Abstract
This study was conducted on Holothuria polii, Holothuria tubulosa, and Holothuria mammata collected from five stations with different depths in the Northern Mediterranean Sea. The body walls and guts of these holothurians were examined in terms of interactions of 10 metals (iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), chromium (Cr), cobalt (Co), vanadium (V), nickel (Ni), cadmium (Cd), and lead (Pb)) and one metalloid (arsenic (As)) using a multivariate analysis, and interspecies differences were determined. The multivariate analysis of variance (MANOVA) revealed significant differences between the species in terms of metal(loid) accumulations. The principal component analysis (PCA) showed a more association between H. tubulosa and H. polii with regard to the accumulation. The cluster analysis (CA) located Pb concentrations of the guts to the farthest place from all elements regardless of the species. A correlation analysis displayed that the element concentrations of the guts were more closely related to each other compared with those of the walls. The most inconsistent element in terms of correlations was the gut Fe contents. Accordingly, while Fe concentrations of H. mammata and H. tubulosa were correlated with all elements (except Pb) in divalent metal transporter 1 (DMT1) (divalent cation transporter 1 (DCT1) or natural resistance-associated macrophage protein 2 (NRAMP2)) belonging to the NRAM protein family, this was not the case in H. polii. Consequently, significant relationships between accumulated metal(loid)s that changed by tissues and sea cucumber species were observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Holothurians are important organisms for particularly coastal ecosystems. They are deposit feeders and play significant roles in recycling nutrients, stimulation of microalgal growth, and mixing the upper sediments (MacTavish et al. 2012). So far, 1400 species from marine waters including 37 from the Mediterranean Sea have been recorded (Aydın 2008; Conand 2006). Being invertebrate, over 66 species of these animals are commercially fished in about 40 countries and generally exported to the Asian markets (Gonzalez-Wanguemert et al. 2014; Purcell et al. 2012).
Since sea cucumbers are promiscuous sediment feeders, they are potential indicators for metal(loid) accumulation (Givianrad et al. 2014; Liu et al. 2016; Mohammadizadeh et al. 2015; Turk Culha et al. 2016). Other advantages that increase their indicator values are that they are slow-moving, bulky that allows easy dissection, long-lived, and widespread.
While some metals are non-essential or toxic, others can be extremely essential for the continuation of life. Metals are required by living organisms for important roles with their chemical features such as redox reactions (Zitka et al. 2013). About one third of proteins include metals. Of these proteins, 47 % are enzymes and 41 % are those proteins that need metals at the catalytic centers. Metalloenzymes account for 59 % of ligases, 44 % of oxidoreductases, 40 % of transferases, 39 % of hydrolases, 36 % of isomerases, and 36 % of lyases (Andreini et al. 2008; Martinez-Finley et al. 2012; Waldron et al. 2009). All the processes including uptake of metal, their transfer to protein that needs them, localization and deposition of metals at a subcellular level, and re-mobilization when needed are accomplished by the metal transporters (Kramer et al. 2007). Up to date, many transporters such as transferrins, natural resistance-associated macrophage protein (NRAMP) family, and ZIP family have been identified. There are important mechanisms of metal binding and deposition for hemostasis at a subcellular level. For instance, five important mechanisms have been described in crustaceans: (1) complex formation with metallothionein or glutathione, (2) transfer to mitochondria, (3) transition from basolateral membrane to blood, (4) accumulation in lysosomes, and (5) transportation to the endoplasmic reticulum (Ahearn et al. 2004). Essential and non-essential metals in the medium are involved in metabolism and accumulated in organisms using these metabolic pathways. The pathways, however, bind, transport, and transmit more than one metal (Menon et al. 2016; Rodriguez-Hernandez et al. 2015), which can result in an antagonism and synergism during the accumulation between metals and formations of metal groups or clusters. In other words, this situation can dictate the metal accumulation profiles in living organisms. The profiles of indicator organisms are also affected by relationships between the metal(loid)s in the medium. Metal(loid)s can show these kinds of relationships independently from the metabolism effect once accumulated. To eliminate this effect in the present study, samples were collected from five independent stations with different depths ranging between 0 and 20 m.
There are studies in the literature dealing with metal(loid) pollutions and accumulations by holothurians. However, there is a serious scarcity of information about relationships of accumulated metal(loid), i.e., accumulation profile, particularly in sea cucumbers. To this end, the study aimed to fill information gap at two points: (1) are there any relations among metals accumulated in sea cucumbers? (2) If so, do the relations change depending on species?
Materials and methods
Sample collection
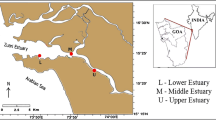
LLLRThe study was carried out in 2015 in the Northern Mediterranean Region. Sea cucumbers were collected by SCUBA diving from five stations (Yeniköy, Edremit, Ayvalık, Dikili, and Hekim Island) with varying depths from 0 to 20 m (Fig. 1). The samples were delivered in sample caps to laboratory.
Metal(loid) analysis
The analytical processes were completed at the laboratory of Environmental Engineering Department, Istanbul University. Sea cucumber samples were eviscerated, and their body walls and guts were separately cleaned and weighed on a watch glass. Aliquots of homogenized specimens were dried in an oven at 115 °C for at least 18 h. The samples were then powdered. Approximately 0.2 g of the samples were transferred to 100-ml Teflon beakers, added with 5 ml of HNO3 (65 %) and 2 ml of H2O2 (30 %) (Merck, Germany), and digested using a microwave digester (CEM MARS Xpress microwave digestion system, USA). A two-step digestion program was used including a 20-min decomposition period with 600 W at 160 °C followed by 20 min with 1200 W at 185 °C. The digested samples were cooled and diluted to 25 ml with deionized distilled water and stored in the refrigerator at approximately −4 °C until measurement with a graphite atomic absorption spectrophotometry (PerkinElmer GF-AAS 600) and flame atomic absorption spectrophotometry (PerkinElmer F-AAS 400) (Neelam et al. 2010). The GF-AAS unit was operated with the Zeeman background correction, and the peak area was integrated for all signals. Fe, Mn, and Zn were determined by F-AAS, while As, Ni, Cu, Cr, Pb, Co, V, and Cd were determined by GF-AAS.
Analysis quality control
All chemicals were of analytical grade. Standard calibration solutions for metal(loid)s (Inorganic Ventures) were prepared in 0.1 % HNO3 starting from 1000 ppm. Laboratory plastic wares and glasses ware cleaned with 1 % HNO3 and rinsed with abundant deionized water before use. One empty acid mix solution from each digestion batch was also included to determine the background of pollution. For every measurement, these samples were used as a blank. The control and blank samples were analyzed before and after every 10 sample as well as after the last sample.
To evaluate the accuracy of the analytical methods and to establish an optimal digestion condition, a reference standard material (LUTS-1) and standard solutions were used. The LUTS-1 was examined as described previously to perform the recovery procedure, and recovery range was between 75 and 118 % for all metals. The accuracy of the measurements was accepted when the repeatability of the measurement for each sample was less than 2 %. Five standard solutions were prepared for the calibration curves, and the R 2 >0.99 condition was achieved. All readings were repeated three times.
Statistical analysis
Tissue and species metal(loid) concentrations were compared with the Kruskal-Wallis and Mann-Whitney U tests at a significance level of P < 0.05, respectively (Conti et al. 2010). Kruskal-Wallis and Mann-Whitney U tests were used due to the number of data available. The Spearman correlation coefficients were determined among the body wall and gut metal(loid) concentrations at significance levels of P < 0.01 and <0.05 (Yap et al. 2010). A multivariate analysis of variance (MANOVA) was employed to determine if accumulations of 11 metals were significantly different between species with or without consideration of tissues (Abbas Alkarkhi et al. 2008). The cluster analysis (CA) was used to reveal metal clusters that were closely associated in terms of accumulation. Accordingly, metal(loid)s having strong relations with each other were located at either in a cluster or adjacent clusters. The CA was performed after Z score transformation at a Euclidean distance according to the Ward method (Lopez et al. 2004). The principal component analysis (PCA) was conducted according to Varmuza and Filzmoser (2009) to determine the projections of similarly accumulated metal(loid)s by species without consideration of tissues. The first two axes explained 82.38 % of variation for Holothuria tubulosa, 81.41 % for Holothuria polii, and 80.40 % for Holothuria mammata. All analyses were carried out using SPSS 21.0 (IBM, USA).
Results and discussions
A close look at the accumulation profiles of each element in the three sea cucumber species revealed that the profiles of the body wall and gut were highly different. This is consistent with the findings of various studies on different invertebrates (Tunca et al. 2013a). One reason of a lesser degree of accumulations of metals and different profiles in the body walls can be that muscle tissue contains a limited amount of metal binding proteins (Guner 2007). It is known that the intestines of sea cucumber have solubilization and bioaccumulation roles (McAloon and Mason 2003). Although lower metal contents, the body wall of sea cucumber includes 58–81 % of the total amount of some metals (Cd, Cu, Pb, Zn), since it accounts for 85–90 % of the whole body (Givianrad et al. 2014). Conversely, the gut accumulates the metals at higher concentrations (Warnau et al. 2006). The later researchers reported that Fe concentration of H. tubulosa gut accounts for 47 % of the whole body. Therefore, the majority of previous studies detected higher levels of metals in the gut compared to the body wall (Liu et al. 2016). In line with these reports, we determined all elements, except Cr and Mn in H. mammata, at higher levels in the gut regardless species.
A comparison of species by tissue basis showed that although no remarkable differences, there are significant differences for some metals (Fig. 2), which is consistent with former studies (Jinadasa et al. 2014; Mohammadizadeh et al. 2015). Considering significantly different elements (Cu, Cd, Cr, Mn) in the current investigation, the species including them in the wall and gut at the lowest amount was H. polii. When H. tubulosa and H. mammata were compared in terms of the gut elements, there were no significant differences, but H. mammata significantly accumulated some metals (Cd, Mn) in their body walls.
There are numerous factors influencing accumulations of elements in living organisms such as features of elements accumulated (essential or non-essential, water solubility, form, etc.), concentration, exposure time, presence of other elements or compounds in the environment, physico-chemical parameters of the medium, environment where an organism live, feeding habits or behaviors, condition of organism, sex, and metabolic rate of organism in some cases. Considering that when the species were collected together from the same region in the present study, differences in metabolic activities between species appeared to be a factor that can explain the species differences. Moreover, an effect of possible differences in feeding habits cannot be ruled out due to varying environment preferences by the species (Aydın 2008; Aydın and Erkan 2015).
Relationships of the accumulated elements regardless of the species were dealt with the PCA and MANOVA in this study based on former studies (Morina et al. 2016). The PCA results concerning the accumulation profile showed a remarkable difference in H. mammata compared with the other species (Fig. 3). Pb and As in H. tubulosa and H. polii formed a separate group from the main group, and to this group, Cr of H. tubulosa and Zn of H. polii joined as a third element. The second component of H. mammata was, however, represented by Mn and Cr. There were significant differences in metal accumulations of the three species (Table 1), which was in concordance with PCA results.
Correlations among the metals of the species are important to better understand the metal(loid) accumulations. The reasons of correlations of metals accumulated in the tissues are that indicator species deposit metal(loid)s in parallel with the environment conditions (Kouba et al. 2010) and use them with a common metabolic pathway (Ahearn et al. 2004). For instance, non-essential Pb+2 and Cd+2 utilize the same metabolic routes with Ca+2 (Martinez-Finley et al. 2012). As a matter of fact deposition and accumulations of non-essential metal(loid)s take place in this way. Moreover, non-essential metal(loid)s are involved in metabolic processes like essential metals by molecular or ionic mimicry mechanisms (Bridges and Zalups 2005). Essential-essential, essential-nonessential, and nonessential-nonessential metal correlations are hereby seen depending on metabolic activities.
When the three species are considered separately, significant correlations among the metal(loid)s of the body wall were the case (Table 2). The strongest correlations were found between V and Co (R 2 = 0.863) in H. mammata and Pb and Co (R 2 = 0.906) in H. tubulosa. Albeit weaker, while the former correlation was also the case for H. tubulosa, the latter was for H. mammata and H. polii. This situation similarly reflected the CA dendrogram as seen in Fig. 4. V concentrations of the gut were related with those of Cu and Cd in the three species with varying strengths. Another important point for V was that it was correlated with more metals in the gut than those in the wall. Although V is essential for many organisms (Da Silva 2012), it is deposited at high levels in a few. This element has been shown to have a role in vanadium-containing proteins (VCPs) that are used in the glycemic control of human with type 2 diabetes and deposited in sea cucumber (Liu et al. 2015). Significant correlations of V with Fe, Cu, Zn, Co, Ni, Cd, and Pb in the present study could be due to common proteins or transport routes to which these elements are involved. First, the isolated protein specific to V is vanadium-binding proteins (vanabins) in Ascidiacea (Ueki et al. 2007; Yoshihara et al. 2005). Vanabins can also show affinity to other elements. Vanabin (vanabin2) selectively binds Fe+3, Cu+2, Co+2, Ni+2, and Zn+2 (Kawakami et al. 2006); vanabin2 reductase binds Co+2, Cu+2, Mn+2, Mo+6, Ni+2, and W+6 (Kitayama et al. 2013; Ueki et al. 2015); vanabin2-binding protein binds Ca+2, Co+2, Cu+2, Fe+3, Mg+2, Mn+2, and Zn+2 (Ueki et al. 2009; Ueki et al. 2015); and VBP-129 binds Fe+3, Cu+2, Co+2, Zn+2, Ca+2, Mg+2, and Mn+2 (Ueki et al. 2015; Yoshihara et al. 2008). The VCPs of sea cucumber were found to bind and deposit V (Liu et al. 2015). Therefore, it would not be wrong to expect that abovementioned elements bind to these proteins. This can also explain the correlations of V with other elements in the current investigation.
Another reason of the associations of V with other metal(loid)s, aside from vanabins (or VCPs), could be low selective proteins with metal binding ability. The most possible protein among these appears to be divalent metal transporter 1 (DMT1) (DCT1 or NRAMP2) belonging to the NRAMP protein family. Homologs of DMT1, a mammalian protein, have been also described from invertebrates (Mims and Prchal 2005; Smyth et al. 2006). DMT1 has actually a strong affinity to Fe, but it can also bind Cd, Co, Mn, Ni, VO (vanadium oxide) (Illing et al. 2012), Cu, and Pb (Garrick et al. 2006). Another transporter with V binding ability is transferrin (Tf) that has specificity to Fe+3 (Costa Pessoa et al. 2015). The Tf is known to bind Cr+3, Cu+2, Ni+2, and Zn+2. Close correlations among elements (that can bind to DMT1 and Tf) were detected in this study with correlation analysis and CA in tissues of the three species.
An important and interesting correlation was found between Ni and Cr in the body walls of the three species albeit at different strengths. However, CA revealed differences between species; i.e., there was not a relation between Ni and Cr in the H. polii body wall compared with other species. One conspicuous issue in terms of these two elements was that close correlations were recorded only in the body walls. The Ni-Cr correlations were also reported for different organisms including a macrophyte species (Phragmites australis) (Rzymski et al. 2014), oysters (Crassostrea sikamea and Crassostrea hongkongensis) (Weng and Wang 2014), crayfish (Astacus leptodactylus) (Fikirdeşici Ergen et al. 2015; Tunca et al. 2013b), and Nile tilapia (Oreochromis niloticus) (Nakayama et al. 2010). While Ni mostly forms a complex with DMT1 (Illing et al. 2012) and Tf (Quarles Jr et al. 2011), Cr forms a complex with sulfate, phosphate (De Oliveira et al. 2016), and Tf (Quarles Jr et al. 2011). The Tf can be a reason of this strong relation of Ni and Cr. It is composed of two homolog regions, N-lobe and C-lobe (Mathies et al. 2015). While both lobes can bind Fe+3, the N-lobe has an affinity for Ni and the C-lobe for Cr (Quarles Jr et al. 2011). At the presence of Fe in the medium, binding strengths of Cr and Ni to Tf are not high enough, but Tf can bind both metals at the absence of competition with Fe, which can help in a better understanding of the correlations.
There are not too many elements like Cr using phosphate transport pathways. Another element involved in metabolism via this pathway is As (Tripathi et al. 2007). In the present study, As appears to have fewer correlations with other elements, which could be due to its low binding properties to carrying proteins. Actually, a close association between As and Cr had been expected through a usage of the phosphate pathways. However, besides this route, Cr can also use sulfate pathways and bind to Tf, which may have reduced a possible correlation in the present study. Accordingly, CA dendrogram shows a big distance between Cr and As in the body wall. However and oppositely, a close placement of these elements in the gut in the dendrogram can suggest a strong correlation, which was further supported by the correlation analysis.
Other noteworthy correlations were between Fe and Cu, Ni, Cd, Co, V, and Zn in the H. mammata gut. It should be underlined that all these elements can bind DMT1. Fe was also correlated with Cu, Ni, V, and Zn in the H. tubulosa gut. Interestingly, metal(loid)s of H. polii gut did not display such a correlation of Fe with others.
All the correlations of Cu were observed in the gut, except one in the body wall of H. mammata and H. polii with Fe. CA displayed a similar pattern. Unlike Cu, Pb showed its correlations only in the body wall and it was the element located at the farthest place from others in the gut.
Conclusions
With this study, metal(loid) accumulation profiles and profile differences of the three sea cucumber species on tissue basis caught from the Northern Mediterranean Sea were investigated. The results suggest that accumulations of metal(loid)s in tissues of the three species were different from each other. This difference is a fact when the comparison is made with or without tissue basis. The differences appear at both metal accumulation amounts and their interactions with each other. These interactions are consistent with the results of previous studies on invertebrates and can be explained at a certain extent with metal-carrying proteins and transport pathways.
References
Abbas Alkarkhi FM, Ismail N, Easa AM (2008) Assessment of arsenic and heavy metal contents in cockles (Anadara granosa) using multivariate statistical techniques. J Hazard Mater 150:783–789
Ahearn GA, Mandal PK, Mandal A (2004) Mechanisms of heavy-metal sequestration and detoxification in crustaceans: a review. J Comp Physiol B 174:439–452
Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218
Aydın M (2008) The commercial sea cucumbers fishery in Turkey SPC Beche de Mer. Information Bulletin 28:40–43
Aydın M, Erkan S (2015) Identification and some biological characteristics of commercial sea cucumber in the Turkey coast waters. International Journal of Fisheries and Aquatic Studies 3:260–265
Bridges CC, Zalups RK (2005) Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 204:274–308
Conand C (2006) Sea cucumber biology: taxonomy; distribution; biology; conservation status. In Bruckner A.W. (ed.). The proceedings of the CITES workshop on the conservation of sea cucumbers in the families Holothuriidae and Stichopodidae OAA Technical Memorandum. 33–50.
Conti ME, Bocca B, Iacobucci M, Finoia MG, Mecozzi M, Pino A, Alimonti A (2010) Baseline trace metals in seagrass, algae, and mollusks in a southern tyrrhenian ecosystem (Linosa Island, Sicily). Arch Environ Contam Toxicol 58:79–95
Costa Pessoa J, Garribba E, Santos MFA, Santos S (2015) Vanadium and proteins: uptake, transport, structure, activity and function. Coord Chem Rev 301:30249–30286
Da Silva JAL (2012) Vanadium in biology: its chemistry and some of its contributions on environmental cycles of chemical elements. In: Vanadium: chemical properties, uses and environmental effects, pp. 1–25.
De Oliveira LM, Gress J, De J, Rathinasabapathi B, Marchi G, Chen Y, Ma LQ (2016) Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulator Pteris vittata. Chemosphere 147:36–43
Fikirdeşici Ergen Ş, Üçüncü Tunca E, Ozkan AD, Ölmez TT, Acaröz E, Altındağ A, Tekinay T, Tunca E (2015) Interactions between metals accumulated in the narrow-clawed crayfish Astacus leptodactylus (Eschscholtz, 1823) in Dikilitaş Lake, Turkey. Chem Ecol 31:455–465
Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, Knöpfel M, Davidson T, Costa M, Paradkar P, Roth JA, Garrick LM (2006) DMT1: which metals does it transport? Biol Res 39:79–85
Givianrad MH, Larijani K, Jamili S, Adeli B (2014) Assessment of heavy metals by ligand-less cloud point extraction in sediment and Holothuria parva (Echinodermata, Holothuroidea). Indian J Geo-Mar Sci 43:825–830
Gonzalez-Wanguemert M, Aydın M, Chantal C (2014) Assessment of target sea cucumber populations from Aegean Sea (Turkey): first insights for a right management of their fisheries. Ocean & Coastal Management 92:87–94
Guner U (2007) Freshwater crayfish Astacus leptodactylus (Eschscholtz, 1823) accumulates and depurates copper. Environ Monit Assess 133:365–369
Illing AC, Shawki A, Cunningham CL, Mackenzie B (2012) Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem 287:30485–30496
Jinadasa BKKK, Samanthi RI, Wicramsinghe I (2014) Trace metal accumulation in tissue of sea cucumber species; north-western sea of Sri Lanka. American Journal of Public Health Research 2:1–5
Kawakami N, Ueki T, Matsuo K, Gekko K, Michibata H (2006) Selective metal binding by vanabin2 from the vanadium-rich ascidian, Ascidia sydneiensis samea. Biochim Biophys Acta, Gen Subj 1760:1096–1101
Kitayama H, Yamamoto S, Michibata H, Ueki T (2013) Metal ion selectivity of the vanadium(v)-reductase vanabin2. Dalton Trans 42:11921–11925
Kouba A, Buřič M, Kozák P (2010) Bioaccumulation and effects of heavy metals in crayfish: a review. Water Air Soil Pollut 211:5–16
Kramer U, Talke IN, Hanikenne M (2007) Transition metal transport. FEBS Lett 581:2263–2272
Liu Y, Zhou Q, Xu J, Xue Y, Liu X, Wang J, Xue C (2016) Assessment of total and organic vanadium levels and their bioaccumulation in edible sea cucumbers: tissues distribution, inter-species-specific, locational differences and seasonal variations. Environ Geochem Health 38:111–122
Liu Y, Zhou Q, Zhao Y, Wang Y, Wang Y, Wang J, Xu J, Xue C (2015) Enrichment, distribution of vanadium-containing protein in vanadium-enriched sea cucumber Apostichopus japonicus and the ameliorative effect on insulin resistance. Biol Trace Elem Res. doi:10.1007/s12011-015-0517-y
Lopez FJS, Garcia MDG, Vidal JLM, Aguilera PA, Frenich AG (2004) Assessment of metal contamination in Donana National Park (Spain) using crayfish (Procamburus clarkii). Environ Monit Assess 93:17–29
MacTavish T, Stenton-Dozey J, Vopel K, Savage C (2012) Deposit-feeding sea cucumbers enhance mineralization and nutrient cycling in organically-enriched coastal sediments. PLoS One 7:e50031
Martinez-Finley EJ, Chakraborty S, Fretham SJB, Aschner M (2012) Cellular transport and homeostasis of essential and nonessential metals. Metallomics 4:593–605
Mathies G, Gast P, Chasteen ND, Luck AN, Mason AB, Groenen EJJ (2015) Exploring the Fe(III) binding sites of human serum transferrin with EPR at 275 GHz. J Biol Inorg Chem 20:487–496
McAloon KM, Mason RP (2003) Investigations into the bioavailability and bioaccumulation of mercury and other trace metals to the sea cucumber, Sclerodactyla briareus, using in vitro solubilization. Mar Pollut Bull 46:1600–1608
Menon AV, Chang J, Kim J (2016) Mechanisms of divalent metal toxicity in affective disorders. Toxicology 339:58–72
Mims MP, Prchal JT (2005) Divalent metal transporter 1. Hematology 10:339–345
Mohammadizadeh M, Bastami KD, Ehsanpour M, Afkhami M, Mohammadizadeh F, Esmaeilzadeh M (2015) Heavy metal accumulation in tissues of two sea cucumbers, Holothuria leucospilota and Holothuria scabra in the northern part of Qeshm Island, Persian Gulf. Mar Pollut Bull 103:354–359
Morina A, Morina F, Djikanović V, Spasić S, Krpo-Ćetković J, Lenhardt M (2016) Seasonal variation in element concentrations in surface sediments of three rivers with different pollution input in Serbia. J Soils Sed 16:255–265
Nakayama SMM, Ikenaka Y, Muzandu K, Choongo K, Oroszlany B, Teraoka H, Mizuno N, Ishizuka M (2010) Heavy metal accumulation in lake sediments, fish (Oreochromis niloticus and Serranochromis thumbergi), and crayfish (Cherax quadricarinatus) in Lake Itezhi-tezhi and Lake Kariba, Zambia. Arch Environ Contam Toxicol 59:291–300
Neelam V, Hardaway CJ, Richert JC, Sneddon JA (2010) Laboratory controlled study of uptake of copper, lead, and zinc in crawfish (Procambrus clarkii) by inductively coupled plasma optical emission spectrometry. Anal Lett 43:1770–1779
Purcell SW, Samyn Y, Conand C (2012) Commercially important sea cucumbers of the world. FAO Species Catalogue for Fishery Purposes. No. 6. FAO, Rome, 150 pp. 30 colour plates.
Quarles Jr CD, Marcus RK, Brumaghim JL (2011) Competitive binding of Fe3+, Cr3+, and Ni2+ to transferrin. J Biol Inorg Chem 16:913–921
Rodriguez-Hernandez MC, Bonifas I, Torre Alfaro De La MC, Flores-Flores JL, Bañuelos-Hernández B, Patiño-Rodríguez O (2015) Increased accumulation of cadmium and lead under Ca and Fe deficiency in Typha latifolia: a study of two pore channel (TPC1) gene responses. Environ Exp Bot 115:38–48
Rzymski P, Niedzielski P, Klimaszyk P, Poniedzialek B (2014) Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ Monit Assess 186:3199–3212
Smyth DJ, Glanfield A, McManus DP, Hacker E, Blair D, Anderson GJ, Jones MK (2006) Two isoforms of a divalent metal transporter (DMT1) in Schistosoma mansoni suggest a surface-associated pathway for iron absorption in schistosomes. J Biol Chem 281:2242–2248
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Tunca E, Ucuncu E, Kurtulus B, Ozkan AD, Atasagun S (2013a) Accumulation trends of metals and a metalloid in the freshwater crayfish Astacus leptodactylus from Lake Yenicaga (Turkey). Chem Ecol 29:754–769
Tunca E, Ucuncu E, Ozkan AD, Ulger ZE, Tekinay T (2013b) Tissue distribution and correlation profiles of heavy-metal accumulation in the freshwater crayfish Astacus leptodactylus. Arch Environ Contam Toxicol 64:676–691
Turk Culha S, Dereli H, Karaduman FR, Culha M (2016) Assessment of trace metal contamination in the sea cucumber (Holothuria tubulosa) and sediments from the Dardanelles Strait (Turkey). Environ Sci Pollut Res Int. doi:10.1007/s11356-016-6152-0
Ueki T, Kawakami N, Toshishige M, Matsuo K, Gekko K, Michibata H (2009) Characterization of vanadium-binding sites of the vanadium-binding protein vanabin2 by site-directed mutagenesis. Biochim Biophys Acta, Gen Subj 1790:1327–1333
Ueki T, Shintaku K, Yonekawa Y, Takatsu N, Yamada H, Hamada T, Hirota H, Michibata H (2007) Identification of vanabin-interacting protein 1 (VIP1) from blood cells of the vanadium-rich ascidian Ascidia sydneiensis samea. Biochim Biophys Acta, Gen Subj 1770:951–957
Ueki T, Yamaguchi N, Romaidi Isago Y, Tanahashi H (2015) Vanadium accumulation in ascidians: a system overview. Coord Chem Rev 301–302:300–308
Varmuza K, Filzmoser P (2009) Introduction to multivariate statistical analysis in chemometrics. CRC, Boca Raton
Waldron KJ, Rutherford JC, Ford D, Robinson NJ (2009) Metalloproteins and metal sensing. Nature 460:823–830
Warnau M, Dutrieux S, Ledent G, Rodriguez Y, Baena AM, Dúbois P (2006) Heavy metals in the sea cucumber Holothuria tubulosa (Echinodermata) from the Mediterranean Posidonia oceanica ecosystem: body compartment, seasonal, geographical and bathymetric variations. Environ Bioindic 1:268–285
Weng N, Wang W-X (2014) Variations of trace metals in two estuarine environments with contrasting pollution histories. Sci Total Environ 485–486:604–614
Yap CK, Edward FB, Tan SG (2010) Similarities and differences of metal distributions in the tissues of molluscs by using multivariate analyses. Environ Monit Assess 165:39–53
Yoshihara M, Ueki T, Watanabe T, Yamaguchi N, Kamino K, Michibata H (2005) Vanabin P, a novel vanadium-binding protein in the blood plasma of an ascidian, Ascidia sydneiensis samea. Biochim Biophys Acta, Gene Struct Expression 1730:206–214
Yoshihara M, Ueki T, Yamaguchi N, Kamino K, Michibata H (2008) Characterization of a novel vanadium-binding protein (VBP-129) from blood plasma of the vanadium-rich ascidian Ascidia sydneiensis samea. Biochim Biophys Acta, Gen Subj 1780:256–263
Zitka O, Krystofova O, Hynek D, Sobrova P, Kaiser J, Sochor J, Zehnalek J, Babula P, Ferrol N, Kizek R, Adam V (2013) Metal transporters in plants. Springer, Berlin, pp. 19–41
Acknowledgments
This study was supported by Istanbul University’s Research Fund (Project Number: 50025/2352) and Ordu University’s Research Fund (Project Number: AR-1501). We are grateful to Dr. Tatsuya UEKI for his support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editorial Responsible: Elena Maestri
Rights and permissions
About this article
Cite this article
Tunca, E., Aydın, M. & Şahin, Ü. Interactions and accumulation differences of metal(loid)s in three sea cucumber species collected from the Northern Mediterranean Sea. Environ Sci Pollut Res 23, 21020–21031 (2016). https://doi.org/10.1007/s11356-016-7288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7288-7