Abstract
Increasing use of heavy metals in various fields, their environmental persistency, and poor regulatory efforts have significantly increased their fraction in river water. We studied the effect of Musi river water pollution on oxidative stress biomarkers and histopathology in rat after 28 days repeated oral treatment. River water analysis showed the presence of Zn and Pb at mg/l concentration and Ag, As, Ba, Cd, Co, Cr, Cu, Mn, Mo, Ni, Sn, and Sb at μg/l concentration. River water treatment resulted in a dose-dependent accumulation of metals in rat organs, being more in liver followed by kidney and brain. Metal content in both control and low-dose group rat organs was below limit of detection. However, metal bioaccumulation in high- and medium-dose group organs as follows: liver—Zn (21.4 & 14.5 μg/g), Cu (8.3 & 3.6 μg/g), and Pb (8.2 & 0.4 μg/g); kidney—Zn (16.2 & 7.9 μg/g), Cu (3.5 & 1.4 μg/g), Mn (2.9 & 0.5 μg/g), and Pb (2.6 & 0.5 μg/g); and brain—Zn (2.4 & 1.1 μg/g), and Ni (1 & 0.3 μg/g). These metals were present at high concentrations in respective organs than other metals. The increased heavy metal concentration in treated rat resulted significant increase in superoxide dismutase, glutathione peroxidase, glutathione reductase, glutathione S transferase enzymes activity, and lipid peroxidation in a dose-dependent manner. However, glutathione content and catalase activity were significantly decreased in treated rat organs. Histopathological examination also confirmed morphological changes in rat organs due to polluted river water treatment. In conclusion, the findings of this study clearly indicate the oxidative stress condition in rat organs due to repeated oral treatment of polluted Musi river water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
River water is a natural resource that supports livelihood for diverse group of people. Cultivation of leafy vegetables, rice, fodder grass along with livestock rearing and fishing are practiced using river water (Buechler et al. 2002). In India, there has been a substantial rise in the pollution of various rivers during the past few decades (Fatima and Ahmad 2006). The enormously increasing population, industries, urbanization, lack of environmental awareness, untreated effluent discharge from industries and municipalities, and use of non-biodegradable pesticides and chemical fertilizers are causing water pollution. As a consequence of such unmanaged anthropogenic activities, many contaminants like heavy metals (Beg and Ali 2008; Chary et al. 2008), polycyclic aromatic hydrocarbons (Malik et al. 2011), and pesticides (Kaushik et al. 2010) have been found in most of the Indian rivers. These pollutants have been shown to be hazardous even at trace concentrations; hence, the river pollution may deserve more public health concern (Zhang et al. 2012). Organic pollutants can be partially removed from the river water by self-purification and accessible dilution (Chary et al. 2008). However, inorganic pollutants, in particular heavy metals, are of great concern due to their non-biodegradability, high persistence, and bioaccumulation at various trophic levels (Scalon et al. 2010). Many studies have shown that prolonged irrigation of agricultural land with river water polluted with sewage and industrial waste has resulted in heavy metal accumulation in soil, vegetables, cereals, and milk (Li et al. 2006; Raj et al. 2006; Singh et al. 2010).
Intake of contaminated food and water is the major route of human exposure to heavy metals (Nabulo et al. 2011; Singh et al. 2010). Following their entry into the human body, heavy metals tend to accumulate in various organs causing potential health hazards (Li et al. 2013). Intracellular accumulation of redox-active metals, viz., iron, copper, chromium, and cobalt, catalyzes free-radical reactions that disrupt the electron transport chain in mitochondria, causing continuous generation of reactive oxygen species (ROS). Disturbance of oxidant/antioxidant balance leads to oxidative stress that subsequently induces DNA damage, lipid peroxidation, and protein modification (Espín et al. 2014). Heavy metal-induced oxidative stress has been implicated in the pathogenesis of numerous diseases like cancer, cardiovascular disease, diabetes, atherosclerosis, neurological disorders, and chronic inflammation (Alissa and Ferns 2011; Jomova and Valko 2011; Uttara et al. 2009). On the other hand, redox-inert metals such as cadmium, zinc, and arsenic exhibit their toxicity and carcinogenicity by diminishing cellular glutathione levels and binding to sulfhydryl groups of proteins (Jomova and Valko 2011). Moreover, the consumption of heavy metal-contaminated food can also deplete some essential nutrients that are required to maintain immunological defenses in the body (Iyengar and Nair 2000; Srinivasan and Reddy 2009). Earlier studies reported that heavy metal-polluted river water exposure induced oxidative stress in various biological models. Farombi et al. (2007) reported that African cat fish collected from the Ogun river of Nigeria showed significant alterations in oxidative stress biomarkers due to heavy metal accumulation in various organs. Similarly, Indian fresh water fish Wallago attu brought from river Yamuna exhibited oxidative stress condition in various organs due to river pollution (Pandey et al. 2003). Male rats exposed to polluted Yangtze river water induced reproductive toxicity by substantially increasing percentage of abnormal sperms (Zhao et al. 2009).
The river Musi spreads over 8000 km2 and flows through Hyderabad, the most economically developed and fifth largest city in India. This river is providing water for agriculture and industries for decades. There are approximately 22 villages in its basin, and about 40,500-ha land is irrigated with its water (Srinivasan and Reddy 2009; van der Hoek 2004). However, the Hyderabad city discharges about 600 million liters per day of untreated sewerage water into Musi river. In addition, 14 industrial estates drain their partially/untreated industrial effluents into Musi (Pullaiah 2013). Hence, Musi river could serve as a useful model for toxicological evaluation of heavy metal pollution. In order to assess the daily and long-term adverse effects of Musi river water pollution, Wistar rats were treated daily for 28 days. The study was aimed to determine the heavy metals’ biodistribution in treated rat organs, their effect on oxidative stress biomarkers, and histomorphology.
Materials and methods
Nicotinamide adenine dinucleotide phosphate (β-NADPH), pyrogallol, glutathione reductase, ethylenediaminetetraacetic acid disodium salt (Na2EDTA), trichloroacetic acid (TCA), 2-thiobarbituric acid (TBA), 5-sulphosalicylic acid, sodium citrate, 5,5-dithiobis-2-nitrobenzoic acid (DTNB), 1-chloro-2,4-dinitrobenzene (CDNB), reduced glutathione (GSH), hydrogen peroxide (H2O2), hydrochloric acid (HCl) nitric acid (99.9 %) trace metals grade perchloric acid (99.9 %) trace metals grade, and n-butanol were purchased from Sigma-Aldrich.
Study site and water sampling
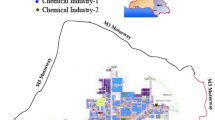
The study was conducted in and around Hyderabad, a megacity with 7.8 million urban residents. Musi river passes through middle of the city. One thousand milliliters of water sample was collected from each of eight different sites throughout the length of the Musi within the city. All the samples post collection were pooled and used for the study. The objective of choosing different sampling sites and pooling the samples was to represent the complete picture of pollution in the river. A scaled geographical map of the course of Musi river showing the study area and sampling points is shown in Fig. 1. The sampling sites are highly polluted points by the effluents from small to large-scale industries like fabric, painting, batteries, dyes, plastic recycling, pharmaceuticals, leather processing, metal surface treatment, etc. In addition, the river also serves as a recipient of sewage and agricultural waste. Along the river bank, large-scale vegetable production is practiced, which is mostly supplied to the vegetable markets in the city.
Animals and treatment
All procedures were reviewed and approved by Institutional Animal Ethics Committee of our organization. Healthy adult female albino Wistar rats aged 8–10 weeks were procured from National Institute of Nutrition, Hyderabad, India. Animals were acclimatized to laboratory conditions for 7 days prior to the start of experiments. Animals were maintained under standard laboratory conditions like temperature of 22 ± 2 °C, the relative humidity of 50–60 % and constant 12-h light and 12-h dark cycles. The animals were fed on commercial pellet diet and water ad libitum. The study and doses were designed according to OECD guidelines 407 (2008). The rats were randomly divided into four different groups of five animals each.
Control | Regular drinking water |
High dose | Undiluted Musi river water |
Medium dose | 1:10 diluted Musi river water |
Low dose | 1:100 diluted Musi river water |
The above doses of water were made available to the rat ad libitum for 28 days. The test animals were observed for symptoms and mortality every day and also body weights were recorded every week. The animals were sacrificed on the termination of the treatment after being anesthetized by pentothal and isoflurane. Liver, kidney, and brain were dissected out, weighed, and rinsed in ice-cold physiological saline, then perfused with cold potassium chloride buffer (1.15 % KCl and 0.5 mM EDTA), homogenized in potassium phosphate buffer (KPB, 0.1 mol l−1, pH 7.4). The tissue homogenate was then centrifuged at 15,000 rpm for 30 min to remove debris. The clear upper supernatant was collected and stored in aliquots at −85 °C until used. A part of each organ was fixed in 10 % formalin for histopathological study.
Lipid peroxidation
Lipid peroxidation (LPO) in rat organs was estimated according to the method described by Wills (1969). Tissue homogenate and 2 ml of 0.375 % thiobarbituric acid—15 % trichloroacetic acid reagent was taken in a test tube, boiled in water bath at 95 °C for 20 min. The solution was then cooled, and 3 ml of n-butanol was added to extract the formed color complex. The absorbance of the pink color was measured at 532 nm (Spectramax plus, Molecular Devices). The amount of malondialdehyde (MDA) was calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1 and expressed as nmol of MDA per gram wet tissue.
Reduced glutathione
Reduced glutathione (GSH) quantity was measured according to the procedure of Jollow et al. (1974) procedure. Equal volumes of tissue homogenate and sulfosalicylic acid (4 % w/v) were mixed and kept in ice for 1 h, centrifuged at 10,000 rpm for 10 min. 0.5 ml of supernatant was mixed with 0.5 ml of DTNB (4 mg/ml) and 2 ml KPB (0.1 mol l−1, pH 7.4). The yellow color developed was read at 412 nm. The amount of GSH present was expressed as microgram GSH per gram wet tissue.
Superoxide dismutase
Superoxide dismutase (SOD) activity was estimated in tissue supernatant using the method of Marklund and Marklund (1974). The assay mixture was prepared by mixing 3 ml of 50 mmol l−1 Tris-HCl buffer (pH 8.2) containing 1 mmol l−1 diethylene-triaminepenta acetic acid, 100 μl of 10 mmol l−1 pyrogallol in 10 mmol l−1 HCl and 10 μl of tissue supernatant. The change in the absorbance was recorded at 420 nm for 5 min. The enzyme activity was expressed as units per milligram protein. The protein content in the tissue supernatant was estimated by Lowry et al. (1951) method.
Catalase
The catalase (CAT) activity was estimated according to the method described by Aebi (1984). The assay mixture contained 0.063 % H2O2 in 0.1 mol l−1 KPB pH 7.4 and 10 μl of tissue supernatant. The decrease in absorbance was measured for 1 min at 240 nm. The enzyme activity was expressed as millimole of H2O2 decomposed per minute per milligram protein using a molar extinction coefficient of 43.6 M−1 cm−1. The activity was expressed as units per milligram protein.
Glutathione peroxidase
Glutathione peroxidase (GPx) activity was measured following the procedure given by Paglia and Valentine (1967) procedure. In a cuvette, 750 μl of KPB (0.1 mol l−1, pH 7), 60 μl NADPH (2.25 mmol l−1 in 0.1 % NaHCO3), 15 μl of glutathione reductase (7.1 μl/ml), and 25 μl reduced glutathione (11.52 mg/ml) were taken. The reaction was initiated by adding 50 μl supernatant and 100 μl of hydrogen peroxide (1.5 mmol l−1). The decrease in absorbance at 340 nm was measured for 1 min. The enzyme activity was expressed as micromole of NADPH oxidized per minute using molar extinction coefficient of 6.22 × 103 mmol−1 cm−1. The GPx activity was expressed in units per milligram protein.
Glutathione reductase
The glutathione reductase (GR) activity was assayed following the method of Carlberg and Mannervik (1985). Eight hundred microliters of 50 mmol l−1 KPB, 100 μl of 1.0 mmol l−1 oxidized glutathione, 100 μl of 0.15 mmol l−1 ß-NADPH, and 20 μl of tissue supernatant were taken in a cuvette. The reaction mixture was immediately mixed by inversion, and the decrease in the absorbance was recorded for 1 min at 340 nm. The enzyme activity was expressed as micromole ß-NADPH decomposed per minute per milligram protein using a molar extinction coefficient of 6.22 × 103 mmol−1 cm−1. The GR activity was expressed in units per milligram protein.
Glutathione S transferase
The glutathione S transferase (GST) activity was determined according to Habig et al. (1974) procedure. 2.75 ml KPB (0.1 mol l−1 pH 6.5), 0.1 ml GSH (75 mmol l−1), 0.1 ml CDNB (30 mmol l−1 in 95 % ethanol), and 0.05 ml supernatant were taken in a cuvette. The change in absorbance was recorded at 340 nm for 1 min. The enzyme activity was expressed as micromole CDNB conjugate per minute per milligram protein using a molar extinction coefficient of 9.6 × 103 M−1 cm−1. The GST activity was expressed in units per milligram protein.
Heavy metal analysis in water sample and biodistribution in rat organs
Throughout the study, the animals were kept in individual metabolic cages along with food and water ad libitum. Urine and feces of five individual rats were collected separately for 28 days. One milliliter of water (control & polluted), 1 ml of urine and 0.1 g of liver, kidney, brain, and feces from each rat were independently digested in nitric acid overnight. Samples were then heated at 80 °C for 10 h followed by additional heating at 130 °C for 30 min. Finally, in the presence of 0.5 ml of 70 % perchloric acid, the samples were again heated for 4 h and evaporated nearly to dryness. The samples were dissolved in 5 ml of deionized water and filtered. Inductively coupled plasma mass spectrometry (ICP-MS) was used to analyze Pb, Zn, Ag, As, Ba, Cd, Co, Cr, Cu, Mn, Mo, Ni, Sn, and Sb contents in the samples. For standardization and validation, certified reference materials were procured from National Institute of Standard Technology (NIST).
Histopathological examination
A part of liver, kidney, and brain were collected from each rat separately, fixed in 10 % formalin for studying structural abnormalities. The tissues were processed in Leica TP 1020 tissue processor, embedded in paraffin blocks using Leica EG 1160 paraffin embedder. The paraffin blocks were cut into ribbons of 4 μm using Microm HM 360 microtome. The slides were stained in hemotoxylin and eosin using Microm HMS-70 stainer. The permanent slides were made and evaluated for histopathological changes under Olympus BX51 microscope. The slides were coded to avoid possible bias before analysis.
Analysis of data
All the data were expressed as means ± standard deviation (SD). The P values were calculated using GraphPad Prism statistical software by one-way ANOVA followed by a Dunnett’s test to compare the groups (control vs treated). A value of P < 0.05 was considered to be statistically significant.
Results
Body and organ weights
It was observed that the treatment of the animals with Musi river water affected both the body weight and organ weights when compared with animals that were exposed to normal drinking water (Fig. 2). The results clearly indicated that the high- and medium-dose river water-treated groups showed a decline in body weight across the study. In addition, the weights of liver and kidney were also significantly decreased. However, the low-dose group did not show any significant changes in body and organ weights.
Lipid peroxidation
The effect of various dilutions of Musi water on lipid peroxidation in various organs is presented in Fig. 3a. Significant dose-dependent increase in lipid peroxidation was detected in liver, kidney, and brain of rats treated with high and medium doses. No significant change was observed in low-dose-treated organs.
Reduced glutathione
The alterations in GSH level were observed in various organs presented in Fig. 3b. Liver, kidney, and brain showed significant dose-dependent decrease in GSH levels at high- and medium-dosed rats except low dose.
Superoxide dismutase activity
The effect of Musi river water on SOD activity in experimental groups is shown in Fig. 4a. A significant increase in SOD activity was observed with increase in the dose level. High and medium doses of Musi river water significantly induced SOD activity in liver and kidney. Only high-dose treatment in brain showed significant increase in the SOD activity.
Catalase activity
The activity of CAT in polluted river water-treated rat organs is presented in Fig. 4b. High- and medium-dose groups showed significant decrease in CAT in liver, kidney, and brain. However, lower dose did not affect the CAT activity in all the organs.
Glutathione peroxidase activity
The GPx activity in liver, kidney, and brain of rat treated with different dilutions of Musi river water is presented in Fig. 4c. Significant increase in GPx activity was observed in liver at all the treated dose levels when compared with controls. However, kidney and brain showed significant increase in GPx activity only at high- and medium-dose levels.
Glutathione reductase
The effect of polluted Musi river water treatment on GR activity of treated rats is presented in Fig. 4d. Glutathione reductase activity was significantly increased in liver, kidney, and brain of both high- and medium-dose groups. No significant difference in the GR activity was observed in rats exposed to low-dose level.
Glutathione S transferase activity
The GST activity in liver, kidney, and brain of Musi river water-treated rats is presented in Fig. 4e. In liver, a significant dose-dependent increase in GST activity was observed at all the doses. In kidney and brain, both medium and high dose brought significant induction in GST activity.
On the whole, 28 days repeated oral treatment of rats with Musi river water resulted in a significant dose-dependent increase in SOD, GPx, GR, GST enzymes activity, and LPO in liver, kidney, and brain. On the other hand, GSH and CAT activity were significantly decreased.
Metal analysis in water sample and biodistribution in rat organs
The summary of analyzed metal content in drinking and Musi river water and their distribution in treated rat organs are presented in Table 1. The data of certified reference materials are given in Table 2. Among 14 metals analyzed, the drinking water used for control group showed the presence of only Ba (0.002 mg/l) and Zn (0.003 mg/l). Whereas the river water analysis revealed the presence of Zn (2.73 mg/l), Pb (5.41 mg/l), Ba (0.16 mg/l), Mn (0.32 mg/l), Ni (0.46 mg/l), Cr (0.48 mg/l), Cu (0.67 mg/l), Sb (0.001 mg/l), Co (0.005 mg/l), Ag (0.01 mg/l), Cd (0.01 mg/l), As (0.01 mg/l), Mo (0.03 mg/l), and Sn (0.06 mg/l). The organ biodistribution studies have shown that the heavy metal concentration in control and low-dose group rat organs was below the detection limit. However, a dose-dependent accumulation of metals was observed in the organs of medium- and high-dose groups. Liver showed Zn (21.4 & 14.5 μg/g), Cu (8.3 & 3.6 μg/g), and Pb (8.2 & 0.4 μg/g) at high concentration in high- and medium-dose groups, respectively, remaining metals were present in the range of 0.09–1 μg/g concentration. In high- and medium-dose groups, kidney showed Zn (16.2 & 7.9 μg/g), Cu (3.5 & 1.4 μg/g), Mn (2.9 & 0.5 μg/g), and Pb (2.6 & 0.5 μg/g) in high concentration, rest of the metals were found in 0.01–0.5 μg/g range. In brain, high- and medium-dose groups showed Zn (2.4 & 1.1 μg/g) and Ni (1 & 0.3 μg/g) at high concentration of the remaining metals present at 0.01–0.9 μg/g range. In urine, Zn (11 & 3.7 μg/ml), Pb (3.6 & 0.9 μg/ml), Ni (3.3 & 1.3 μg/ml), and Cu (2.7 & 1.7 μg/ml) were present in high concentrations in both high- and medium-dose groups, respectively, remaining metals were present at 0.1–2 μg/ml range. Similarly, in feces, Zn (30.4 & 11 μg/g), Cu (6.7 & 3.9 μg/g), Pb (5.7 & 0.8 μg/g), Mn (4.3 & 0.4 μg/g), and Ni (3 & 0.6 μg/g) were eliminated at high concentration than the other metals.
Histopathological changes in organs
The potential toxicity of polluted river water after 4-week repeated oral exposure was evaluated in liver, kidney, and brain. The histology of liver showed that exposure to polluted water in high and medium doses caused excessive focal hemorrhage and dilated central vein, while the low-dosed and control groups did not show any of these changes (Fig. 5). In the kidney, histological alterations were noted as focal necrosis and focal tubular damage. The brain organ revealed extensive focal vacuolation in both high- and medium-dose groups. However, the low-dose-treated rat organs showed normal histology.
Photomicrographs of various organs of rats after 28 days repeated oral treatment with Musi river water. Upper panel represents normal architecture of liver, kidney, and brain from control rats. Lower panel represents liver, kidney, and brain from high-dose group rats showing pathological changes pointed by arrows. Observation was made at ×40 magnification
Discussion
In the present study, we analyzed the possible oxidative damage and antioxidant response of rat after treatment with polluted river water for 28 days through oral route. Our results showed that the Musi river water contained Zn, Pb, Mn, Ni, Cr, Cu, Sb, Co, Ba, Ag, Cd, As, Mo, and Sn at high concentrations. This indicates that the Musi river is being polluted by the untreated effluents from industries and urban sewage. Biodistribution study revealed that the metal accumulation in treated rat organs was in a dose-dependent manner. The liver showed higher metal accumulation followed by kidney and brain. Further, it was also observed that the proportion of metal elimination was more through urine and feces than accumulation in organs. The biodistribution was in proportion to the metal concentration in the water sample, i.e., Zn, Pb, Mn, Ni, Cr, and Cu were present at high concentration in river water accumulated more when compared to other metals.
Previous studies showed that the treatment/natural exposure to heavy metals had induced oxidative stress in fish (Farombi et al. 2007; Pandey et al. 2003), Pied flycatcher nestlings (Berglund et al. 2007), Griffon vulture (Espín et al. 2014), water birds (Martinez-Haro et al. 2011), and rat (Farombi et al. 2012). In our study, we also observed that the rats treated with the polluted river water suffered severe oxidative stress. Further, the treated rats showed decreased body and organ weights indicating severity of heavy metal toxicity in river water. The toxicological effects of heavy metals are primarily attributed to their ability to generate free radicals in biological system. It is known that ROS are generated either directly via the catalytic production of superoxide by the Haber–Weiss and Fenton reactions or indirectly by other mechanisms during heavy metal exposure (Mittal and Flora 2006). Excessive generation of ROS surpasses the natural antioxidant defenses and exert toxicity through lipid peroxidation, which is considered as a first step of cellular membrane damage. The level of lipid peroxidation is widely used as an indicator of ROS-mediated oxidative damage (Tabrez and Ahmad 2009). Our results indicated significant and dose-dependent elevation of MDA (marker of lipid peroxidation) levels in liver, kidney, and brain of treated rats indicating that polluted water increased the production of ROS. GSH is an important antioxidant that protects cells against ROS-mediated oxidative stress (Espín et al. 2014). Administration of polluted river water caused significant and dose-dependent depletion of GSH in all the treated organs, demonstrating that GSH was utilized for neutralization of ROS.
Antioxidant enzyme activities in metal-stressed experimental organisms are highly variable, depending on the species, metals, dose, and exposure time; however, these altered enzyme activities reflect the modified redox status of the stressed cells (Márquez-García and Córdoba 2009). Among the antioxidant enzymes, SOD is considered as the first line of defense against oxygen toxicity owing to its inhibitory effects on superoxide radical immediately after its formation (Prabhakar et al. 2012). In the current study, the SOD and GPx activities were increased significantly, and CAT activity was significantly decreased in all the organs of treated rats exposed to polluted water. The elevated SOD enzyme in organs indicates a protective response towards the increased ROS. The observed decrease in CAT activity could be credited to increased superoxide radical (O2 −) production by the pollutants. It was reported that the CAT may be directly inhibited by superoxide radicals as well as by direct binding of some metals like Cd to thiol groups of the enzyme (Roméo et al. 2000). CAT and GPx have a similar kind of function in catalyzing H2O2. In general, the rate of H2O2 production is mainly balanced by GPx, but at increased H2O2 levels, CAT becomes more prominent in decomposing H2O2 (Berglund et al. 2007). Moreover, high H2O2 concentrations are supposed to inactivate SOD enzyme. However, SOD activity was increased in this study. This could be due to maintenance of low level of H2O2 by GPx, CAT, and GSH.
GR activity has been reported as a useful biomarker of redox cycling as it is the crucial enzyme for the regeneration of GSH from GSSG (Tabrez and Ahmad 2009). A significant increase in GR activity in liver, kidney, and brain of polluted water-treated rats could be attributed to high demand for regeneration of GSH. GST catalyzes the conjugation of GSH with xenobiotic compounds and cytotoxic aldehydes, produced during lipid peroxidation. Hence, an induction of GST activity is an indication of an increased GST-dependent detoxification process in neutralization of heavy metals. As observed in the present study decreased GSH content which reflects high utility of GSH in conjugation reactions of heavy metals by GST (Espín et al. 2014). Histopathological study also revealed adverse effects on morphology of treated rat organs due to exposure of polluted Musi river water.
Our results are in accordance with Tabrez and Ahmad (2009) who showed that waste water collected from industrial areas of Aligarh and Saharanpur cities in India, resulted significant increase in LPO, SOD, CAT, GR and GST activities in rat liver treated for 15 days. Similarly, Cyprinus carpio, a fish collected from heavy metal-polluted Ataturk Dam lake, showed increased lipid peroxidation and CAT activity, decreased GSH content, and SOD activity (Karadag et al. 2014). Berglund et al. (2007) study indicated that pied flycatcher surrounding ore smelter plant, a heavy metal-contaminated area showed signs of oxidative stress evidenced by upregulated hepatic antioxidant defense as increased GR and CAT activities and slightly but not significantly elevated lipid peroxidation and GST activity.
When comparing the present study with the studies on pure metal effect on oxidative stress, Mittal and Flora (2006) showed that mice exposure to arsenic led to a significant increase in the level of LPO in liver and kidney along with a concomitant decrease in the activities of SOD, CAT, and GPx. Similarly, when mice are exposed to a mixture of heavy metals (Pb, Hg, Cd, and Cu), the levels of GSH and SOD in the kidney and testis were significantly declined along with significant histopathological changes (Al-Attar 2011). In another study, when rats were daily injected with aluminum chloride in saline at a dose of 10 mg/kg for 10 days resulted in an increase in LPO, in most of the brain regions, which was accompanied by a decrease in the activity of SOD, CAT, and GPx (Sánchez-Iglesias et al. 2009).
Conclusion
The findings of this study indicate that Musi river is polluted with various heavy metals which tend to accumulate in different organs of rats over the course of 28 days repeated oral exposure. The extent of heavy metals accumulation was dose-dependent and directly correlated with the histopathology in different organs of rats. The toxic effects of heavy metals may be attributed to excessive generation of ROS and poor antioxidant defense in rats loaded with metal pollutants from the river water. Thus, the potential toxicity of polluted water emphasizes the importance of regular monitoring of river waters, particularly when it is used for drinking or irrigation. Further, long-term studies are warranted to evaluate the toxicological profiles of heavy metals-polluted waters.
References
Aebi H (1984) Catalase. In: Packer L (Ed), Methods in enzymology, Academic pres, Orlando. 105:121–126
Al-Attar AM (2011) Vitamin E attenuates liver injury induced by exposure to lead, mercury, cadmium and copper in albino mice. Saudi J Biol Sci 18:395–401
Alissa EM, Ferns GA (2011) Heavy metal poisoning and cardiovascular disease. J Toxicol 2011:1–21
Beg KR, Ali S (2008) Chemical contaminants and toxicity of Ganga river sediment from up and down stream area at Kanpur. Am J Environ Sci 4:362–366
Berglund AM, Sturve J, Förlin L, Nyholm NE (2007) Oxidative stress in pied flycatcher (Ficedula hypoleuca) nestlings from metal contaminated environments in northern Sweden. Environ Res 105:330–339
Buechler S, Gayathri Devi M, Raschid-Sally L (2002) Livelihoods and wastewater irrigated agriculture along the Musi River in Hyderabad City, Andhra Pradesh, India. Urban Agric Mag 8:14–17
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Chary NS, Kamala CT, Raj DS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 69:513–524
Espín S, Martínez-López E, Jiménez P, María-Mojica P, García-Fernández AJ (2014) Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus). Environ Res 129:59–68
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health 4:158–165
Farombi EO, Akintunde JK, Nzute N, Adedara IA, Arojojoye O (2012) Municipal landfill leachate induces hepatotoxicity and oxidative stress in rats. Toxicol Ind Health 28:532–541
Fatima RA, Ahmad M (2006) Genotoxicity of industrial wastewaters obtained from two different pollution sources in northern India: a comparison of three bioassays. Mutat Res 609:81–91
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Iyengar V, Nair P (2000) Global outlook on nutrition and the environment: meeting the challenges of the next millennium’. Sci Total Environ 249:331–346
Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzeneoxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Karadag H, Fırat Ö, Fırat Ö (2014) Use of oxidative stress biomarkers in Cyprinus carpio L. for the evaluation of water pollution in Ataturk Dam Lake (Adiyaman, Turkey). Bull Environ Contam Toxicol 92:289–293
Kaushik A, Sharma HR, Jain S, Dawra J, Kaushik CP (2010) Pesticide pollution of River Ghaggar in Haryana, India. Environ Moni Assess 160:61–69
Li Y, Wang YB, Gou X, Su YB, Wang G (2006) Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J Environ Sci 18:1124–1134
Li X, Chen Z, Chen Z, Zhang Y (2013) A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 93:1240–1246
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Malik A, Verma P, Singh AK, Singh KP (2011) Distribution of polycyclic aromatic hydrocarbons in water and bed sediments of the Gomti River, India. Environ Monit Assess 172:529–545
Marklund S, Marklund G (1974) Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Márquez-García B, Córdoba F (2009) Antioxidative system and oxidative stress markers in wild populations of Erica australis L. differentially exposed to pyrite mining activities. Environ Res 109:968–974
Martinez-Haro M, Green AJ, Mateo R (2011) Effects of lead exposure on oxidative stress biomarkers and plasma biochemistry in waterbirds in the field. Environ Res 111:530–538
Mittal M, Flora SJ (2006) Effects of individual and combined exposure to sodium arsenite and sodium fluoride on tissue oxidative stress, arsenic and fluoride levels in male mice. Chem Biol Interact 162:128–139
Nabulo G, Black CR, Young SD (2011) Trace metal uptake by tropical vegetables grown on soil amended with urban sewage sludge. Environ Pollut 159:368–376
OECD guideline 407 (2008) Guideline for the testing of chemicals: repeated dose 28—day oral toxicity study in rodents. Organization for Economic Cooperation and Development: Paris
Paglia DE, Valentine WN (1967) Studies on qualitative and quantitative characterization of erythrocytes glutathione peroxidase. J Lab Clin Med 70:158–169
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309:105–115
Prabhakar PV, Reddy UA, Singh SP, Balasubramanyam A, Rahman MF, Indu Kumari S, Agawane SB, Murty US, Grover P, Mahboob M (2012) Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J Appl Toxicol 32:436–445
Pullaiah C (2013) Assessing the economic impact of water pollution—a case study of Musi River Hyderabad, India. Int Res J Social Sci 2:18–23
Raj BG, Patnaik MC, Babu PS, Kalakumar B, Singh MV, Shylaja V (2006) Heavy metal contaminants in water-soil-plant-animal continuum due to pollution of Musi river around Hyderabad in India. Indian J Anim Sci 76:131–133
Roméo M, Bennani N, Gnassia-Barelli M, Lafaurie M, Girard JP (2000) Cadmium and copper display different responses towards oxidative stress in the kidney of the sea bass Dicentrarchus labrax. Aquat Toxicol 48:185–194
Sánchez-Iglesias S, Méndez-Alvarez E, Iglesias-González J, Muñoz-Patiño A, Sánchez-Sellero I, Labandeira-García JL, Soto-Otero R (2009) Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDA-induced model of Parkinson’s disease. J Neurochem 109:879–888
Scalon MC, Rechenmacher C, Siebel AM, Kayser ML, Rodrigues MT, Maluf SW, Rodrigues MA, Silva LB (2010) Evaluation of Sinos River water genotoxicity using the comet assay in fish. Braz J Biol 70:1217–1222
Singh A, Sharma RK, Agrawal M, Marshall FM (2010) Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem Toxicol 48:611–619
Srinivasan JT, Reddy VR (2009) Impact of irrigation water quality on human health: a case study in India. Ecol Econ 68:2800–2807
Tabrez S, Ahmad M (2009) Effect of wastewater intake on antioxidant and marker enzymes of tissue damage in rat tissues: implications for the use of biochemical markers. Food Chem Toxicol 47:2465–2478
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
van der Hoek W (2004) A framework for a global assessment of the extent of wastewater irrigation: the need for a common wastewater typology. In: Scott CA, Farunqui NI, Raschid-Sally L (eds) Wastewater Use in Irrigated Agriculture. Confronting the Livelihood and Environmental Realities. CAB International, International Water Management Institute, International Development Research Centre, Trowbridge, pp 11–24
Wills ED (1969) Lipid peroxide formation in microsomes. Relation of hydroxylation to lipid peroxide formation. Biochem J 113:333–341
Zhang Y, Zhang X, Wu B, Cheng S (2012) Evaluating the transcriptomic and metabolic profile of mice exposed to source drinking water. Environ Sci Technol 46:78–83
Zhao D, Cheng S, Wu B, Zhang Y, Zhang X, Li W, Cui Y (2009) Toxicity of the Yangtze River source of drinking water on reproductive system of male mice (Mus musculus). Ecotoxicology 18:729–735
Acknowledgments
The authors UAR and PVP thank ICMR, New Delhi, for financial support through ICMR-SRF fellowship. The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia, for funding the work through the research group project No. RGP-VPP-009.
Compliance with ethical standards
All procedures were reviewed and approved by Institutional Animal Ethics Committee of our organization (File No. IICT/BIO/TOX/PG/21/03/2014).
Conflict of interest
Authors do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Reddy, U.A., Prabhakar, P.V., Rao, G.S. et al. Biomarkers of oxidative stress in rat for assessing toxicological effects of heavy metal pollution in river water. Environ Sci Pollut Res 22, 13453–13463 (2015). https://doi.org/10.1007/s11356-015-4381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4381-2