Abstract
The aim of the present study was to evaluate the phytotoxicity of olive mill wastewater (OMW) after being treated by the white-rot fungus Coriolopsis gallica. For this, the effect of irrigation with treated OMW (TOMW) and untreated OMW (UOMW) on tomato plants (Lycopersicon esculentum) for 3 weeks was studied. The control plants were irrigated with distilled water. Agronomic tests were performed in pot experiments in a greenhouse using the randomized complete block (RCB) experimental design. The relative leaf height (RLH), as a morphological parameter, and the content of total phenols in the roots and total chlorophyll [Cha + Chb] and reducing sugars in the leaves, as physiological parameters, were selected as responses of the experimental design. The results obtained showed that [Cha + Chb] in the leaves of tomato growth under TOMW was enhanced by 36.3 and 19.4 % compared to the plant growth under UOMW and to the controls, respectively. Also, reducing sugar concentrations were closed to those of the control plants, ranging from 0.424 to 0.678 g/L for the different dilutions tested. However, the plants irrigated with UOMW showed lower reducing sugar concentrations ranging from 0.042 to 0.297g/L. The optimum RLH (0.537) was observed in the plants irrigated with TOMW diluted at (1:4), this value being higher than that observed in the controls (0.438). Our study proved that the irrigation with TOMW significantly improved tomato growth and photosynthesis activity over those irrigated with UOMW. Optimization of TOMW as a fertilizer was obtained for a dilution of 1:4. From the obtained results, it can be concluded that OMW treated by C. gallica holds potential to be used as a fertilizer for tomato plants.

ᅟ Please provide a caption for the graphical abstract.The graphical abstract is improved and sent as attachment Please replace it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Olive mill wastewater (OMW) represents one of the most contaminating effluents produced by the agro-food industries (Cardinali et al. 2010). At present, a low cost-effective practice of OMW management is its land spreading for both disposal and fertirrigation purposes (Mekki et al. 2006; Hanifi and El Hadrami 2009). OMW is rich in water, organic matter, nitrogen, phosphorous, potassium, and magnesium which are favorable for agricultural soils. In this perspective, several studies have found positive effects of OMW on soil fertility (Komilis et al. 2005; Ouzounidou et al. 2010; Nair et al. 2014). However, negative effects on physicochemical soil properties (Buchmann et al. 2015), accumulation of salts, reduced water infiltration (Chartzoulakis 2016), and phytotoxic effects on seed germination and plant growth (Saadi et al. 2013; Massoudinejad et al. 2014) are limiting constrains for the application of OMW to soil. The high content in recalcitrant phenolic compounds (PCs) is responsible for the phytotoxic and microbial growth inhibition effects of the OMW. Indeed, PCs exhibit phytotoxic properties and may cause alterations in the N cycle and soil microbial activity as well as contamination of surface and groundwater (Asfi et al. 2012; Justino et al. 2009).
Earlier studies on plant response to OMW were typically limited to seed germination and plant growth (Casa et al. 2003; Mekki et al. 2006). Recently, morphological and physiological plant responses of OMW application to soil irrigation have been investigated (Asfi et al. 2012). Thus, the effects of OMW on seed germination, shoot and root elongation, biomass production, nutrient uptake and translocation, ascorbic acid content, polyphenols, photosynthetic pigments, and photosynthetic performance of spinach to understand the mechanisms of phytotoxicity were reported by Ouzounidou et al. (2012).
Certain studies have proposed the treatment of OMW prior to its land disposal (Barbera et al. 2014) in order to reduce its environmental impact, paying special attention to the reduction of its phenolic content by the development of efficient treatment technologies. Mekki et al. (2013) indicated that OMW treated (TOMW) by white-rot fungi had a significant fertilizing potential that could be advantageously used in agronomy. Nevertheless, an evaluation of the phytotoxicity of TOMW is needed to assess treatment efficacy and to promote its use in agriculture healthily.
Biological OMW treatment techniques have been considered as alternative environmentally friendly methods, easy to control, and with low impact on ecosystems. Thus, the treatment of OMW with ligninolytic fungi and their phenol-oxidizing enzymes has been the subject of many studies in the last decades (Dias et al. 2004; Duarte et al. 2014).
In previous studies, Daâssi et al. (2014a) reported that fungal treatment of OMW with Coriolopsis gallica, a white-rot fungus able to discolor and significantly dephenolize several types of phenolic compounds, reduced its phytotoxicity on radish (Raphanus sativus) seeds. This strain showed a flawless ability for OMW decontamination. Therefore, the present work was undertaken to evaluate the phytotoxicity of OMW, previously treated by C. gallica, and the impact of its application on the irrigation of tomato plants (Lycopersicon esculentum) through the study of certain physiological parameters.
Material and methods
Microorganism
The fungus C. gallica (KJ 412304), previously isolated from decaying wood in the vicinity of Bousalem, Northwest Tunisia, was selected to perform the present study for its ability to dephenolize and discolor OMW (Daâssi et al. 2014a,b). The strain was maintained on 2 % mal extract agar (MEA) Petri plates at 4 °C and subcultured every 2 months.
OMW samples
OMW was obtained from an olive oil production plant in Sfax, Tunisia, which uses a traditional discontinuous process for the extraction of olive oil. The samples were stored at −20 °C until analysis.
Biological treatment of OMW
The strain was first precultivated for 2 days and then the homogenized mycelium was transferred to the liquid medium at 2 % (v/v) level for the OMW treatment.
Fungal inocula (plugs of 6 mm in diameter) were obtained from 7-day-old mycelia grown on MEA medium (30 g/L, pH 5.5). The plugs were transferred into 250-mL Erlenmeyer flasks containing 50 mL of liquid OMW medium (30 % v/v) (Daâssi et al., 2014). The OMW medium contained (g/L): (NH4)2SO4, 6; KH2PO4, 1; MgSO4–7H2O, 0.5; KCl, 0.5; trace element solution, 1 mL. The trace element solution composition (g/L) was as follows: B4O7Na2–10H2O, 0.1; CuSO4–5H2O, 0.01; FeSO4–7H2O, 0.05; MnSO–7H2O; 0.01; ZnSO4–7H2O, 0.07; (NH4)6Mo7O24–4H2O, 0.01.
Physicochemical analyses
Total phenolic compounds (TPCs) in the UOMW and TOMW were estimated by the Folin-Ciocalteau’s reagent using gallic acid as a standard (Box 1983). The ion compositions of OMW were measured in a chemically digested sample by atomic absorption spectrometry, and the chemical oxygen demand (COD) was determined according to APHA (1995) standard methods. Reducing sugars (RS) were determined using the method of Miller (1959). The electronic conductivity (EC) and the hydrogen potential (pH) were measured with a conductivity meter (Jenway mod. 4200) and a pH meter (Hanna instruments mod. pH 211), respectively.
TPCs in UOMW and TOMW by C. gallica culture extract were examined by reversed-phase high performance liquid chromatography (RP-HPLC) using an Agilent 1100 series chromatograph. Compounds were separated on a Macherey-Nagel C-18 column (EC 250/4 mm Nucleosil® 100–5 μm) 40 °C. The flow rate was 0.750 mL/min. The mobile phase used was 0.05 % acetic acid in water/acetonitrile (90/10, v/v) (solvent A) versus acetonitrile (solvent B) for a total running time of 51 min, with the following gradient: solvent B (10 %) for 5 min, then increased to 80 % over 15 min, maintained at 80 % for 20 min, and increased to 100 % in 1 min until completion of the analysis. Compounds were detected using a DAD-UV/VIS detector at 320 nm.
Tomato growth conditions
The seeds of tomato (L. esculentum) were sown on 15 March 2014 in alveolus plates filled with peat for 3 weeks. At the end of March, the seedlings were transferred into pots containing 500 g of peat and transplanted to a greenhouse (three plants per pot). Twelve days after transplanting, the pots were fertilized with 10 mL of full-strength fertilizer solution and afterward treated weekly with 10 mL of OMW. The treatment of the plants with OMW was performed 3 days after the fertigation and the irrigation with water was performed four times per week. The fertilizer solution consisted of 34.94 g of potassium nitrate (K2O: 46 % and N: 13 %), 14.19 g of ammonium nitrate (N: 33.5 %), 4.2 mL of phosphoric acid (1.6–53 %), and 20.833 g of magnesium sulfate (12 %) (Reymov et al. 2002).
The cultivated peat soil is an insoluble and hygroscopic product. It is characterized by solid aspect, black color, cation exchange capacity (70 meq/100g), pH of 4.2, and contains (weight/g dry weight): moisture (72 to 74 %), proteins (7.5 %), fat matter (1.5 %), fiber (28 %), and organic matter (85 %).
Experimental device RCB
A complete randomized experimental design with three blocks and three replicates was applied in this study (Table in supplementary materials). The UOMW was initially diluted to 30 %. TOMW and UOMW were diluted in distilled water (v/v) at different proportions (1:2; 1:4; 1:8, and 1:12). The pH of TOMW and UOMW was adjusted to 7.0 to avoid any change related to their acidity.
Collection of samples
At the end of tomato cultivation, the plants were uprooted from the pots using a fine jet of water, washed thoroughly with running deionized water and blot dried. Different parts of the plant were separated manually (leaves, stem, roots), cut into small pieces, and dried in an oven at 100 °C for 24 h.
RLF estimation
Five plants were selected at the end of tomato cultivation. The length (L) of all leaves was measured with a simple ruler. Plant height and the insertion height of the petiole from each leaf was also obtained to calculate the relative leaf height by the equation
where RLH is the relative leaf height, LH is the leaf height (cm), that is, the distance between the soil surface and the node corresponding to the leaf, and PH is the plant height (cm) (Blanco and Folegatti, 2003).
Photosynthetic pigment analysis
For the determination of chlorophyll a and chlorophyll b, the method of MacKinney (1941) was used. Ten milligrams of fresh plant material (sheet without midrib) was cut into small pieces, ground in 2 mL of acetone (80 %), and then centrifuged for 10 min at 14,000 rpm at 4 °C. The supernatant was recovered, 2 mL of acetone (80 %) was added, and the pellet was centrifuged again at 4 °C (10 min, 14,000 rpm). The absorbance of the supernatant was measured at 645 and 663 nm (Lichtenthaler and Buschmann 2001; Ouzounidou et al. 2006). Data were expressed as milligrams per gram of fresh weight (FW) of tomato.
Extraction of PCs from tomato roots
Five grams of homogenized roots were extracted with 50 mL of acetone in a conical flask under stirring (600 rpm) for 1 h at room temperature (22 °C). The root extracts were then filtered. The extraction process was done in triplicate.
Five grams of the dried samples were placed in the filter cartridge of a classical Soxhlet apparatus and extracted with 170 mL of acetone for 2 h. Extracts were cooled to room temperature. The extraction process was performed in triplicate (Tomsone and Kruma 2013).
Determination of TPC
The total phenolic content (TPC) in root extracts was determined by the Folin-Ciocalteu method (Singleton et al. 1999). Calibration curves were performed with gallic acid. Absorption was determined at a wavelength of 725 nm with a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan). Data were expressed in milligram equivalents of gallic acid per gram of fresh tomato material.
Reducing sugars analysis in tomato leaves
Tomato leaves were cut into small pieces, weighed, placed separately in 10-mL glass vials containing 80 % (v/v) ethanol, and then heated at 60 °C for 30 min. The extract was then filtered and diluted with 80 % (v/v) ethanol up to 20 mL (Khan et al. 2000). The filtrate was diluted tenfold with 80 % (v/v) ethanol. Reducing sugars were determined by the 3,5-dinitrosalicylic acid (DNS) method (Miller 1959). One milliliter of the leave extract was added to 1 mL of DNS, shaken in vortex, boiled for 5 min, and cooled to room temperature on an ice bath. The optical density of the samples was measured at 540 nm with a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan).
Statistical analysis
Design expert, Statistica® 7.0 Stat Soft was used for the experimental designs and statistical analysis of the experimental data.
Results and discussion
Physicochemical properties of the OMW
The chemical properties of the UOMW and TOMW greatly differed from each other for several of the measured parameters (Table 1). As expected, UOMW was an acidic effluent (pH = 5.4) with a high phenolic content (3.5 g/L) and high chemical oxygen demand (COD = 26 g/L), showing the toxic properties of this material. In addition, the data presented in Table 1 showed that UOMW was rich in sodium (0.532 g/L). This high concentration of salt could cause a nutrient imbalance and saline stress altering plant growth. Tomato growth is reduced under the influence of salt stress (Maggio et al. 2010). Fortunately, some characteristics of OMW are favorable for agriculture since this effluent is rich in organic matter, P, K, and Mg.
On the other hand, TOMW had a pH of 6.3 and its content in phenolics was lower than 0.5 g/L. Accordingly, Daâssi et al. (2014b) recently demonstrated the significant abatement of polyphenols in quantity and quality of OMW after being treated by C. gallica culture using high performance liquid chromatography coupled to electrospray time-of-flight mass spectrometry (HPLC-ESI-TOF-MS) analysis (Chromatogram in supplementary materials).
Assessment of the OMW phytotoxicity
Qualitative parameters
The appearance of leaves reflects the health state of plants. Leaves with green color, without necrosis or discoloration indicate good photosynthesis process and the distribution of the nutriment throughout the plant (Shah et al. 2011). During the irrigation of tomato cultures with TOMW and UOMW, the pigmentation and morphology of the leaves were observed (Figs. 1 and 2). In Fig. 1, the difference of color in tomato leaves before and after the irrigation with TOMW is shown. It was observed that the irrigation promoted leaf pigmentation of tomato plants compared to those before irrigation as well as to the controls (irrigated with water) (Fig. 2). These results are in accordance with those reported by Mekki et al. (2006), who mentioned that the irrigation of various plant species (tomato, chickpeas, beans, wheat, and barley) with OMW previously treated with Phanarochaete chrysosporium did not cause any adverse effect on the morphology of the tested plants.
Though, the appearance of leaves is insufficient to assess the impact of OMW on tomato. Hence, a quantitative morphological parameter (relative leaf height) and physiological parameters such as photosynthetic pigment (chlorophyll a and chlorophyll b) and reducing sugars in tomato leaves and phenolic content in the roots were studied.
Quantitative parameters
Statistical analysis
The experiments of tomato irrigation with OMW were applied using a complete randomized experimental design with three blocks and three replicates using the Statistica® 7.0 Stat Soft program to estimate the coefficients of the model. A total of 45 runs with different combinations of fungal treatment (A) and dilutions (B) were designed (Table 2). The responses, measured after 3 weeks of irrigation, were named as R1, R2, R3, and R4 and corresponded to RLF, the concentration of TPCs in the roots and (Cha + Chb) and RS in the leaves, respectively.
The experimental results were analyzed by standard ANOVA (Table 3). The adjusted sum of squares values (R 2) were 86, 90, 87, and 87 % for R1, R2, R3, and R4, respectively. At the same time, a relatively low value of the coefficient of variation (CVR1 = 9.7 %; CVR2 = 11.27 %; CVR3 = 8.56 %; CVR4 = 17.59 %) indicated a better accuracy and reliability of the experiments.
Graphical interpretation
The impact of irrigation using UOMW and TOMW on tomato plants for a period of 3 weeks was assessed. The tested responses were R1, R2, R3, and R4.
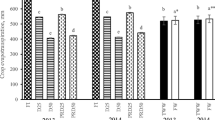
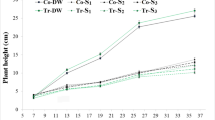
Response R1: RLH
From the data in Fig. 3, it can be observed that tomato growth was enhanced under the irrigation with TOMW. Thus, the maximum height was 31.27 cm which is higher than that of the controls (27.38 cm) (data not showed). The optimum RLH (0.537) was observed in plants irrigated with TOMW diluted at 1:4, this value being higher than those irrigated with UOMW (0.282) and even than the controls (0.438). This proves the fertilizing effect of TOMW on tomato plants. The above results are supported by the findings of Mekki et al. (2013) who reported an improvement in plant growth and a similar or even better dry productivity of plants irrigated with TOMW than of plants irrigated with water.
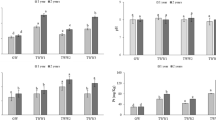
Response R2: Phenols
The data presented in Fig. 4 revealed that the irrigation of tomato plants with UOMW presented a high phenolic content in the roots (from 413 to 722 mg gallic acid/L) compared to the plants irrigated with TOMW (from 283 to 590 mg gallic acid/L) and to controls (265 mg gallic acid/L). Such results could be based on the high initial load of TPC in UOMW. Similar findings were observed by Martín et al. (2002) who emphasized that during OMW spread, plant roots could absorb OMW phenols. In addition, as the results showed, the dilution of OMW was found essential to reduce the initial organic load and the phenolic content in tomato roots associated to the phytotoxicity properties (Sayadi and Ellouz 1995; Paraskeva and Diamadopoulos 2006).
Furthermore, the amount of phenols in control pots can be explained by the basal level of phenolic load in vegetable materials. In fact, phenolic compounds are ubiquitous in plants and are involved in the features of woods and barks, plant defense, flower color, and flavor properties. Recently, the phenolic compounds from plants have aroused considerable interest due to their antioxidant properties (Cheynier, 2012).
According to Fig. 4, it has been found that the content of total phenols gradually decreased with dilution in both UOMW and TOMW. This observation is in agreement with the results found by Asfi et al. (2012), who reported that the exposure to OMW dilutions of 1:20 and 1:10 resulted in overaccumulation of total polyphenols in spinach roots plants. A similar behavior was observed by El Hadrami et al. (2004) who found that crude and undiluted OMW was lethal when applied to crops of maize (Zea mays L.) chickpea (Cicer arietinum L.), tomato, and wheat.
Response R3: Cha + Chb
The results of the variations of chlorophyll a + b contents in tomato leaves, obtained by using different combinations of OMW treatments and dilutions, are summarized in Fig. 5. It is worth mentioning that the total amount of chlorophyll a and b in leaves of tomato growth under TOMW was enhanced by 36.3 and 19.4 % compared to the plants grown under UOMW and to the controls, respectively. Furthermore, the maximum pigmentation observed in tomato irrigated with TOMW was about 171.84 and 174.36 mg/g at a dilution of 1/2 and 1/4, respectively. These concentrations were significantly higher than those obtained with UOMW (135.62 and 135.29 mg/g at the dilutions of 1/2 and 1/4, respectively) and those of the control (140.55 mg/g). According to Ouzounidou et al. (2010), the loss of chlorophyll content in tomato leaves irrigated with UOMW may be attributed to the interference of the polyphenols present in OMW in the formation of chlorophyll. Several researches attribute OMW toxicity to their phenolic compounds specially p-coumaric acid and ferulic acid which present phytotoxic effects toward plants (Yang et al., 2004). Besides, the increase observed in leaf pigmentation of tomato irrigated with TOMW can be related to the low content of total phenolics in TOMW (0.322 g/L) and its nutrient fertilizing contents such as nitrogen (1.96 g/L), potassium (1.205 g/L), magnesium (439.55 mg/L), and calcium (1.269 g/L). Hence, the OMW biochemical properties determined a significant variation in the photosynthesis activity of plants as showed by the chlorophyll a and b concentrations. These findings are in accordance with those found by López-Piñeiro et al. (2007), who reported that the composition of TOMW, containing organic carbon, total nitrogen, and available potassium, enriched poor soils and increased crop yields. Also, Asfi et al. (2012) and Ouzounidou et al. (2008) emphasized that spinach and pea plants grown under raw and diluted OMW revealed nutrient deficiency symptoms, since the uptake and translocation of calcium, iron, magnesium, and potassium were impeded.
The good development of tomato irrigated with TOMW was probably related to the presence of certain organic acids (e.g., humic and fulvic acids) resulted from the association of OMW biodegradation products that provide humification processes. As pointed out by Daâssi et al. (2014a,b), the catalytic efficiency of laccases was the one responsible for OMW biodegradation and the association of elements (probably organic acids) for the humification process. Some studies (Komilis et al. 2005, Mekki et al. 2006) found that the irrigation with TOMW caused significant shifts in the structure and function of microbial communities which in turn influenced soil fertility.
Response R4: Reducing sugars
Sugars are synthesized in the non-photochemical reactions of photosynthesis. To evaluate the effect of OMW application on the photosynthesis activity of plants, reducing sugar concentrations in leaves of tomato plants were estimated (Fig. 6).
For plants irrigated with TOMW, reducing sugar concentrations were comparable to those of the controls, ranging from 0.424 to 0.678 g/L at different dilutions. However, for OMW non-treated plants, reducing sugar concentrations were lower, ranging from 0.042 to 0.297 g/L. The drop in reducing sugar levels in plants irrigated with UOMW can be explained by an inhibition of the biosynthesis of reducing sugars as a result of their high concentrations of phenolic compounds (Ouzounidou et al. 2010).
The positive effects of the TOMW fertirrigation seemed evident, provided the photosynthetic activity of tomato can be further efficiently used for humification purposes in agriculture.
Optimization of fertilizing treated OMW
An optimization process was carried out to apply TOMW as an organic fertilizer for tomato growth using the Design-Expert version 7.0 (STAT-EASE Inc., Minneapolis, USA) software.
The statistical study showed that the application of TOMW revealed a fertilizer value of tomato plants by improving their total chlorophyll contents (a + b) and reducing sugars in the leaves. However, the results obtained under the effect of treated OMW showed fluctuations for certain dilutions.
The optimization was based on the following three objectives: the minimization of the phenolic compounds in roots and the maximization of the total chlorophyll (a + b) content and the reducing sugar content in tomato leaves (Table 4).
The analysis of the statistical data (Table 4) showed that the best condition for OMW application as a fertilizer was the growth of tomato under treated OMW at the dilution of 1:4. This condition led to a minimum value of phenolic content in roots (398 mg gallic acid/L) and maximum values in total chlorophylls (a + b) and reducing sugars (0.554 and 178.3626 mg/g, respectively) in leaves. Therefore, at least regarding the monitored parameters, we can assert that OMW, especially treated by the C. gallica strain, may be utilized as organic amendment in agriculture under controlled conditions at a suitable dilution.
Conclusions
Based on statistical analysis of morphological and physiological parameters, tomato plants were reported to tolerate C. gallica treated-OMW (TOMW) irrigation diluted at 1:4. The test of TOMW application impacts as an organic fertilizer showed positive effects on tomato growth. Thus, the application of OMW, mainly after removal of its phenolic components, may be suggested as a good strategy for effective management of OMW.
References
APHA (1995) Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington (DC, USA)
Asfi M, Ouzounidou G, Moustakas M (2012) Evaluation of olive oil mill wastewater toxicity on spinach. Environ Sci Pollut Res 19(6):2363–2371
Barbera AC, Maucieri C, Ioppolo A, Milani M, Cavallaro V (2014) Effects of olive mill wastewater physico-chemical treatments on polyphenol abatement and Italian ryegrass (Lolium multiflorum Lam.) germinability. Water Res 52:275–281
Blanco FF, Folegatti MVA (2003) New method for estimating the leaf area index of cucumber and tomato plants. Hortic Bras 21(4):666–669
Box JD (1983) Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res 17(5):511–525
Buchmann C, Felten A, Peikert B, Muñoz K, Bandow N, Dag A, Schaumann GE (2015) Development of phytotoxicity and composition of a soil treated with olive mill wastewater (OMW): an incubation study. Plant Soil 386(1):99–112
Cardinali A, Cicco N, Linsalata V, Minervini F, Pati S, Pieralice M, Tursi N, Lattanzio V (2010) Biological activity of high molecular. J Agric Food Chem 58(15):8585–8590
Casa R, D’Annibale A, Pieruccetti F (2003) Reduction of the phenolic components in olive-mill wastewater by enzymatic treatment and its impact on durum wheat (Triticum durum Desf.) germinability. Chemosphere 50(8):959–966
Chartzoulakis KS (2016) The potential of saline and residual water use in olive growing. Acta Hortic 1057:257–273
Cheynier V (2012) Phenolic compounds: from plants to foods. Phytochem Rev 11(2):153–177
Daâssi D, Belbahri L, Vallat A, Woodward S, Nasri M, Mechichi T (2014a) Enhanced reduction of phenol content and toxicity in olive mill wastewaters by a newly isolated strain of Coriolopsis gallica. Environ Sci Pollut Res 21(3):1746–1758
Daâssi D, Lozano-Sánchez J, Borrás-Linares I, Belbahri L, Woodward S, Zouari-Mechichi H, Mechichi T, Nasri M, Segura-Carretero A (2014b) Olive oil mill wastewaters: phenolic content characterization during degradation by Coriolopsis gallica. Chemosphere 113:62–70
Dias AA, Bezerra RM, Pereira AN (2004) Activity and elution profile of laccase during biological decolorization and dephenolization of olive mill wastewater. Bioresource Technol 92(1):7–13
Duarte KR, Justino C, Panteleitchouk T, Zrinek A, Freitas AC, Duarte AC, Rocha-Santos TAP (2014) Removal of phenolic compounds in olive mill wastewater by silica-alginate-fungi biocomposites. Int J Environ Sci Te 11(3):589–596
El Hadrami A, Belaqziz M, El Hassni M, Hanifi S, Abbad A, Capasso R, Gianfreda L, El Hadrami I (2004) Physico-chemical characterization and effects of olive oil mill wastewaters fertirrigation on the growth of some Mediterranean crops. J Agron 3:247–254
Hanifi S, El Hadrami I (2009) Olive mill wastewaters: diversity of the fatal product in olive oil industry and its valorisation as agronomical amendment of poor soils: a review. J Agron 8(1):1–13
Justino CI, Duarte K, Loureiro F, Pereira R, Antunes SC, Marques SM, Gonçalves F, Rocha-Santos TAP, Freitas AC (2009) Toxicity and organic content characterization of olive oil mil wastewater undergoing a sequential treatment with fungi and photo-Fenton oxidation. J Hazard Mater 172(2-3):1560–1572
Khan AA, McNeilly T, Collins C (2000) Accumulation of amino acids, proline, and carbohydrates in response to aluminum and manganese stress in maize. J Plant Nutr 23(9):1303–1314
Komilis DP, Karatzas E, Halvadakis CP (2005) The effect of olive mill wastewater on seed germination after various pretreatment techniques. J Environ Manage 74(4):339–348
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids-measurement and characterization by UV–VIS, Current Protocols in Food Analytical Chemistry (CPFA), supplement 1. John Wiley, New York, pp F4.3.1–F4.3.8
López-Piñeiro A, Murillo S, Barreto C, Muñoz A, Rato JM, Albarrán A, García A (2007) Changes in organic matter and residual effect of amendment with two-phase olive-mill waste on degraded agricultural soils. Sci Total Environ 378(1-2):84–89
MacKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Maggio A, Barbieri G, Raimondi G, De Pascale S (2010) Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J Plant Growth Regul 29:63–72
Martín J, Sampedro I, García-Romera I, García-Garrido JM, Ocampo JA (2002) Arbuscular mycorrhizal colonization and growth of soybean (Glycine max) and lettuce (Lactuc sativa) and phytotoxic effects of olive mill residues. Soil Biol Biochem 34(11):1769–1775
Massoudinejad MR, Arman K, Aghayani E (2014) Ecological risk assessment to olive oil mill wastewater (OMW) with bioassay on plant species. Ecol Environ Conserv 20(1):229–234
Mekki A, Dhouib A, Aloui F, Sayadi S (2006) Olive wastewater as an ecological fertiliser. Agron Sustain Dev 26(1):61–67
Mekki A, Dhouib A, Sayadi S (2013) Effects of olive mill wastewater application on soil properties and plants growth. Int J Recycl Organic Waste Agr 2(1):15
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Nair N, Altieri R, Esposito A, Saville K (2014) Recent studies on preparation of humified compost using olive mill waste for horticultural purposes. Acta Hortic 1018:465–470
Ouzounidou G, Moustakas M, Symeonidis L, Karataglis S (2006) Response of wheat seedlings to Ni stress: effects of supplemental calcium. Arch Environ Contam Toxicol 50(3):346–352
Ouzounidou G, Asfi M, Sortirakis N, Papadopoulou P, Gaitis F (2008) Olive mill wastewater triggered changes in physiology and nutritional quality of tomato (Lycopersicon esculentum Mill.) depending on growth substrate. J Hazard Mater 158(2-3):523–530
Ouzounidou G, Zervakis GI, Gaitis F (2010) Raw and microbiologically detoxified olive mill waste and their impact on plant growth. Terr Aquatic Environ Toxicol 4(1):21–38
Paraskeva P, Diamadopoulos E (2006) Technologies for olive mill wastewater (OMW) treatment. J Chem Technol Biot 81(9):1475–1485
Reymov АМ, Namazov SS, Mirzaqulov XC, Beglov BM (2002) Nitrogen-phosphorus-calcium fertilizers on the base of the phosphorities of Central Kyzylkum and nitric acid. Reports of Academy of Sciences of Uzbekistan 5:50–52 (in Russian)
Saadi I, Raviv M, Berkovich S, Hanan A, Aviani I, Laor Y (2013) Fate of soil-applied olive mill wastewater and potential phytotoxicity assessed by two bioassay methods. J Environ Qual 42(6):1791–1801
Sayadi S, Ellouz R (1995) Role of lignin peroxidase and manganese peroxidase from Phanerochaete chrysosporium in the decolorization of olive mill waste-waters. Appl Environ Microb 61(13):1098–1103
Shah AR, Khan TM, Sadaqat HA, Chatha AA (2011) Alterations in leaf pigments in cotton (Gossypium hirsutum) genotypes subjected to drought stress conditions. Int J Agric Biol 13(6):902–908
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method in Enzymol 29:152–178
Tomsone L, Kruma Z (2013) Comparison of different solvents for isolation of phenolic compounds from horseradish (Armoracia Rusticana L.) leaves. Res Rural Devt 1:104–110
Yang CM, Chang IF, Lin SJ, Chou CH (2004) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Orza stevia) seedlings: II. Stimulation of consumption-orientation. Bot Bull Acad Sin 45:119–125
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Daâssi, D., Sellami, S., Frikha, F. et al. Assessment of Coriolopsis gallica-treated olive mill wastewater phytotoxicity on tomato plants. Environ Sci Pollut Res 23, 15370–15380 (2016). https://doi.org/10.1007/s11356-016-6615-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6615-3