Abstract
Barks from Prosopis juliflora (acacia) were collected in 12 sites of different geological contexts over the volcanic Fogo Island (Cape Verde). Elemental contents of Ba, Br, Co, Cr, Fe, K, Na, Zn and some rare earth elements (REE)—La, Ce, Sm, Eu, Tb, Yb, and Lu, were obtained for biological samples and topsoils by using k 0-standardized and comparative method of instrumental neutron activation analysis (INAA), aiming the evaluation of chemical elements uptake by acacia bark. This first biomonitoring study of Fogo Island showed that, in general, significant accumulations of trace elements present in high amounts in these soils occur. This can be partially explained by the semi-arid climate with a consequent bioavailability of chemical elements when rain drops fall in this non-polluted environment. REE enrichment factors (EFs) increase with the decrease of ionic radius. Heavy REE (HREE) are significantly enriched in bark, which agrees with their release after the primary minerals breakdown and the formation of more soluble compounds than the other REE, and uptake by plants. Among the potential harmful chemical elements, Cr appears to be partially retained in nanoparticles of iron oxides. The high EFs found in tree barks of Fogo Island are certainly of geogenic origin rather than anthropogenic input since industry and the use of fertilizers is scarce.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the chemical elements that occur in terrestrial ecosystems, some of them are present as trace elements in soils (<0.1 wt%), resulting from processes such as chemical weathering of the parent material, volcanic eruptions, and/or forest fires. The main active agents in weathering are water, carbon dioxide, and oxygen. The weathering process is slow and continuous, producing dilute solutions of trace elements that are transported into the groundwater and eventually into the rivers, and then to the ocean, when rainfall and drainage are adequate. In regions of limited rainfall or poor drainage, the products of weathering accumulate locally (Bañuelos and Ajwa 1999). Trace elements occur incorporated in primary and secondary minerals, and their concentrations in soils reflect the nature of the parent material, climate, and drainage conditions (Förstner 1995). The accumulation of these elements in surface soils is due to their complexation by organic material (adsorption), as a result of cycling through vegetation or atmospheric deposition. The behavior of some trace elements in soils can be very complex because they can occur in different oxidation states, being soluble, exchangeable, adsorbed, or co-precipitated with carbonates, sulfides, phosphates, and hydroxides (Bañuelos and Ajwa 1999). The availability of trace elements in soils may result in bigger uptakes by plants, which presents a health risk to wildlife, and especially to human beings, with the ingestion of those plant tissues (Kabata-Pendias 2001).
Several studies have been performed in order to assess the fate and behavior of the trace elements in the environment all over the world, namely the bioaccumulation of trace elements. These studies have been carried out in lichens and in diverse parts of plants, like tree barks, grown in different types of soils, including the volcanic soils (Baxter et al. 2012; Cruz et al. 2013; De Nicola et al. 2003; Freitas et al. 2006; Godinho et al. 2008; Pacheco et al. 2001, 2002; Pacheco and Freitas 2009; Prudêncio 2007; Shagal et al. 2012; Wilson et al. 2009). Numerous works have shown that tree bark is an efficient biomarker to evaluate past organic (Clarkson et al. 2002; Hermanson and Hites 1990; Hermanson and Johnson 2007; Wen et al. 2009) and inorganic contamination (Lahd Geagea et al. 2007, 2008a, b; Senhou et al. 2002), along with “natural enrichment” on metals and other trace elements in soils, and atmosphere. This is the case of soils from recent or even active volcanic regions, like Cape Verde archipelago, where some trace elements may occur in very high amounts that can have an impact on the health of plants and animals growing in (Marques et al. 2012, 2014a, b).
Due to the volcanic origin of the Cape Verde islands, there was never a land connection and the islands must have been colonized from other parts of the world. The natural vegetation of this archipelago has been destroyed by agriculture practices, wood cutting, overgrazing, and more recently by afforestation. Within the different types of vegetation, a forest of Acacia albida previously covered the dry parts of this environment. Nowadays, this species disappeared and was replaced by Prosopis juliflora (Frahm et al. 1996). Acacias grow and survive in dry environments and have an important function sustaining the ecological and hydrological stability of arid and semi-arid ecosystems (Ludwig et al. 1999).
In the present work, barks of acacia (P. juliflora) were collected in different geological formations of the Fogo Island (Cape Verde). The corresponding soils were also studied. Chemical analyses of bark and soils were performed by instrumental neutron activation analysis (INAA) allowing to obtain the concentration of Ba, Br, Co, Cr, Cs, Fe, K, Na, Zn, and rare earth elements (REE).
The main goals of this study are as follows: (i) the evaluation of the chemical elements uptake, and the establishment of the chemical patterns/enrichment factors of the tree bark grown in volcanic soils in the semi-arid conditions of Cape Verde, and (ii) the bark response to differences in soil composition, the geographic location, and climate conditions. Thus, this work is a first approach to evaluate chemical elements uptake, including metals that can be a threat to health, by using tree bark as biomarker in the active volcanic island of Fogo (Cape Verde).
Material and methods
Studied area
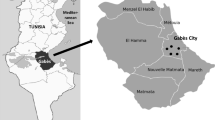
The Cape Verde archipelago is located in the Atlantic Ocean, some 500–800 km westwards of Africa coast, and comprises nine inhabited islands and several islets (with a total area of 4033 km2). Fogo is the fourth bigger island of Cape Verde (area of 476 km2) and is located in the south-western part of the archipelago (Fig. 1a). This island is an active stratovolcano rising 2829 m above sea level. The Fogo Island has a semi-arid climate with an average temperature of about 25 °C, which can reach the 0 °C in Chã das Caldeiras (December and January); the rain occurs between July and October and the precipitation is largely influenced by elevation and wind- and leeward conditions. The north-east part of the island is considered the humid region, with a precipitation higher than 600 mm/year, and the arid regions are located in the south-west, with a precipitation of 160 mm/year. The vegetation of this semi-arid island is restricted and occurs mostly in humid valleys or windward regions (Olehowski et al. 2008). The volcanic rocks of Fogo are included in three main stratigraphic units: (a) a carbonatite unit exposed in fluvial valleys near S. Filipe (the oldest rocks of the island), (b) a major volcanic sequence related to the sub-aerial shield-building of the volcano (nephelinites and associated lavas with layers of scoria or tuffs, previous to the caldera formation), and (c) a post caldera sequence including several pre-historic and historic eruptions (Madeira and Brum da Silveira 2005; Madeira et al. 2005; Torres et al. 1998). Deposits (de/Lahar) also occur associated with the post-caldera formation, with no precise age attributed. Soils are in general incipient, and paleosoils occur mainly in the western part of the island (Marques et al. 2014a).
Twelve acacia bark samples, as well as the soil underneath them, were collected in November 2010 in different country areas of Fogo Island (Cape Verde), corresponding to all the geological formations of the island: five samples in soils developed in the pre-caldera formation (carbonatite, nephelinites and associated lavas (herein referred as nephelinites), pyroclasts, and tuffs); and seven samples in soils from the post-caldera formation (lavas, pyroclasts, and deposits) (Fig. 1b, Table 1).
Sampling, treatment, and analysis
Thin layers of the acacia bark (not exceeding 0.5 cm) were carefully removed at an average height of 180 cm above the ground level, and away from the road. At the base of the trees, topsoil samples (0–20 cm depth) were also collected, using a stainless steel shovel to avoid contamination, and stored in polyethylene bags.
Prior to analysis, the bark trees were rinsed with deionized water during 30 s for the complete removal of dust and epiphytes, freeze-dried, and ground in Teflon™ mill. Pellets of ≈500 mg were done for k 0-standardized INAA. Long (5 h) irradiations of the bark trees were performed in the Portuguese Research Reactor (RPI). Details of the irradiation conditions and measurements were described elsewhere (De Corte 1987, 2001; Freitas and Martinho 1989a, b; Freitas 1993; Pacheco et al. 2001). In the case of the soils, the whole sample was obtained by sieving through a 2-mm nylon mesh sieve, dried, and ground in agate mortars for chemical analysis. The total contents were obtained by comparative INAA method. Two reference materials were used, namely soil GSS-4 and sediment GSD-9 from the Institute of Geophysical and Geochemical Prospecting (IGGE). Reference values were taken from data tabulated by Govindaraju (1994). Two aliquots of each standard were used for internal calibration, and standard checks were performed (QA/QC). The samples and standards were prepared for analysis by weighing 200–300 mg of powder into cleaned high-density polyethylene vials. The irradiations (6 h) were also performed in the RPI (Dung et al. 2010). More details of the analytical method are given in Dias and Prudêncio (2007), Gouveia et al. (1992), Gouveia and Prudêncio (2000), and Prudêncio (2009). The precise and accurate concentration of 15 chemical elements was obtained (Ba, Br, Co, Cr, Fe, K, Na, Zn, and REE—La, Ce, Sm, Eu, Tb, Yb, and Lu).
Enrichment factors
In order to evaluate the enrichment of the chemical elements in plants with respect to the soil, enrichment factors (EF) were calculated, using Fe as conservative element according to:
where EF x stands for the enrichment factor of the element X in the tree bark, after having its concentration normalized to Fe concentrations in the corresponding soil sample. Iron was selected for normalization purpose due to its precise and accurate determination by INAA and its conservative behavior. In fact, the Fe3+ tends to remain in soils during the weathering processes in this type of soils/arid climate as shown by Marques et al. (2014b). Though unit or near unit EFs are usually considered unexceptional, the EF > 10 is accepted as a substantial enrichment over the natural background (Chiarenzelli et al. 2001; Galinha et al. 2010).
Results and discussion
The enrichment factors of the chemical elements in acacia barks and the chemical elements contents obtained by INAA in the underneath topsoils of Fogo Island are given in Tables 1 and 2, respectively.
In general, the results point to a significant bioaccumulation in bark of most of the chemical elements studied taking into consideration that EF > 10 is a reasonable enrichment factor by plants. Exceptions were found for Co, La, and Sm, which are not enriched in any of the acacia barks (Fig. 2).
K and Na
Potassium and Na, considered as essential or beneficial elements for the plant growth (Alloway 2009; ITRC 2001; Lucas 2001), occur in high amounts in the soils of the Fogo Island (Fig. 3a), particularly in those developed in the post-caldera formation. A high content of K in the soil developed on carbonatite was also found. Concerning bioaccumulation, all the acacia barks studied show high accumulation of K regardless of the geological context where the tree grows. This behavior could be expected since K participates in soil biological processes, plays an important role in water regulation, and has a rapid uptake in warm and aerated soils (Maurice 2009). Nevertheless, some variations in the EFK are observed in acacia bark of the Fogo Island, with a tendency for higher enrichment factors in barks collected in the pre-caldera formation, particularly in the nephelinites (older soils), and in deposits (reaching an EFK = 3246) (Fig. 3b) despite the lower contents of K in these soils. This could be expected since K is available for plants uptake in volcanic soils probably due to its easy mobilization from the vitreous phase during weathering processes (Zharikova and Golodnaya 2009), and to the low amounts of minerals able to incorporate K namely alkali feldspars and secondary clay minerals. Besides, the EFK found in the Fogo Island are very high when compared with tree barks grown in other volcanic environments namely in the Atlantic Pico Island (Azores, Portugal) (Pacheco and Freitas 2009), which can be partially explained by climate differences, particularly the lower precipitation in Cape Verde.
The acacia bark presents a curious behavior in the uptake of Na. Like K, a higher bioaccumulation in the soils from the pre-caldera formation (EFNa > 10) and a lower Na uptake in the soils from the post-caldera formation were found (EFNa < 10) (see Fig. 3b). This may be partially explained by the higher weathering degree of primary minerals like plagioclase and nepheline in the older soils with a consequent Na release and bioavailability. However, acacia barks collected on soils developed on lava of the post-caldera formation (23-M) and on deposits (24-M) from the north-east part of the island also show a high EFNa, due to the rough topography and/or the higher precipitation in this part of the island, with a partial elution of this element after breakdown of primary minerals. The EFNa in acacia bark of Fogo Island may reach high values when compared with the ones found in Pico Island, Azores (Pacheco and Freitas 2009).
Cr, Co, and Zn
Concerning the first transition elements (Cr, Co, and Zn), despite the high contents found in soils, the acacia barks present low contents, particularly of Co (Fig. 4a). In fact, there is no preferential uptake of Co by acacia in the different geological formations, being the EFCo < 10 for all the trees studied in this work (see Fig. 2). The results obtained indicate that in Fogo Island, the uptake of Zn by bark appears not to depend significantly on the parent rock/soil type. In fact, there is no significant difference in the EFZn of the tree barks grown in soils of the post-caldera (more recent soils) and pre-caldera (older soils) formations, with an EFZn > 10 for all the acacia bark studied, except for the one grown in carbonatites where the highest amount of Zn in soil was measured (Fig. 4b). Zinc is an essential nutrient and its soluble forms are the easiest to be uptake from soils by plants, being this process dependent of the plant type and the main soil conditions (composition and pH) (Alloway 2009). According to Loska et al. (2005), Zn is one of the most mobile elements in soil, and its content is affected by weathering of the parent material, precipitation, decomposition of living matter, and the use of soil fertilization. The deficiency on this element in the soil hinders plant growth, causes interveinal chlorosis and yellowing on young leaves, and reduces leaf size (Kabata-Pendias 2001). The high EFZn of tree barks found in Fogo, when compared with other Atlantic islands (Pacheco and Freitas 2009), can be explained by the higher content and availability of this element in this island. It should be noted that in a recent study performed by Marques et al. (2012) high contents of Zn and an aqua regia extraction of ≈50 % of this element were found in the volcanic topsoils of Santiago Island (Cape Verde). The occurrence of high contents of this element in soils of Fogo (68.8 to 232 mg/kg) is certainly from the natural background due to Zn compatibility during fractionation, through magmatic processes, and its enrichment in volcanic rocks. Also, the Zn adsorption in secondary Fe or Mn oxides has been suggested (Zampella and Adamo 2010), and the presence of Fe oxides including as nanoparticles was recently described by Marques et al. (2014b) in this island. A considerable translocation of Zn from soils to leaves was observed in other volcanic soils by De Nicola et al. (2003) in Vesuvius National Park.
Chromium occurs in higher amounts in soils developed in the pre-caldera formation, with exception of carbonatite (see Table 2 and Fig. 4a). In a previous study of topsoils of Fogo Island (Marques et al. 2014b), this was already mentioned and explained based on the higher concentration of Fe oxide nanoparticles in the older soils, where Cr appears to be concentrated. Thus, despite the high EFCr found in most of the trees studied (see Table 1), the acacia barks from pre-caldera formation do not show the same trend, pointing the results to non-availability of the Cr portion incorporated in Fe oxides nanoparticles. In this way, Fe oxides appear to play an important role in the retention of Cr, which is considered of risk to environment (Marques et al. 2014b; Shanker et al. 2005).
Br and Ba
Bromine occurs in higher amounts in the older soils, except the one developed on carbonatite (Fig. 5), and is generally enriched in the acacia barks (all samples have EFBr > 10) (see Table 1). The high EFBr in tree bark grown in carbonatite soil may be partially due to the breakdown of fluorapatite and release of Br, where this element may be replacing F in the mineral structure (Marques et al. 2014b). Despite the low contents of Br in the younger soils of the post-caldera formation, this element is in general more accumulated in bark; this can be in part explained by the geographic position of the sampling sites—higher windward north and east areas, and more precipitation. In this part of the island, the parent rock/age factor appears to have no significance in soils as referred by Olehowski et al. (2008). However, the glassy component of the soil may also contribute for a higher availability of Br; also volcanic ash soils tend to accumulate Br in its humic acid (Yamada 1968). Acacia barks from the eastern and northeastern part of the island present the highest Br accumulation (EFBr > 2000), indicating that the contribution from the marine aerosol has to be taken in account since dominant winds blow from NE (60–80 %) (INMG 2010; Olehowski et al. 2008). In fact, tree barks collected in sites of high altitude and wind exposure have high EFBr, which can be explained by the higher Br content in marine submicrometer aerosol (that can reach higher altitudes) and precipitation (Sander et al. 2003).
Barium was found in high amounts in the topsoils of Fogo Island (from 370 to 1050 mg/kg) and a high bioaccumulation of this element seems to occur (EFBa > 10) regardless of the geological context (Fig. 6 and Table 1) (Pearson correlation coefficient ρ = 0.045). Nevertheless, in the soils from the post-caldera formation, there is a tendency to higher Ba accumulation in the acacia bark. The different bioavailability of this element in soils may be due to its presence in different mineral phases, such as feldspars or barite. The occurrence of significant concentrations of Ba in volcanic soils of Cape Verde (Santiago Island) has already been mentioned by Marques et al. (2012). It should be noted that Ba is a toxic element to most plants, and its uptake may increase human and animals exposure through vegetable consumption (Monteiro et al. 2011).
Rare earth elements
The REE patterns relative to chondrites (values of Anders and Grevesse (1989) multiplied by 1.36, obtained by Korotev (1996a, b)) of soils are shown in Fig. 7. The higher contents of REE are found in two soils from the pre-caldera formation: soil 11-SF (collected in nephelinites) and soil 6-SF (collected in carbonatites). The other studied soils show similar REE patterns, with lower REE contents. A slight negative Ce anomaly occurs in soils 11-SF and 67-CF (Ce/Ce* = 0.80 and 0.79, respectively).
The enrichment factor of REE vs. atomic number for acacia barks is shown in Fig. 8 (the acacia bark collected in site 11-SF was ignored since only a few REE contents in the bark were obtained). The REE patterns are in general similar for acacia grown in different geological formations with an increase from light REE (LREE)—those from La to Sm (i.e., lower atomic numbers and masses), to heavy REE (HREE)—those from Gd to Lu (higher atomic number and masses). A significant EF for the HREE was found, reaching EFLu = 4439. Concerning LREE and middle REE (MREE)—those from Pm to Ho, particularly from La to Eu, the acacia barks present in general low EF (EF < 10), with exception of Ce in acacia collected in nephelinite (site 89-SF) where a significant positive anomaly occurs (see Fig. 8).
The significant enrichment in HREE may be explained by the preferential uptake of these elements by bark after the pyroxenes breakdown, where smaller ions of the HREE substitute more readily than the LREE during magma processes. Also, HREE form soluble complexes more stable than the other REE partially explaining their presence in higher amounts in soil solutions (Henderson 1996). The LREE may be partially incorporated in iron oxides and clay minerals, being less bioavailable (Compton et al. 2003; Prudêncio et al. 2011). Moreover, the positive Ce anomaly found in some of the acacia barks may be related with a partial Ce oxidation after the REE release from primary mineral phases and a favored uptake of Ce4+ by this type of plants. Previous studies have reported the uptake of cerium oxide nanoparticles (nCeO2) by plants and their physiological impacts (Hernandez-Viezcas et al. 2013; Lopez-Moreno et al. 2010a, b; Rico et al. 2013; Zhang et al. 2012). A similar trend for a higher retention of Ce relative to La in plants, compared to groundwater, was previously observed in the arid environment of AlKhod area, Oman (Sehmi et al. 2009), and in some tropical trees of Brazil (Nakanishi et al. 1997).
Despite the low solubility of the REE, their compounds are mobile under specific geochemical conditions, depending on pH, temperature, redox potential and the availability of potential ligands (Henderson 1996). The results obtained in this work show that REE may be significantly fractionated during biological processes in the volcanic soils of Fogo Island.
A previous work performed by Pacheco and Freitas (2009) in the Atlantic Pico volcanic island (Azores, Portugal) showed that, in general, the EFs of the chemical elements studied are lower than the ones found in Fogo Island of Cape Verde. This can be explained mainly by differences in the climate, particularly the higher precipitation in Azores compared to the semi-arid environment of Cape Verde archipelago. Despite the high EFs in Fogo tree barks, the origin of the chemical elements (some that can be a threat to health) is certainly from the natural background rather than anthropogenic inputs since industry and the use of fertilizers is scarce.
Thus, the chemical elements may be significantly accumulated by plants grown in volcanic soils of Fogo Island, which can be explained mainly by the soil composition together with the semi-arid climate and a consequent bioavailability of chemical elements when rain drops fall in this environment with unimportant anthropogenic contamination input.
The results obtained in this work indicate that acacia bark can be used as effective biomonitor reflecting cumulative effects in environmental control, detecting anomalous concentrations of metal(loids) both from soil and atmosphere.
Conclusions
The acacia barks grown in diverse volcanic soils in the semi-arid environment of the Fogo Island (Cape Verde archipelago, Atlantic ocean) present high enrichment factors of the majority of the chemical elements studied (essential or not to plants), regardless of the geological formation where they grow. A significant bioaccumulation of essential elements to the plant growth, particularly K and Zn, occur as could be expected. A high uptake of Br was also observed for the majority of the trees, partially explained by marine/atmospheric contribution. In a lower scale, Ba and Cr also show a high bioavailability in the different types of soils where plants were collected. The HREE are preferentially uptake by tree barks relative to the other REE. A positive Ce anomaly was found in some tree barks indicating the uptake of nanoparticles of CeO2 which can be expected since oxidation is the main chemical weathering process in the semi-arid climate of Fogo Island.
The high availability of chemical elements in the volcanic soils of Fogo Island is certainly due to long periods with no precipitation, leading to their accumulation and uptake by plants when water interferes. This bioaccumulation is surely from the natural background since no significant anthropogenic inputs occur in the island. Among the mineral phases present in soils, iron oxides nanoparticles can play an important role in the retention of potentially harmful elements like Cr.
As long-lived organisms, acacia barks of Fogo Island can be used as biomonitors to detect concentration of trace elements both from soils and/or atmospheric origin, and the results obtained in this work can be used as a benchmark if anthropogenic activities increase in the future to evaluate eventual pollution.
References
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Heal 31:537–548. doi:10.1007/s10653-009-9255-4
Anders E, Grevesse N (1989) Abundances of the elements: meteoritic and solar. Geochim Cosmochim Acta 53:197–214, http://www.ucolick.org/~woosley/ay220-11/papers/ag89.pdf
Bañuelos GS, Ajwa HA (1999) Trace elements in soils and plants: an overview. J Environ Sci Heal A 34(4):951–974. doi:10.1080/10934529909376875
Baxter I, Hermans C, Lahner B, Yakubova E, Tikhonova M (2012) Biodiversity of mineral nutrient and trace element accumulation in Arabidopsis thaliana. PLoS ONE 7(4), e35121. doi:10.1371/journal.pone.0035121
Chiarenzelli JR, Aspler LB, Dunn C, Cousens B, Ozarko DL, Powis KB (2001) Multi-element and rare earth element composition of lichens, mosses, and vascular plants from the Central Barrenlands, Nunavut, Canada. Appl Geochem 16:245–270. doi:10.1016/S0883-2927(00)00027-5
Clarkson PJ, Larrazabal-Moya D, Staton I, McLeod CW, Ward DB, Sharifi VN, Swithenbank J (2002) The use of tree bark as a passive sampler for polychlorinated dibenzo-p-dioxins and furans. Int J Environ An Chem 82:843–850. doi:10.1080/0306731021000102301
Compton JS, White RA, Smith M (2003) Rare earth element behavior in soils and salt pan sediments of a semi-arid granitic terrain in the Western Cape, South Africa. Chem Geol 201:239–255. doi:10.1016/S0009-2541(03)00239-0
Cruz J, Silva MO, Dias MI, Prudêncio MI (2013) Groundwater composition and pollution due to agricultural practices in Sete Cidades Volcano (Azores, Portugal). Appl Geochem 29:162–173. doi:10.1016/j.apgeochem.2012.11.009
De Corte F (1987) The k 0-standardization method—a move to the optimization of neutron activation analysis (Aggrégé Thesis). Institute for Nuclear Sciences, University of Gent, Gent
De Corte F (2001) The standardization of standardless NAA. J Radioanal Nucl Chem 248:13–20. doi:10.1023/A:1010601403010
De Nicola F, Maisto G, Alfani A (2003) Assessment of nutritional status and trace element contamination of holm oak woodlands through analyses of leaves and surrounding soils. Sci Total Environ 311:191–203. doi:10.1016/S0048-9697(03)00132-3
Dias MI, Prudêncio MI (2007) Neutron activation analysis of archaeological materials: an overview of the ITN NAA laboratory, Portugal. Archaeometry 49(2):381–391. doi:10.1111/j.1475-4754.2007.00308.x
Dung HM, Freitas MC, Santos JP, Marques JG (2010) Re-characterization of irradiation facilities for k0-NAA at RPI after conversion to LEU fuel and re-arrangement of core configuration. Nucl Instrum Meth A 622:438–442. doi:10.1016/j.nima.2010.02.057
Förstner U (1995) Metal speciation and contamination of soil. CRC, Boca Raton, pp 1–33
Frahm JP, Lindlar A, Sollman P, Fischer E (1996) Bryophytes from the Cape Verde Islands. TROP Bryol 12:123–153, http://core.ac.uk/download/pdf/14529978.pdf
Freitas MC (1993) The development of k 0-standardized neutron activation analysis with counting using a low energy photon detector (PhD Thesis). Institute for Nuclear Sciences, University of Gent, Gent
Freitas MC, Martinho E (1989a) Neutron activation analysis of reference materials by the k 0-standardization and relative methods. Anal Chim Acta 219:317–322. doi:10.1016/S0003-2670(00)80363-3
Freitas MC, Martinho E (1989b) Accuracy and precision in instrumental neutron activation analysis of reference materials and lake sediments. Anal Chim Acta 223:287–292. doi:10.1016/S0003-2670(00)84093-3
Freitas MC, Pacheco AMG, Dionísio I, Sarmento S, Baptista MS, Vasconcelos MTSD, Cabral JP (2006) Multianalytical determination of trace elements in atmospheric biomonitors by k 0-INAA, ICP-MS and AAS. Nucl Instrum Meth A 564:733–742. doi:10.1016/j.nima.2006.04.008
Galinha C, Freitas MC, Pacheco AMG (2010) Enrichment factors and transfer coefficients from soil to rye plants by INAA. J Radioanal Nucl Chem 286:583–589. doi:10.1007/s10967-010-0803-2
Godinho RM, Wolterbeek HT, Verburg T, Freitas MC (2008) Bioaccumulation behaviour of transplants of the lichen Flavoparmelia caperata in relation to total deposition at a polluted location in Portugal. Environ Pollut 151:318–325. doi:10.1016/j.envpol.2007.06.034
Gouveia MA, Prudêncio MI (2000) New data on sixteen reference materials obtained by INAA. J Radioanal Nucl Chem 245:105–108. doi:10.1023/A:1006748407917
Gouveia MA, Prudêncio MI, Morgado I, Cabral JMP (1992) New data on the GSJ reference rocks JB-1a and JG-1a by instrumental neutron activation analysis. J Radioanal Nucl Chem 158:115–120. doi:10.1007/BF02034778
Govindaraju K (1994) Compilation of working values and sample description for 383 geostandards. Geostandard Newslett 18:1–158
Henderson P (1996) Chapter 1. The rare earth elements: introduction and review. In: Jones AP, Wall F, Terry Williams C (eds) Rare earth minerals: chemistry, origin and ore deposits. Chapman & Hall, London, pp 1–9
Hermanson MH, Hites RA (1990) Polychlorinated biphenyls in tree bark. Environ Sci Technol 24:666–671. doi:10.1021/es00075a008
Hermanson MH, Johnson GW (2007) Polychlorinated biphenyls in tree bark near a former manufacturing plant in Anniston, Alabama. Chemosphere 68:191–198. doi:10.1016/j.chemosphere.2006.11.068
Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL (2013) In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 7:1415–1423. doi:10.1021/nn305196q
INMG (2010) Segunda comunicação nacional de cabo verde para as mudanças climáticas. Ministério do Ambiente, Desenvolvimento Rural e Recursos Marinhos. Inst Nac Meteo Geofís. www.sia.cv/ (in Portuguese)
ITRC (Interstate Technology & Regulatory Council) (2001) Phytotechnology technical and regulatory guidance document. http://www.itrcweb.org/Guidance/GetDocument?documentID=64
Kabata-Pendias H (2001) Trace elements in soils and plants. CRC, Boca Raton
Korotev RL (1996a) A self-consistent compilation of elemental concentration data for 93 geochemical reference samples. Geostandard Newslett 20:217–245. doi:10.1111/j.1751-908X.1996.tb00185.x
Korotev RL (1996b) On the relationship between the Apollo 16 ancient regolith breccias and feldspathic fragmental breccias, and the composition of the prebasin crust in the Central Highlands of the Moon. Meteorit Planet Sci 31:403–412, http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19980018585.pdf
Lahd Geagea M, Stille P, Millet M, Perrone T (2007) REE characteristics and Pb, Sr and Nd isotopic compositions of steel plant emissions. Sci Total Environ 373:404–419. doi:10.1016/j.scitotenv.2006.11.011
Lahd Geagea M, Stille P, Gauthier-Lafaye F, Perrone T, Aubert D (2008a) Baseline determination of the atmospheric Pb, Sr and Nd isotopic compositions in the Rhine valley, Vosges mountains (France) and the Central Swiss Alps. Appl Geochem 23:1703–1714. doi:10.1016/j.apgeochem.2008.02.004
Lahd Geagea M, Stille P, Gauthier-Lafaye F, Millet M (2008b) Tracing of industrial aerosol sources in an urban environment using Pb, Sr, and Nd isotopes. Environ Sci Technol 42:692–698. doi:10.1021/es071704c
Lopez-Moreno ML, De La Rosa G, Hernanez-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2010a) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol 44:7315–7320. doi:10.1021/es903891g
Lopez-Moreno ML, De la Rosa G, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2010b) X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem 58:3689–3693. doi:10.1021/jf904472e
Loska K, Wiechuła D, Pelczar J (2005) Application of enrichment factor to assessment of zinc enrichment/depletion in farming soils. Commun Soil Sci Plan 36:1117–1128. doi:10.1081/CSS-200056880
Lucas Y (2001) The role of plants in controlling rates and products of weathering: importance of biological pumping. Ann Rev Earth Pl Sci 29:135–163. doi:10.1146/annurev.earth.29.1.135
Ludwig JA, Tongway DJ, Marsden SG (1999) Stripes, strands or stipples: modelling the influence of three landscape banding patterns on resource capture and productivity in semi-arid woodlands, Australia. Catena 37:257–273. doi:10.1016/S0341-8162(98)00067-8
Madeira J, Brum da Silveira A (2005) Geomorphic and structural analysis of the Fogo Island Volcano (Cape Verde). Abstract Volume of SAL2005 International Workshop on Ocean Island Volcanism, Sal, Cape Verde
Madeira J, Munhá J, Tassinari CGC, Mata J, Brum da Silveira A, Martins S (2005) K/Ar ages of carbonatites from the island of Fogo (Cape Verde). Actas da XIV Semana da geoquímica e VII Congresso de geoquímica dos países de língua Portuguesa, 475–478. http://www.researchgate.net/profile/Jose_Madeira3/publication/257425711_KAr_ages_of_carbonatites_from_the_Island_of_Fogo_(cape_Verde)/links/00b7d52541fc8d7abe000000.pdf
Marques R, Prudêncio MI, Rocha F, Cabral Pinto MS, Silva MMVG, Ferreira da Silva E (2012) REE and other trace and major elements in the topsoil layer of Santiago Island, Cape Verde. J Afr Earth Sci 64:20–33. doi:10.1016/j.jafrearsci.2011.11.011
Marques R, Prudêncio MI, Waerenborgh JC, Rocha F, Dias MI, Ruiz F, Ferreira da Silva E, Abad M, Muñoz AM (2014a) Origin of reddening in a paleosol buried by lava flows in Fogo Island (Cape Verde). J Afr Earth Sci 96:60–70. doi:10.1016/j.jafrearsci.2014.03.019
Marques R, Waerenborgh JC, Prudêncio MI, Dias MI, Rocha F, Ferreira da Silva E (2014b) Iron speciation in volcanic topsoils from Fogo Island (Cape Verde)—iron oxide nanoparticles and trace elements concentrations. Catena 113:95–106. doi:10.1016/j.catena.2013.09.010
Maurice PA (2009) Environmental surfaces and interfaces from the nanoscale to the global scale. Wiley, New Jersey
Monteiro FA, Nogueiro RC, Melo LCA, Artur AG, da Rocha F (2011) Effect of barium on growth and macronutrient nutrition in Tanzania guineagrass grown in nutrient solution. Soil Sci Plant Anal 42:1510–1521. doi:10.1080/00103624.2011.581725
Nakanishi TM, Takahashi J, Yagi H (1997) Rare earth element, Al, and Sc partition between soil and Caatinger wood grown in north-east Brazil by instrumental neutron activation analysis. Biol Trace Elem Res 60(3):163–174. doi:10.1007/BF02784437
Olehowski C, Naumann S, Fischer D, Siegmund A (2008) Geo-ecological spatial pattern analysis of the island of Fogo (Cape Verde). Glob Planet Chang 64:188–197. doi:10.1016/j.gloplacha.2008.09.006
Pacheco AMP, Freitas MC (2009) Trace-element enrichment in epiphytic lichens and tree bark at Pico Island, Azores, Portugal. J Air Waste Manag 59:411–418. doi:10.3155/1047-3289.59.4.411
Pacheco AMG, Freitas MC, Barros LIC, Figueira R (2001) Investigating tree bark as an air-pollution biomonitor by means of neutron activation analysis. J Radioanal Nucl Chem 249(2):327–331. doi:10.1023/A:1013293814789
Pacheco AMG, Barrosa LIC, Freitas MC, Reis MA, Hipólito C, Oliveira OR (2002) An evaluation of olive-tree bark for the biological monitoring of airborne trace-elements at ground level. Environ Pollut 120:79–86. doi:10.1016/S0269-7491(02)00130-6
Prudêncio MI (2007) Biogeochemistry of trace and major elements in a surface environment (volcanic rock, soil, mosses, lichens) in the S. Miguel Island, Azores. Port J Radioanal Nucl Chem 271(2):431–437. doi:10.1007/s10967-007-0227-9
Prudêncio MI (2009) Ceramic in ancient societies: a role for nuclear methods of analysis. In: Koskinen AN (ed) Nuclear chemistry: new research. Nova Science, New York, pp 51–81
Prudêncio MI, Dias MI, Waerenborgh JC, Ruiz F, Trindade MJ, Abad M, Marques R, Gouveia MA (2011) Rare earth and other trace and major elemental distribution in a pedogenic calcrete profile (Slimene, NE Tunisia). Catena 87:147–156. doi:10.1016/j.catena.2011.05.018
Rico CM, Hong J, Morales MI, Zhao L, Barrios AC, Zhang J, Peralta-Videa JR, Gardea-Torresdey JL (2013) Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol 47:5635–5642. doi:10.1021/es401032m
Sander R, Keene WC, Pszenny AAP, Arimoto R, Ayers GP, Baboukas E, Cainey JM, Crutzen PJ, Duce RA, Onninger GH, Huebert BJ, Maenhaut W, Mihalopoulos N, Turekian VC, Van Dingenen R (2003) Inorganic bromine in the marine boundary layer: a critical review. Atmos Chem Phys 3:2963–3050. doi:10.5194/acp-3-1301-2003
Sehmi K, Abdalla OAE, Khirbash S, Khan T, Asaidi S, Farooq S (2009) Mobility of rare earth elements in the system soils-plants-groundwaters: a case study of an arid area (Oman). Arab J Geosci 2:143–150. doi:10.1007/s12517-008-0024-y
Senhou A, Chouak A, Cherkaoui R, Moutia Z, Lferde M, Elyahyaoui A, El Khoukhi T, Bounakhla M, Embarche K, Gaudry A (2002) Sensitivity of biomonitors and local variations of element concentrations in air pollution biomonitoring. J Radioanal Nucl Chem 254:343–349. doi:10.1023/A:1021688203179
Shagal MH, Maina HM, Donatus RB, Tadzabia K (2012) Bioaccumulation of trace metals concentration in some vegetables grown near refuse and effluent dumpsites along Rumude-Doubeli bye-pass in Yola North, Adamawa State. GARJEST 1(2):018–022
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753. doi:10.1016/j.envint.2005.02.003
Torres PC, Madeira J, Silva LC, Brum da Silveira A, Serralheiro A, Mota Gomes A (1998) Carta geológica da ilha do Fogo (República de Cabo Verde). Erupções históricas e formações enquadrantes. LATTEX, Departamento de Geologia da Fac. de Ciências da Univ. de Lisboa. Escala 1–25000
Wen S, Yang F, Li JG, Gong Y, Zhang XL, Hui Y, Wu YN, Zhao YF, Xu Y (2009) Polychlorinated dibenzo-p-dioxin and dibenzofurans (PCDD/Fs), polybrominated diphenyl ethers (PBDEs), and polychlorinated biphenyls (PCBs) monitored by tree bark in an E-waste recycling area. Chemosphere 74:981–987. doi:10.1016/j.chemosphere.2008.10.002
Wilson B, Pyatt B, Denton G (2009) An evaluation of the bioavailability and bioaccumulation of selected metals occurring in a wetland area on the volcanic island of Guam, Western Pacific Ocean. J Environ Sci 21:1547–1551. doi:10.1016/S1001-0742(08)62453-5
Yamada Y (1968) Occurrence of bromine in plants and soil. Talanta 15(11):1135–1141
Zampella M, Adamo P (2010) Chemical composition and Zn bioavailability of the soil solution extracted from Zn amended variable charge soils. J Environ Sci 22(9):1398–1406. doi:10.1016/S1001-0742(09)60266-7
Zhang P, Ma Y, Zhang Z, He X, Zhang J, Guo Z, Tai R, Zhao Y, Chai Z (2012) Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano 6:9943–9950. doi:10.1021/nn303543n
Zharikova EA, Golodnaya OM (2009) Available potassium in volcanic soils of Kamchatka. Eurasian Soil Sci 42(8):850–860. doi:10.1134/S1064229309080031
Acknowledgments
The authors would like to thank the reviewers for their careful and constructive reviews that considerably improved the manuscript. Grateful acknowledgments are made to financial support made by the project UID/GEO/04035/2013, and also to the staff of the Portuguese Research Reactor (RPI) of CTN/IST for their assistance with the neutron irradiations. C2TN/IST authors are thankful to the FCT (the Portuguese Science and Technology Foundation) support through the UID/Multi/04349/2013 project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Marques, R., Prudêncio, M.I., Freitas, M.d.C. et al. Chemical element accumulation in tree bark grown in volcanic soils of Cape Verde—a first biomonitoring of Fogo Island. Environ Sci Pollut Res 24, 11978–11990 (2017). https://doi.org/10.1007/s11356-015-5498-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5498-z