prednisolone was detected in the pharmaceutical wastewater. Steroids have been associated with deterioration of endocrine and reproductive function. Studies have proposed increased risk of breast, prostate, and testicular carcinomas on exposure to endocrine disrupting substances like prednisolone (Kudłak and Namieśnik 2008). Phenolic compounds have been identified as potent environmental pollutants. Pharmaceutical, petroleum, and chemical industries have contributed in the discharge of a large amount phenolic compounds. Phenolic compounds led to the generation of free radicals in organs like the liver leading to the generation of phenoxy radicals and intermediate metabolites resulting in generation of superoxide radicals and hydrogen peroxide (Michałowicz and Duda 2007). Toluene has been found associated with the increase in formation of reactive oxygen species, and decrease in oxidative stress markers like SOD, CAT, and glutathione S-transferase when different concentrations of toluene were exposed to Drosophila melanogaster was exposed to different concentrations of toluene (Singh et al. 2009).
Non-enzymatic antioxidants such as vitamin A, C, and E are effective antioxidants and had a potential to mitigate oxidative stress induced injuries (Olas and Wachowicz 2002) (Chaudiere and Ferrari-Iliou 1999). Several studies have reported that vitamin E therapy mitigated oxidative damage in the liver and kidney (Onyema et al. 2006), (Rao et al. 2006). This study is in agreement with previous investigations that vitamin E do play some role in relieving oxidative stress and improving the biomarkers related to stress. Although ameliorating effects of vitamin E on the activities of SOD and CAT were observed, yet H2O2 concentration in control group was higher than those of other groups. Although increase in SOD activity leads to an increase production of H2O2. However, some heavy metals like arsenic have an interesting pathway of increasing the production of H2O2 via mechanisms other than SOD induction which is explicated by oxidation of As (III) to As (V). High level of H2O2 causes induction of CAT activity. It is suggested that under physiological conditions arsenic can directly produce H2O2. These heavy metals also cause the production of free radicals usually through reactions independent of CAT activity such Fenton’s reactions which further affects H2O2 concentration in the liver and kidney tissues (Jomova and Valko 2011; Jurczuk et al. 2004).
Environmental metals like lead, chromium, and cadmium are known hepatotoxic agents (Cui et al. 2004), (Mousa 2004), (Ramm 2005). They have a potential to cause chronic renal diseases (Shaikh et al. 1999), (Barbier et al. 2005). It was found that a mixture of heavy metals can induce oxidative stress in the liver, kidney, brain, and erythrocytes. The mechanism involved might be lipid peroxidation along with decreasing ability of antioxidative defense systems in male rat (Jadhav et al. 2007a), (Jadhav et al. 2006). The final observation of the study suggested that mixture of heavy metals may cause liver injury on prolonged exposure and poses a health risk. The kidney particularly proximal tubules has been a potential first target of toxicity caused by heavy metals (Barbier et al. 2005), (Madden and Fowler 2000). It was accessed that oxidative stress inducing potential of heavy metals was maximum in the kidney as lipid peroxidation and diminution in enzymatic and non-enzymatic antioxidant status was maximum (Jadhav et al. 2007b).
In vivo toxicological studies should include animals from both sexes. However, gender dependent effects may be prominent in some studies. There are strong evidences of higher antioxidant activity in female than male. Female animals are also less prone to oxidative stress than male (Chakraborti et al. 2007).
Wastewater from industries should be treated before expulsion into the environment or open water bodies. Currently, there is no wastewater treatment plant operational in the industrial areas of Lahore. There should be strict legislation regarding this issue. It is estimated that half of the wastewater produced worldwide is discarded without specific treatment. Different methods used to treat pharmaceutical wastewater can be categorized as biological processes such as aerobic and anaerobic treatments, advance treatments which include membrane technology, activated carbon, and membrane distillation processes. Advanced oxidation processes included ozone treatment, photo catalysis, and Fenton oxidation etc. Hybrid technologies have also been employed for the removal of heavy metals from pharmaceutical wastewater (Gadipelly et al. 2014). Several methods have been proposed to remove certain pharmaceutical compounds like caffeine, cyclophosphamide, and diethyl meta-toluamide, and there concentrations were reduced markedly from wastewater using ozone-based processes (Kim and Tanaka 2010). Nalidixic acid a persistent fluoroquinolone was removed efficiently from wastewater of pharmaceutical industries using photo-Fenton processes, and a resultant decrease in toxicity was observed (Sirtori et al. 2009).
Conclusion
The present study concluded that pharmaceutical wastewater might be associated with generation of oxidative stress and alteration in levels of T-SOD, CAT, and H2O2 in the liver, kidney, and plasma specimen, and this stress may be attributed to the pathological changes induced in these organs. However, non-enzymatic antioxidant vitamin E may improve oxidative stress. There is a strong urge that pharmaceutical wastewater must be detoxified through treatment plants before emission into environment. Treated and untreated wastewaters must be characterized not only on chemical basis but short-term toxicity test must also be employed to determine their toxicity potentials.
Statistical analysis
Results were expressed as mean ± standard error mean (n = 5). One-way ANOVA test at p = 0.05 was applied on the data using GraphPad Prism 6, followed by a Tuckey’s post hoc analysis.
References
Akhtar MF, Ashraf M, Anjum AA, Javeed A, Sharif A, Saleem A, Akhtar B (2016a) Textile industrial effluent induces mutagenicity and oxidative DNA damage and exploits oxidative stress biomarkers in rats. Environ Toxicol Pharmacol 41:180–186. doi:10.1016/j.etap.2015.11.022
Akhtar MF et al (2016b) Toxicity appraisal of untreated dyeing industry wastewater based on chemical characterization and short term bioassays. Bull Environ Contam Toxicol 96:502–507. doi:10.1007/s00128-016-1759-x
Andreozzi R, Marotta R, Pinto G, Pollio A (2002) Carbamazepine in water: persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res 36:2869–2877
Andreozzi R et al (2004) Effects of advanced oxidation processes (AOPs) on the toxicity of a mixture of pharmaceuticals. Water Science & Technology 50:23–28
Banos G, Medina-Campos ON, Maldonado PD, Zamora J, Pérez I, Pavón N, Pedraza-Chaverrí J (2005) Antioxidant enzymes in hypertensive and hypertriglyceridemic rats: effect of gender. Clin Exp Hypertens 27:45–57
Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P (2005) Effect of heavy metals on, and handling by, the kidney. Nephron Physiology 99:105–p110
Chakraborti A, Gulati K, Banerjee BD, Ray A (2007) Possible involvement of free radicals in the differential neurobehavioral responses to stress in male and female rats. Behav Brain Res 179:321–325
Chaudiere J, Ferrari-Iliou R (1999) Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol 37:949–962
Cui X et al (2004) Subchronic exposure to arsenic through drinking water alters expression of cancer-related genes in rat liver. Toxicol Pathol 32:64–72
Ejaz S, Chekarova I, Cho JW, Lee SY, Ashraf S, Lim CW (2009) Effect of aged garlic extract on wound healing: a new frontier in wound management. Drug Chem Toxicol 32:191–203
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food Chem Toxicol 42:1563–1571
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539. doi:10.2174/1568026013394831
Gadipelly C, Pérez-González A, Yadav GD, Ortiz I, Ibáñez R, Rathod VK, Marathe KV (2014) Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res 53:11571–11592
Jadhav S, Sarkar S, Tripathi H (2006) Cytogenetic effects of a mixture of selected metals following subchronic exposure through drinking water in male rats. Indian J Exp Biol 44:997
Jadhav S, Sarkar S, Patil R, Tripathi H (2007a) Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: a biochemical and histopathological study in male rats. Arch Environ Contam Toxicol 53:667–677
Jadhav SH, Sarkar SN, Kataria M, Tripathi HC (2007b) Subchronic exposure to a mixture of groundwater-contaminating metals through drinking water induces oxidative stress in male rats. Environ Toxicol Pharmacol 23:205–211
Jemec A, Drobne D, Tišler T, Sepčić K (2010) Biochemical biomarkers in environmental studies—lessons learnt from enzymes catalase, glutathione S-transferase and cholinesterase in two crustacean species. Environ Sci Pollut Res 17:571–581
Ji J, Wu Z, Liu Q, Zhang Y, Ye M, Li M (1991) An ultramicroanalytic and rapid method for determination of superoxide dismutase activity. Journal of Nanjing Railway Medical College 10:27–29
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Jurczuk M, Brzóska MM, Moniuszko-Jakoniuk J, Gałażyn-Sidorczuk M, Kulikowska-Karpińska E (2004) Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol 42:429–438
Kim I, Tanaka H (2010) Use of ozone-based processes for the removal of pharmaceuticals detected in a wastewater treatment plant. Water Environment Research 82:294–301
Kudłak B, Namieśnik J (2008) Environmental fate of endocrine disrupting compounds—analytical problems and challenges. Crit Rev Anal Chem 38:242–258
Lakshmi B, Sudhakar M, Aparna M (2013) Protective potential of black grapes against lead induced oxidative stress in rats. Environ Toxicol Pharmacol 35:361–368
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luo Z, Wang B, Liu M, Jiang K, Liu M, Wang L (2014) Effect of dietary supplementation of vitamin C on growth, reactive oxygen species, and antioxidant enzyme activity of Apostichopus japonicus (Selenka) juveniles exposed to nitrite. Chin J Oceanol Limnol 32:749–763
Madden EF, Fowler BA (2000) Mechanisms of nephrotoxicity from metal combinations: a review. Drug Chem Toxicol 23:1–12
Mates J (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Michałowicz J, Duda W (2007) Phenols—sources and toxicity. Pol J Environ Stud 16:347–362
Mousa SA (2004) Expression of adhesion molecules during cadmium hepatotoxicity. Life Sci 75:93–105
Olas B, Wachowicz B (2002) Resveratrol and vitamin C as antioxidants in blood platelets. Thromb Res 106:143–148
Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO (2006) Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys 43:20
Patlolla AK, Barnes C, Yedjou C, Velma V, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24:66–73
Ramm GA (2005) Ruddell RG Hepatotoxicity of iron overload: mechanisms of iron-induced hepatic fibrogenesis. In: Seminars in liver disease. vol 4. pp 433–449
Rao MV, Parekh SS, Chawla SL (2006) Vitamin-E supplementation ameliorates chromium-and/or nickel induced oxidative stress in vivo. J Health Sci 52:142–147
Santra A, Das Gupta J, De B, Roy B, Guha Mazumder D (1999) Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol 18:152–155
Sengupta T, Chattopadhyay D, Ghosh N, Das M, Chatterjee G (1990) Effect of chromium administration on glutathione cycle of rat intestinal epithelial cells. Indian J Exp Biol 28:1132–1135
Shaikh ZA, Vu TT, Zaman K (1999) Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol 154:256–263
Sharif A et al (2016) Pharmaceutical wastewater being composite mixture of environmental pollutants may be associated with mutagenicity and genotoxicity. Environ Sci Pollut Res 23:2813–2820
Singh MP, Reddy MK, Mathur N, Saxena D, Chowdhuri DK (2009) Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: role of ROS generation. Toxicol Appl Pharmacol 235:226–243
Sirtori C, Zapata A, Oller I, Gernjak W, Agüera A, Malato S (2009) Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res 43:661–668
Smythies J (1999) The neurotoxicity of glutamate, dopamine, iron and reactive oxygen species: functional interrelationships in health and disease: a review—discussion. Neurotox Res 1:27–39
Susa N, Ueno S, Furukawa Y, Sugiyama M (1996) Protective effect of vitamin E on chromium (VI)-induced cytotoxicity and lipid peroxidation in primary cultures of rat hepatocytes. Arch Toxicol 71:20–24
Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Xu Z et al (2013) Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem Toxicol 58:1–7
Zaidi SKR, Banu N (2004) Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta 340:229–233
Zhang Y, Luo Y, Hou Y-X, Jiang H, Chen Q, Tang H-R (2008) Chilling acclimation induced changes in the distribution of H2O2 and antioxidant system of strawberry leaves. Agric J 3:286–291
Acknowledgments
The authors wish to thank Chairman Department of Pharmacology and Toxicology for his kind support and valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experimental protocols were performed in compliance with Institutional Guidelines for the Care and Use of Laboratory Animals, UVAS, Lahore, Pakistan.
Funding
The research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sharif, A., Ashraf, M., Javeed, A. et al. Oxidative stress responses in Wistar rats on subacute exposure to pharmaceutical wastewater. Environ Sci Pollut Res 23, 24158–24165 (2016). https://doi.org/10.1007/s11356-016-7717-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7717-7