Abstract
The economy of an industrialized country is greatly dependent on fossil fuels. However, these nonrenewable sources of energy are nearing the brink of extinction. Moreover, the reliance on these fuels has led to increased levels of pollution which have caused serious adverse impacts on the environment. Hydrogen has emerged as a promising alternative since it does not produce CO2 during combustion and also has the highest calorific value. The biohythane process comprises of biohydrogen production followed by biomethanation. Biological H2 production has an edge over its chemical counterpart mainly because it is environmentally benign. Maximization of gaseous energy recovery could be achieved by integrating dark fermentative hydrogen production followed by biomethanation. Intensive research work has already been carried out on the advancement of biohydrogen production processes, such as the development of suitable microbial consortium (mesophiles or thermophiles), genetically modified microorganism, improvement of the reactor designs, use of different solid matrices for the immobilization of whole cells, and development of two-stage process for higher rate of H2 production. Scale-up studies of the dark fermentation process was successfully carried out in 20- and 800-L reactors. However, the total gaseous energy recovery for two stage process was found to be 53.6 %. From single-stage H2 production, gaseous energy recovery was only 28 %. Thus, two-stage systems not only help in improving gaseous energy recovery but also can make biohythane (mixture of H2 and CH4) concept commercially feasible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although fossil fuels meet the energy demands for most of the countries today, their contribution toward environmental degradation and climate change due to greenhouse emissions have raised serious global concerns over their usage. Also, with the current rate of consumption of petroleum and coal, the exhaustion of these reserves has necessitated the search for an alternate source of energy (Soetaert and Vandamme 2009). Thus, this has triggered focus on renewable and development of greener technologies to fulfill the growing energy demands (Das and Verziroglu 2001). In the recent past, hydrogen has emerged out as a clean, carbon neutral, and renewable source of energy which has the highest energy density (143 GJ ton−1) and, on combustion, produces only water as by-product. At present, approximately 368 trillion cubic meters of hydrogen is produced commercially for various purposes (Pandu and Joseph 2012) using processes such as steam methane reforming, oil/naphtha reforming of refinery/chemical industrial off-gases, coal gasification, and water electrolysis (Baghchehsaree et al. 2010). However, these processes rely directly or indirectly on nonrenewable energy sources, consume lot of energy, and also have high carbon footprint. For the quest of clean renewable energy solutions, many technologies have been explored viz. bio-oil production by hydrothermal liquefaction, biomass gasification, pyrolysis of petroleum for methane production, etc. One such concept gained importance in recent times is Hythane. Hythane® is a combination of hydrogen, and methane was first developed by Hydrogen Components, Inc. (HCI) (http://edeninnovations.com/hythane-fuel/hythane-fuel-history).
The combination of hydrogen and methane as vehicular fuels proposes many advantages (Moreno et al. 2012):
-
1.
Lower flammability of methane limits its fuel efficiency. Addition of hydrogen could improve the lean flammability range significantly.
-
2.
In lean air/fuel mixtures, flame speed of methane is low, whereas hydrogen has 8-fold more flame speed.
-
3.
Hydrogen is a powerful combustion stimulant for accelerating the methane combustion within an engine, and hydrogen is also a powerful reducing agent for efficient catalysis at lower exhaust temperatures.
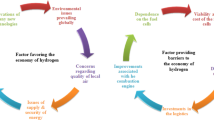
Biological process for clean energy gaseous energy generation encompasses biohydrogen and biomethane production. The carbon footprint of biohydrogen and biomethane production processes is still less as compared to chemical processes (Korres et al. 2010). Dark fermentative hydrogen production is known for their high rate and yield as compared to other biological processes (Roy et al. 2012). Biohydrogen can be produced from organic wastes at ambient temperature and atmospheric pressure (Benemann 1997) thereby generating a sustainable process that subsequently helps in waste stabilization. The major routes for biological hydrogen production are photolysis of water (direct and indirect biopholysis by blue green algae and microalgae), oxidation of organic acids by photo-fermentation and dark fermentation (using mesophilic or thermophilic bacteria). Nevertheless, each of the abovementioned processes are associated with their respective advantages and limitations. Biophotolysis of water and photo-fermentation yield very low rate of hydrogen production, and internal lighting requires additional energy input. Also, scaling up of these processes is difficult. Dark fermentation, on the other hand, is independent of light energy, requires moderate process conditions, and is less energy consuming (Das et al. 2008). Thus, dark fermentation process is considered as most promising method for biohydrogen production amongst all other processes. After dark fermentative H2 production, the spent media thus produced have high amount of short-chain fatty acids viz. acetate, butyrate, propionate, etc. These volatile fatty acids could be a suitable substrate for methanogens (Fig. 1). Thereby, integration of biohydrogen with biomethane process under the eponym of biohythane could help in improvement of gaseous energy recovery. Prior to subjection of spent media for biomethanation, the pH of it should be adjusted to a range of 7 to 7.8. Moreover, the dissolved H2 in the media also influences the growth of hydrogenotrophic methanogens.
Gaseous energy recovery in terms of only H2 might not be sufficient to make this process commercially viable. Only 20 to 30 % of total energy can be recovered through H2 production. On integration with photo-fermentation, theoretically, 12 mol mol−1 glucose could be recovered. Many challenges are associated with such integrated systems such as scaling up problem, shading effect of pigments produced by photofermentative organisms (Miyake et al. 1998). Thus, biohythane production provides encouraging opportunity for converting organic residues rich in carbohydrates, fats, and proteins for clean energy generation.
The present study gives a comprehensive insight of different microorganisms involved in both biohydrogen and biomethane production. Furthermore, the intra species interaction amongst the methanogens reveals the complexity of biohythane process.
Biochemistry behind biohythane production
Biochemistry of dark fermentative H2 production
The principle substrate for dark fermentative H2 production is glucose as it is the simplest sugar that is preferred by most of the microbes. Thus, the complex substrates are first hydrolyzed to simple sugars like glucose which is further metabolized via glycolytic pathway to pyruvate by dark fermentative bacteria. During this process, the microbes gain energy in the form of ATP, while pyruvate gets converted to acetate and butyrate releasing H2 as by-product. The mechanism of H2 production differs among the obligate and facultative anaerobes. In the metabolism of obligate anaerobes, first, the pyruvate-ferredoxin oxidoreductase (PFOR) enzyme oxidizes pyruvate to acetyl coenzyme A (acetyl-CoA). This step requires ferredoxin (Fd) reduction (Eq. 1). The Fd is further oxidized by [FeFe] hydrogenase resulting in the formation of H2 (Eq. 2).
In contrast, the metabolism of facultative anaerobic bacteria (Enterobacter sp., E. coli, etc.) and pyruvate oxidation are catalyzed by pyruvate formate lyase (PFL) resulting in the formation of acetyl-CoA and formate which is catalyzed (Knappe and Sawers 1990) (Eq. 3):
The formate is then further cleaved to produce carbon dioxide and hydrogen which is catalyzed by formate hydrogen lyase (FHL) enzyme (Eq. 6) (Stephenson and Stickland 1932).
Stoichiometrically, it is observed that 4 mol of H2 per mole of glucose can be produced if acetate is the sole end product of pyruvate oxidation (Eq. 5), whereas H2 yield of only 2 mol mol−1 glucose consumed can be produced if butyrate is the sole end product (Eq. 6).
Thus, the dark fermentative H2 production is limited by thermodynamic constraints, and the final yield of H2 produced depends upon the fate of pyruvate oxidation step.
Role of hydrogenase in facultative microorganisms
Facultative anaerobe such as in E. coli have four types of nickel-iron [NiFe] hydrogenase: hydrogenase-1 (Hyd-1), hydrogenase-2 (Hyd-2), hydrogenase-3 (Hyd-3), and hydrogenase-4 (Hyd-4). The uptake hydrogenases Hyd-1 and Hyd-2 are encoded by hya and hyb operons, respectively (Menon et al. 1991). A bidirectional hydrogenase (Hyd-3) encoded by hyc operon involved in both H2 production and consuming hydrogen (Maeda et al. 2007). It catalyzes the degradation of formate to molecular hydrogen under acidic pH (6.2–6.5). There is no role of Hyd-4 in H2 production, but it is required normal cellular function. The monocistronic fdhF gene codes for formate dehydrogenase-H (FDH-H). The hycBCDEFGH genes code for components of the formate hydrogen-lyase system which accepts electrons from FDH-H and reduces proton to evolve hydrogen. The hyp operon codes for the five different proteins which are involved in the maturation of hydrogenases. One of the important genes of this system is fhlA which codes for the central regulator of the formate regulon. The FHLA belongs to transcriptional activator protein group. It helps in expression of FHL system. The formate binds to upstream activator site and consequently activates FDH-H and other component of the FHL system (Leonhartsberger et al. 2002).
Role of hydrogenase in obligate anaerobe
The obligate anaerobes possess an enzyme known as [FeFe] hydrogenase. It catalyzes the formation of molecular H2 by mediating transfer of electrons from ferredoxin (Fd) to proton. The pyruvate produced during glycolysis gets converted to acetyl-CoA and CO2. This reaction is catalyzed by pyruvate ferredoxin oxidoreductase (PFOR). The yield expected via this pathway is 2 mol mol−1 glucose consumed (Khanna and Das 2013). Additional 2 mol of hydrogen can be produced by the oxidation of NADH formed during glycolysis. Thus, the overall theoretical yield is 4 mol mol−1 glucose consumed. The NADH transfers its electron to ferredoxin (Fd) through NADH: ferredoxin oxidoreductase (NFOR) and itself get oxidized (Fig. 2) (King et al. 2006). Conversely, lower hydrogen yields have been observed in obligate anaerobes. The presence of competing pathways and metabolites such as acetate, butyrate, lactate, or ethanol plays a crucial role in decreasing the overall yields. Formation of these metabolites utilizes the reducing equivalents (NADH). Ethanol production involves consumption of 4 mol of NADH. Similarly, lactate and butyrate production is associated with 2 mol of NADH consumption. Thus, there is lesser availability of NADH for PFOR complex to form molecular hydrogen.
Biochemistry of methanogenesis
Role of MCR in methane production
The principle enzyme system involved in biomethane production is methyl-coenzyme M reductases. The energy metabolism of methanogenic archaea proceeds by a stepwise reduction of coenzyme bound and activated C1 intermediates. The central metabolite methyl-coenzyme M (CH3–SCoM) reacts with the electron donor coenzyme B (HS–CoB) to form methane and the heterodisulfide CoM–S–S–CoB (Fig. 3). This reaction is catalyzed by methyl-coenzyme M reductase (MCR). This reaction takes place under strict anaerobic conditions by methanogenic microorganisms (Friedrich 2005). It requires a nickel-porphinoid prosthetic group, coenzyme F430. MCR is a hexamer which comprises 2 alpha, 2 beta, and 2 gamma subunits. It also has two identical nickel porphinoid active sites (Ermler 2005). The N-terminal region of alpha subunit has a ferredoxin-like alpha/beta-sandwich fold with a duplicated beta-alpha-beta topology. The binding of coenzyme M appears to induce specific conformational changes that suggest a molecular mechanism by which the enzyme ensures that methyl-coenzyme M enters the substrate channel prior to coenzyme B, as required by the active-site geometry (Grabarse et al. 2001).
Hydrogenotrophic methanogenesis
The CO2 and H2 are produced during acidogenic dark fermentation. These gasses could prove to be energy source in the metabolic pathway of special group of methanogen. Methanogenic bacteria solely depend on CO2 and H2 as energy sources (Balch et al. 1979a, b). However, there are few exceptions such as Methanothrix sp. which utilize H2 during acetate metabolism (Huser et al. 1982). Methanolobus tindarius utilize methylamine and methanol along with H2 for methane production (Konig and Stetter 1982), similarly, Methanosphaera stadtmaniae, reduction of methanol with H2 to produce methane (Miller and Wolin 1985).
In methanogenic ecosystems, the partial pressure of hydrogen is usually between 1 and 10 Pa and the associated free energy change (ΔG′) from CO2 and H2 lies in the range of −20 to −40 kJ mol−1. However, in vivo ATP synthesis from ADP and inorganic phosphate requires at least 50 kJ mol−1 of free energy (Thauer and Morris 1984). Thus, less than 1 mol ATP per mol, CH4 can be produced under the regular physiological conditions during growth. Significant literature studies have suggested existence of chemiosmotic mechanism-mediated coupling of exergonic formation of CH4 and endergonic phosphorylation of ADP. The catalytic formation of N-formylmethanofuran from methanofuran, CO2, and H2 is majorly governed by three enzyme complexes:
-
The F420-nonreducing (NiFe) hydrogenase, responsible for catalyzing the reduction of an unknown electron acceptor
-
A formylmethanofuran dehydrogenase, which catalyzes the reversible reduction of CO2 + MFR to CHO-MFR with an unknown one-electron donor
Microbial characteristics on biohydrogen and biomethane production process
Microbial insight on dark fermentative H2 producing microorganisms
Research on biohydrogen production came into prominence in the early twenty-first century (Benemann 1996).
Mesophilic dark fermentative H2 production
Usually, H2 is produced in anaerobic conditions by a wide variety of microorganisms; however, they differ in their hydrogen-producing capability. In different parts of the world, several hydrogen-producing species were discovered that belonged to different domains of microbes such as anaerobes, facultative anaerobes, methylotrophs, and photosynthetic bacteria (Nandi and Sengupta 1998). Of the abovementioned domains, strict and facultative anaerobic chemoheterotrophs belonging to Clostridia and Enterobacteriaceae sp. have proven to be the most promising H2-producing microbes.
Hydrogen production using Clostridium sp.
These are fermentative, spore-forming obligate anaerobes which have low G + C content and are Gram-positive, rod-shaped group of bacteria. They are considered industrially more important than other anaerobic bacteria because they have lower doubling time and can withstand unfavorable conditions (heat shock, physical stress, etc.). The first industrial application of Clostridia strain came into existence during World War 1 for solvents and alcohol production (Weizmann and Rosenfeld 1937). H2 is obtained as one of the by-product in such solventogenic processes, and one of the highest H2 yielding microbe belongs to this group (Taguchi et al. 1994). Some of the newly isolated Clostridium sp. such as C. butyricum, C. welchii, C. beijerinckii, and C. pasteurianum have been reported for H2 production either individually or as synthetic mixed consortia. C. beijerinckii AM21B isolated from termite gut has produced the highest H2 yield of 1.8 to 2.0 mol mol−1 glucose (Taguchi et al. 1994), and it is reported that this strain is capable of utilizing a wide range of other carbohydrates, such as fructose, glucose, sucrose, cellobiose, galactose, lactose, xylose, and arabinose. Cellulose and hemicellulose which are predominant in lignocellulosic biomass can also be used as substrates for H2 production by Clostridium sp. (Wei et al. 2014).
Hydrogen production using Enterobacter sp.
These bacteria are Gram-negative, rod-shaped, motile (peritrichous flagellated), or nonmotile, facultative anaerobes which have higher growth rates as compared to obligate anaerobes. Like Clostridium sp., Enterobacter sp. can also utilize a wide range of carbon sources; however, they are resistant to lower traces of dissolved oxygen. Also, the H2 production is not inhibited by high partial pressure of H2 (Tanisho et al. 1987). Nevertheless, they have lower H2 yield when compared to Clostridium sp. using glucose as substrate. Yield and rate of H2 production of l.0 mol mol−1 glucose and 21 mmol L−1 h−1, respectively, were observed in batch fermentation (Tanisho 1998).
Hydrogen production using Escherichia coli
These are Gram-negative, rod-shaped, motile bacteria with low G + C content and they produceH2 primarily from formate. Under anoxic conditions, formate is converted to H2 and CO2 which is catalyzed by enzyme complex formate lyase (FHL) (Stickland 1929). This FHL is a membrane-bound multienzyme complex comprising of two sub units viz. a formate dehydrogenase and a hydrogenase. The H2 yield using E. coli was 0.9–1.5 mol mol−1 glucose (Blackwood et al. 1956). E. coli has been used extensively as key organism for genetic manipulation for the improvement of H2 production. In recent times, overexpression of formate hydrogen lyase (FHL) in E. coli showed 2.5-fold improvements in H2 production (Yoshida et al. 2005). Inactivation of twin-arginine translocation system helped in further improvement in H2 production in E. coli strain MC4100 (Penfold et al. 2006). Furthermore, selective knockout of frdC (encoding for furmarate reductase), ldhA (lactate dehydrogenase), fdnG (formate dehydrogenase), ppc (phosphoenolpyruvate carboxylase), narG (nitrate reductase), focA (formate transporter), hyaB (the large subunit of hydrogenase 1), aceE (pyruvate dehydrogenase), mgsA (methylglyoxal synthase), and hycA (a regulator of the transcriptional regulator FhlA) led to improvement in H2 production using glycerol as substrate (Tran et al. 2014).
Hydrogen production using Citrobacter sp.
These groups of facultative anaerobes also belong to Enterobacteriaceae family which are Gram-negative bacilli with low G + C content. They produce H2 both chemolihotrophically and organotrophically. Citrobacter sp.Y19 has been reported to produce 15 mmol L−1h−1 of H2 from CO and H2O under chemolihotrophic conditions (Jung 2002), while under Chemoorganotrophic conditions with glucose as substrate, it gave H2 yield of 1.1 mol mol−1 of glucose (Vatsala 1992).
Hydrogen production using Bacillus sp.
These are generally Gram-positive, facultative mesophilic bacterium which grow mostly at 30 °C. However, they can survive much higher temperatures, and in unfavorable conditions, they form spores. Under genus Bacillus, several potent H2-producing microbes have been identified. The Bacillus licheniformis isolated from cattle dung produced H2 yield of 0.5 mol mol−1 glucose following the lactic acid pathway (Kalia and Purohit 2008). The Bacillus coagulans isolated from sewage sludge produces H2 yield of 2.2 mol mol−1 glucose which was higher as compared to Bacillus licheniformis (Kotay and Das 2007). The ease of handling these organisms and their nontoxicity toward dissolved oxygen makes them industrially advantageous over strict anaerobes like Clostridium and methanogens. Our research group has also isolated many potential H2-producing mesophilic microorganisms such as Bacillus coagulans IIT-BT S1, Klebsiella pneumoniae IIT-BT 08, and Citrobacter freundii IIT-BT L139 that were isolated from different sources (Kumar and Das 2000). Among the above isolates, highest H2 yield of 2.2 mol mol−1 glucose consumed was observed with K. pneumoniae IIT-BT 08 under optimized process parameters and 10 g L−1 glucose as substrate (Kumar and Das 2000).
Thermophilic dark fermentative H2 production
As compared to mesophilic system, the kinetics and stoichiometry of H2 production are much more favorable at thermophilic temperatures. Additionally, it helps in reducing the risk of methanogenic and pathogenic contaminations that arise either from inoculum or the feedstock. Another benefit of H2 production process in thermophillic regimes is that it is less affected by the partial pressure of hydrogen (pH2) in the liquid phase. Many industries discharge high-temperature effluents that are rich in organic content such as distillery industry effluents, sugar industries wastewater, and food processing. These effluents cannot be directly expelled in water bodies as they can cause a serious threat to environment. Also, cooling process is not economical, and in the process, biological activity might be lost (Jo et al. 2008). Thus, these high temperature effluents can be utilized for H2 production by thermophilic bacteria. On the basis of optimum growth temperature, the thermophilic bacteria are classified as
-
Moderate thermophiles (45 to 55 °C)
-
True thermophiles (55 to 75 °C)
-
Extremophiles (above 75 °C)
Different domains of thermophilic H2-producing species have been identified such as Clostridia, Thermotoga, Thermoanaerobacterium, Thermoanaerobacter, and Caldicellulosiruptor sp. (Zeidan et al. 2010).
Hydrogen production using Thermoanaerobacterium sp.
These are Gram-negative straight rods that obligate anaerobes with motile peritrichous flagella having low G + C content. They are interrelated with Clostridium species and were first isolated from Frying Pan Springs in Yellowstone National Park in 1993 (Lee et al. 1993). They have the ability to degrade xylan and produce H2, and under nutritionally deprived conditions, they form spores. Apart from H2, they are reported to produce diverse metabolic end products such as ethanol, acetate, CO2, and lactate.
Hydrogen production using Thermoanaerobacter sp.
These are obligate anaerobes, Gram-positive rods that are irregular and non-spore-forming (exception Thermoanaerobacter finnii) (Wiegel and Ljungdahl 1981). These genera along with Thermoanaerobium were identified as the first thermophilic anaerobic bacteria which produced hydrogen, ethanol, acetate, CO2, and lactate as sugar fermentation products. Butyrate production, however, was not reported by these species. These microorganisms can utilize a variety of sugars but cannot degrade cellulose. The theoretical maximum H2 yield of 4 mol mol−1 glucose consumed has been reported by the species of these genera (Khanna and Das 2013).
Hydrogen production using thermophilic Clostridium sp.
These are rod-shaped, Gram-positive, motile, often spore-forming, and obligate anaerobic organisms that have gained much importance in biofuel research in recent years. They belong to the phylum Firmicutes. They contain cellulose enzyme that has the ability to degrade cellulose and can ferment lignocellulosic biomass to H2. The highest H2 yield of 1.6 mol mol−1 hexose consumed was observed in the case of using cellulose as carbon source (Levin et al. 2006).
Hydrogen production using Caldicellulosiruptor sp.
These species are obligatory anaerobic non-spore-forming Gram-positive bacteria that are isolated from the natural habitats like hot springs and lake sediments. They are characterized within the Bacillus/Clostridium subphylum. These microorganisms could utilize a wide range of substrates such as cellulose, cellobiose, xylan, and xylose with the help of vast repertoire of hydrolytic enzymes (Rainey et al. 1994). Thus, these species are ideal when lignocellulosic wastes are to be used for hydrogen production. The Caldicellulosiruptor saccharolyticus is an extremophile that grows at 70 °C which produces H2, and the predominant metabolite formed by this organism is acetate and lactate. However, it is observed that these species do not produce butyrate as metabolic end product which is in contrast to other thermophilic microbes such as Thermoanaerobacterium sp. The maximum reported H2 production rate for this specie is 5 to 6 mmol L−1 h−1 using paper pulp as substrate (Kadar et al. 2004).
Extremophilic hydrogen production using Thermotoga sp.
They are rod-shaped, Gram-positive obligate anaerobes that grow at 90 °C which is the highest reported temperature for H2 production. They were first isolated from geothermal-heated sea floors in Italy and Azores, and the name of their genus is derived from the presence of a characteristic outer sheet like structure called toga. They are usually found in high temperature, pressure, and sulfur containing environments and can use elemental sulfur or thiosulfate or both as electron source. Their metabolic end products include acetate, hydrogen, and CO2, with ethanol in very trace amounts. Some of the examples of potent hydrogen producing species under this genera are Thermotoga maritima and Thermotoga neoplanita (Finkelstein et al. 2004).
Biomethane producing microorganisms
A distinct understanding of the complex methanogenesis process was obtained with a clear knowledge of syntrophism among different anaerobic microorganisms. In contrast to other microbes, methanogens have a characteristic ability to produce methane and hydrocarbons, and they belong to group Archaeobacteria. Their distinctive features such as the presence of membrane lipids having isoprenoids ether linkage with glycerol, absence of peptidoglycan containing muramic acid, and discrete ribosomal RNA sequences make them distinguished from Eubacteria (Balch et al. 1979a, b; Raskin et al. 1994).
Methanogens are classified into three groups based on their metabolic pathway of methane production: CO2-reducing, methylotrophs, and aceticlastics. The CO2-reducing methanogens require two-electron reduction steps to convert CO2 or bicarbonate to methane (Rouviere and Wolfe 1988). For this purpose, most of the methanogens use H2 as sole source of electrons which can be obtained either from geological eruption or H2 produced by other hydrogen-producing microbes. However, methanogens rapidly consume H2 under anaerobic conditions suppressing its accumulation in the system. Thus, H2 plays an important role of extracellular intermediate.
Apart from H2, many hydrogenotrophic methanogens use formate as electron donor for reduction of CO2 to CH4 though its concentration remains low in the methanogenic environment (Boone et al. 1989). The ability to oxidize primary and secondary alcohols for reduction of CO2 to CH4 is restricted to only few methanogens (Bleicher et al. 1989). Methylotrophic methanogens utilize substrates that have methyl groups, such as methanol, trimethylamine, and dimethyl sulfide in which the methyl group is transferred to a methyl carrier (ultimately to coenzyme M) to form methane (Hippe et al. 1979). Initially, based on morphological characteristics, methanogens were classified along with nonmethanogens; however, with the eighth edition of Bergey’s Manual, physiological unity of methanogens was recognized, and they were placed under single group (Bryant and Boone 1987). Further unity of methanogens was demonstrated with the introduction of ribotyping method which includes cataloging and sequencing of 16S rRNA. Surprisingly, methanogens exhibited phylogenetic relation with certain groups of microorganisms that belonged to the kingdom Archaeobacteria such as extremehalophiles and extremely thermophilic sulfur-dependent organisms. Thus, a new higher level taxonomic kingdom was introduced called the urkingdom Archaea which included the methanogens and other Archaeobacteria. Under the urkingdom Archaea, the methanogens were classified under Euryarchaeota which also comprise of extremehalopliles, Thermoplasma, and some nonmethanogenic thermophilic extremophiles.
Methanobacteriales
These are usually Gram-positive, rod-shaped methanogens that use CO2 as energy source to reduce methanol to methane. Methanosphaera sp., however, is an exception to the above group as they are cocci in shape and use H2 as energy source. They mainly comprise of two families, Methanobacteriaceae, and Methanothermaceae.
The Methanobacteriaceae family is highly diverse and includes several genera such as Methanobacterium, Methanothermobacter gen. nov., Methanobrevibacter, and Methanosphaera. Among the Methanobacterium, Methanobacterium jormicicum is the oldest species described which has the inability to catabolize formate. This feature contributes toward physiological and morphological differences with Methanobacterium bryantii. Methanobrevibacter spp. are short rods or cocco-bacilli in shape which have complex organic requirements. They are commonly found in the gastrointestinal tract or feces of mammals and use H2 or formate as energy source to reduce CO2 to CH4. Methanosphaera are another member of Methanobacteriaceae family which are Gram-positive, cocci-shaped nonmotile organisms that occur singly or in small groups. They grow by utilizing H2 to reduce methanol (CH3OH) to methane, and their pseudomurien cell wall is composed of serine. Methanosphaera stadmaniae and M. cuniculi belong to this genus (Biavati et al. 1988). Thermophilic Methanobacteriales have separate genus of Methanothermobacter sp. and include Methanothermobacter thermoautotrophicus and Methanothermobacter wollfi. They are depended on H2 and CO2 as energy source and cannot utilize formate. Methanobacterium thermoformicicum, however, is an exception to this group as it is capable of utilizing formate. Methanothermaceae comprise of the thermophilic methanogens that belong to order Methanobacteriales, and the extreme thermophilic methanogens that grows at temperature of 83–85 °C are grouped under the genus Methanothermus. These are rod-shaped methanogens that grow on CO2 and H2 (Lauerer et al. 1986).
Methanococcales
They are coccoid-shaped, halophilic, chemolithotrophic, marine methanogens which include three thermophilic species (Methanothermococcus, Methanocaldococcus, and Methanoignis) and one mesophilic species (Methanococcus). They produce methane by reducing CO2 and use H2 or formate as energy source.
Methanothermococcus thermolithotrophicus is a thermophillic specie that grows at 65 °C and is found in deep sea hydrothermal vents. They belong to the family Methanococcaceae and are motile, Gram-negative cocci. Their cell wall is deprived of muramic acid and glycoprotein (Huser et al. 1982).
Another example of hyperthermophilic marine cooci is Methanocaldococcus jannaschii which require 200-atm pressure and 85 °C temperature for growth. They are also barophilic in nature and were the first organism in the archaea to have its complete genome sequenced. Methanoignis igneus is another hyprethermophilic microorganism that is capable of producing methane by utilizing H2 as energy source.
Methanomicrobiales
This order comprises of three families viz., Methanomicrobiaceae, Methanosarcinaceae, and Methanocorpusculaceae. Methanomicrobiales species use acetic acid as carbon source. Numerous other species have additional, complex nutritional requirements. Mesophilic or thermophilic microorganisms that are also slightly halophilic in nature belong to this order. A protein layer (S-layer) is present in the cell wall of these organisms which is responsible for osmotic sensitivity of these organisms. Such external sheath is also present in Methanospirillum hungateii. Morphologically, they are usually pleomorphic, irregular coccoid, and appear as plate-shaped or rod-shaped cells. They are susceptible to dilute detergents such as 2 % v/v SDS or hypotonic shock. An external sheath is also present in Methanospirillum hungateii which has a helical spiral shape. The Methanocorpusculaceae are cocci-shaped, hydrogenotrophicmethanogens (Methanocorpusculum) which oxidize H2, formate, or alcohols and reduce CO2 to methane. The only representatives of their group are Methanomicrobium mobile and Methanolacinia paynteri. Methanoplanus are Gram-negative, weakly motile, plate-shaped organisms which are acetoclastic in nature and utilize H2 and formate to produce methane (Wildgruber et al. 1982). They comprise of two species, Methanoplanus limicola and Methanoplanus endosymbiosus. Their cell envelop shows hexagonal structure, and they were first isolated from marshy swamps. Methanoculleus bourgensis, M. marisnigri, M. thermophilicus, and M. olentangyi are grouped under genus Methanoculleus, and these microorganisms use H2/formate as methanogenic substrates (Asakawa 2003).
Methanosarcinales
The microorganisms belonging to this group require methyl-group containing compounds such as methanol, methylamines, or methyl sulfides for their nutrition, and thus, they are also known as methylotrophic microorganisms. This genera could be further classified as Methanosarcina, Methanosaeta (Methanothrix), Methanolobus, Methanococcoides, Methanohalophilus, and Methanohalobium (Methanosarcinaceae). These microorganisms have the ability to utilize trimethylamine and dismutate it to ammonia, carbon dioxide, and methane. In a similar manner, they can catabolize methanol into methane and carbon dioxide. Certain species of this group however are hydrogenotrophic or acetoclastic and thus can reduce CO2 to methane or can utilize acetate to methane and carbon dioxide. A very unique feature of the members of this group is that they are methylotrophic and cannot use formate as a catabolic substrate. The family Methanosaetaceae comprise of aceticlastic genus, Methanosaeta (Methanothrix) which utilizes only acetate as energy source. Morphologically, they are Gram-negative, nonmotile rods (length 2.5 to 6.0 μm) with flat ends, and they often form long-chain-like structure. Their rod shape is conferred by an external sheath, and the organisms grow within this sheath (Asakawa 2003).
Methanopyrales ord. nov.
They are classified as a separate group of methanogens which contain a single specie M. kandleri. They are hydrogenotrophic bacteria that reduce CO2 to methane and are usually rod-shaped, Gram-positive microbes that grows at very high temperatures. Their cell wall is composed of a unique type of pseudomurein which contains ornithin in addition to lysine but lacks N-acetylglucosamine (Kurr et al. 1991).
Microbial interactions
Competition or methanogenic substrates: general considerations
The spent media obtained after acetogenic H2 production is rich in volatile fatty acids such as acetate, butyrate, and ethanol which are suitable substrates for methanogens. However, the methanogenic seed cultures are usually cocontaminated with sulfate-reducing bacteria and metal-reducing bacteria (Robinson and Tiedje 1984a, b). Table 1 shows the free energy of different biochemical processes catalyzed by methanogens, metal reducers, and sulfur-reducing microorganisms. Sulfate-reducing bacteria belongs to Gram-negative proteobacteria which can use much greater diversity of electron donors than methanogens. Similarly, hydrogenotrophic microorganisms are Gram-positive eubacteria which can use a variety of substrates which includes complex substrates viz. methoxyl groups of methoxylated aromatic compounds and sugars, and purines. Thus, a habitat where the organic substrate (electron donor) is limiting, a hierarchy for competition is observed between these organisms. If potential electron acceptors for metal-reducing bacteria such as Fe3+ reducers are present in the system, they can outcompete other organisms (Lovley and Phillips 1987). This would be further followed by succession of sulfate-reducing bacteria and methanogens.
Competition for H2 in hydrogenotrophic methanogens.
A considerable amount of H2 is remained in the overhead space as well as in the dissolved form after the first stage of acetogenic hydrogen production. This leftover H2 is consumed during the second-stage methanogenic conditions. To estimate the H2 consumption rate by hydrogenotrophic methanogens, the reaction should be slow enough such that it is not limited by H2 transfer from the gas phase to liquid phase. Therefore, to understand the competition for H2 under anaerobic conditions, the apparent K m values for H2 utilization can be observed. For methanogens and methanogenic habitats, the apparent K m values are in the range of 4–8 μM H2 (550–1100 Pa) (Table 2). The higher K m values probably represent the intrinsic limitations of the uptake hydrogenase for using H2 at lower partial pressures. Under higher loading rates in a bioreactor, the partial pressure of H2 can be observed which might help in determining uptake of H2. Another approach to understand the competition among the anaerobes for H2 uptake could be to correlate the available free energy with threshold partial pressure of H2. The H2 thresholds values have been examined for several hydrogenotrophic anaerobes (Cord-Ruwisch et al. 1988). It was observed that an inverse correlation exists between the available free energy for the reaction and the H2 threshold value which follows an order of acetogens > methanogens > sulfate reducers. Thus, this implies that the presence of sulfate reducing microorganisms can lead to decrease in the partial pressure of H2 to such a low level that even methanogens cannot use it. A more precise explanation of the thermodynamic effect of H2 partial pressure on H2 uptake rate is given by the free energy estimation using Nernst Equation. For a chemical reaction occurring at 25 °C:
The ΔG′ values can be estimated (pH = 7) in kilojoules as expressed in Eq. 9.
where A represents the molar concentration of reactant A, R is the ideal gas constant, and T is the absolute temperature in Kelvin. Assuming HCO3 concentration of 10 mM and methane partial pressure of 0.5 atm for a methanogenesis process involving H2-CO2 reduction, the dependency on free energy (ΔG′) can be calculated (Eq. 10) as
Competition for acetate
Although much information regarding the physiological properties of hydrogenotrophic methanogenic species is unavailable, the properties that favor one species over the other are still debatable. Several reports are available that suggest the role of acetate in methanogenesis. Members belonging to Methanosarcina sp. grow rapidly at high acetate concentrations, while on the contrary, the members belonging to Methanothrix sp., favor low acetate concentration. Dominance of Methanothrix was observed when acetate concentration decreased beyond 1 mM in a thermophilic anaerobic digester (Wiegant et al. 1986; Zinder et al. 1984). The competition for acetate can be explained using both Michaelis-Menton and threshold models (as described in the case of hydrogenotrophic bacteria). The minimum threshold of acetate utilization for Methanosarcina species is typically 0.5 mM and higher, while those for Methanothrix are in the micromolar range. Methanogenesis is favored at a slightly alkaline pH range (7.2–8.0) and, at such pH range, acetate dissociates poorly. Thus, this undissociated acetic acid at these pH values is responsible for such threshold values.
Obligate interspecies H2/formate transfer
Symbiotic association is one of the highlights of anaerobic methanogenesis which include symbiotic organisms that oxidize ethanol to acetate and Methanobacterium that use electron from H2 to reduce CO2 to CH4. Also, coexistence of a H2 producing microorganism and an H2-oxidizing organism is possible by breaking single substrate, a phenomenon known as syntrophisim. Thus, the hydrogen transfer could be facilitated by the physical juxtaposition between hydrogen consumers and producers (Conrad et al. 1985). In a coculture of Syntrophomonas wolfei with Methanospirillum hungatei in the presence of formate, the Syntrophomonas sp. grew faster; however, it grew slowly when it was cocultured with Methanobacterium bryantii that cannot utilize formate (McInerney et al. 1981). Thus, in a two-stage biomethanation system, the physiological differences between the two genera play a vital role. In the case of thermophilic methanogenesis, acetate and propionate oxidizing microorganisms could couple with Methanobacterium thermoautotrophicum (which could use formate). At such high temperatures, the partial pressure of H2 would be higher, and also, high temperature facilitates diffusion. Thus, under thermophilic conditions, the formate concentration may be insignificant.
Interspecies acetate transfer
The accumulation of acetate under anaerobic conditions could be utilized by acetoclastic methanogens. During syntrophic reactions, acetate is a major product, and accumulation of acetate could affect the thermodynamics of methane production. Two moles of acetate and H2 are produced with the syntrophic degradation of butyrate; therefore, a 10-fold change in acetate concentration has the same effect as H2 on reaction thermodynamics. When Methanosarcina barkeri was bio-augmented to a Syntrophomonas wolfei-Methanospirillum hungatei coculture, butyrate degradation was facilitated (Beaty and McInerney 1989). As compared to dissolved H2 concentration, acetate concentration is usually higher; therefore, acetate turnover is probably not perturbed as H2 turnover. Obligate interspecies acetate transfer was better explained using acetone degrading methanogenic enrich culture (Platen and Schink 1987). The mixed culture was dominated by filamentous Methanothrix sp. when acetone was provided as sole carbon and energy source. In this process, acetone was first catabolized by carboxylation to acetoacetate which then split into two moles of acetate and then finally converted to methane. With an external addition of acetate and bromoethane sulfonate (BES) to the system, the acetone degradation was inhibited. The above observations suggested that even though the ΔG for conversion of acetone to acetate is −34.2 kJ/rxn, the efficiency of acetone-degrading microbes was dependent on acetate degradation.
Process parameters affecting biohythane production process
Role of pH on dark fermentation
pH is one of the most important chemical parameters which may affect the most of the biochemical processes as it not only governs the efficiency of enzymatic machinery of the microorganisms, but it also plays a crucial role in maintaining the oxidation-reduction potential of the cells. Many enzymes are involved in the metabolic pathway of H2 production in which the glycolytic enzymes and supporting enzymes (Fe-Fe H2ase, formate lyase, etc.) are the key players. Since all the enzymes have an optimum pH for their maximum activity, studying the role of pH in H2 production becomes imperative. During the course of dark fermentation, the pH profile changes due to the accumulation of metabolites like volatile fatty acids. This change in pH affects the functioning of enzymatic machinery involved in H2 production and at very low pH (3.8–4.2), and H2 production gets ceased. The accumulation of volatile fatty acids is detrimental for the microorganisms as the cell membrane’s integrity is destroyed which eventually leads to disruption of maintenance of internal pH (Khanal et al. 2004). Moreover, at low pH, a metabolic shift toward solventogenesis from acidogenesis occurs. In H2 production process, enriched mixed consortia or anaerobic sludge are used as source of as inoculum, and at low pH, the methanogens and other H2 consuming microbes are suppressed. To understand the importance of pH during H2 production, various studies have conducted controlled pH experiments and observed that H2 production and substrate conversion improved under controlled pH. In the controlled pH ranges of 5.5 to 6.5, a significant improvement in H2 production was observed with highest H2 yield of 1.72 mol mol−1 of xylose at a pH of 6.5 (Calli 2008). Maintaining pH inside the reactor in the case of packed bed reactors (PBR) with whole cell immobilized system is difficult. Moreover, the variation in pH along the length of the PBR is nonlinear. Thus, during continuous operation, infusing feed of higher pH is recommended to prevent drastic pH drop inside the PBR (Keskin et al. 2012). A similar observation was made with thermophilic packed bed reactor, where infusion of high pH feed helped in stabilizing H2 production inside the reactor (Roy et al. 2014).
The role of pH in maintaining the oxidation reduction potential during H2 production can be better explained (Eqs. 11 and 12) considering the redox potential of hydrogen that is given as
Therefore, at pH 6 and PH2 of 1 atm,
The gaseous products produced are carbon dioxide and hydrogen which are evolved at a ratio of 2:1. The total gas evolved is quite a few times the gas present in the overhead space which ultimately leads to increment of the partial pressure of H2 (up to 0.33 atm). The redox potential of H2 (E) is 0.340 V at pH 6.0 and 0.3-atm pressure (Tanisho et al. 1989). Above this pH, the metabolic pathway shifts toward solventogenesis. The enzymes complexes such as NADH, ferredoxin oxidoreductase, ferredoxin, and hydrogenase, utilize NADH as electron source for catalysis in many obligates such as Clostridium sp.
Role of temperature on biohythane production
The temperature is considered as one of the curtail factor for any biochemical reaction. For H2 production, it plays a vital role (Hung et al. 2011; Roy et al. 2014). The cellular metabolisms are mediated by enzymatic reactions which are greatly influenced by pH and temperature. Maximum productivity of enzymes is generally observed at an optimum temperature range. Temperature variance from the optimum ranges leads to either denaturation of metabolic and life-supporting enzymes or inactivation of the same. If the temperature is increased by a factor often degrees Celsius, the enzymatic activity generally increases by a factor of doubles. This trend is observed until the optimal temperature is reached and beyond optimal temperature enzymatic activity decreases. Microbes responsible for H2 and methane production have been found in varied temperature ranges. Temperature thus might influence the nutritional requirement, metabolic end product formation, and characteristics of microbial cells. Most of the literature reports on biohydrogen and biomethane production are based on mesophilic dark fermentation (Li et al. 2001). The choice of microorganisms decides the operational temperature of the fermentor. Fermentative microorganisms capable of producing H2 were reported at different temperatures viz. psycrophilic (0–20 °C), mesophilic (20–42 °C), or thermophilic (42–75 °C). Many mesophilic bacterial isolates (such as Clostridium and Enterobacter strains) showed optimal H2 production in temperature range of 37–45 °C (Vindis 2009). The growth rate of the organism gets severely affected in growth temperatures deviates from the optimal ranges. The activation enthalpy of H2 production and thermal deactivation enthalpy could be determined by using modified Arrhenius equation (Fabiano and Perego 2002). The activation enthalpies and entropies are function of temperature. Concomitantly, thermal inactivation could also be estimated using such plot. The Arrhenius equation can be modified and could be expressed in terms of maximum specific hydrogen production (Eq. 13).
Thus, activation and deactivation enthalpy could be expressed as Eqs.14 and 15, respectively
where r m is the maximum specific hydrogen production rate, X g (g L−1) is the cell mass concentration, Y H2/X (mL H2 g cell mass−1) is the yield, ΔH* (kJ mol−1) is the activation enthalpy of fermentation, and A is the Arrhenius pre-exponential factor (Nath et al. 2006). Moreover, influence of temperature on activation entropy and thermal deactivation entropy (ΔS* and ΔS d*, respectively) could be expressed as
where h and K B are Planck’s and Boltzmann’s constant, respectively. In the case of methanogens, temperature plays a vital role in product yield A variation of minute temperature change of 2–3 °C could lead to suppression of methanogens and facilitate acidogens thereby causing accumulation of VFAs in the system. Eventually, it would lead to decrease in pH below 7, and methanogenesis would be suppressed (Speece 1983). The activity of all anaerobic microorganisms is drastically affected by considerable temperature drop and ceases methane production; however, these microorganisms are able to recover with appropriate temperature stabilization. In a case study, a carrier-induced granular sludge bed (CIGSB) reactor was used with sucrose-based artificial wastewater (Lee et al. 2006). The effect of temperature on hydrogen production rate and yield was studied. The H2 production increased with increase in temperature from 30 to 40 °C. On further increasing the operational temperature leads to decrease H2 production (Fabiano and Perego 2002). In any enzymatic process, increase in temperature shows positive kinetic effect up to the threshold temperature beyond which thermal deactivation of biocatalyst takes place. The thermal inactivation of biocatalytic reactions controlling the metabolic pathway leads to decrease in efficiency of CIGBS.
Role of partial pressure on biohythane production
Hydrogen and methane are gaseous products that get dissolved in the fermentation broth during production pathways that are very sensitive to hydrogen partial pressure. The partial pressure of hydrogen inside the reactor increases as it starts getting accumulated in the head space. As H2 is the product of dark fermentation, its accumulation would inhibit the product formation which is in accordance with Le Chatelier’s principle. This increase in partial pressure also contributes toward metabolic shift during fermentation. It leads to formation of reduced end products such as ethanol, propionate, lactate, butanol, and acetone (Hawkes et al. 2007). Many strategies were used for the removal of H2 from the fermentation system. Decreasing partial pressure by intermittent N2 sparging helped in improving the H2 yield by 68 % (Mizuno et al. 2000). Similarly, sparging of methane also improved H2 yield by removing accumulated H2 from the reactor. Membrane made up of silane or polyvinyltrimethylsilane could selectively absorb H2 from the system. Such properties were used to decrease partial pressure of H2 for improvement of H2 yield (Teplyakov et al. 1985).
Role of HRT on biohythane production
Hydraulic retention time (HRT) is the time that the cells and soluble nutrients are retained inside the reactor. Unlike methanogenesis, H2 production occurs at lower HRTs. Hydraulic retention time is governed by the volume of the reactor and flow rate of feed (HRT is equal to volume of reactor/feed flow rate). For operation of H2 production in continuous mode, the HRT should be optimized. At suitable HRT, highest rate of H2 production and substrate conversion was observed. Very low HRT might also lead to wash out state where all the active cells escape out of the reactors. Thus, optimization of HRT has close relation with specific growth rate of the organism. On working with mixed consortia, which contain methanogens and acidogenic H2 producers, manipulating the HRT might lead to shift in microbial profile inside the reactor. Lower HRT would lead to enrichment of acidogenic H2 producers inside the reactor, and the methanogens would get washed out. Thus, acidic pH (6–6.5) and low HRT could completely suppress methanogens from the mixed consortia. The HRT also plays an influential role in the formation of end metabolites. This property is concomitantly associated with the changes in microbial profile in response to changes in HRT. On lowering HRT from 10 to 6 h, the H2 production was increased in Clostridium sp. and followed by decrease in propionate production (Zhang et al. 2006). On using anaerobic sludge, HRT of 1 day showed higher H2 production and increase in B/A ratio (Mariakakis et al. 2012). No methane was detected in the biogas. Study of HRT helps in designing the experiment and reactor for treatment of industry wastewater, where implementation of low HRT by infusing transient loading improved H2 production and also helped in COD removal. The organic loading rate (OLR) is a function of HRT. Thus, OLR is also considered as one of the parameters in continuous mode of H2 production. The HRT might also prove to be a handy tool in enriching microbial consortia. In a CSTR, microbial population dynamics can be modulated on the basis of their specific growth rate. Manipulation of HRT might help in expelling slow growing microbes from the reactor system. Methanogens and other H2-consuming microbes are slow-growing microorganisms (0.0167–0.02 h−1) (Zhang et al. 2006). Thus, shorter HRTs help in selective enrichment of H2-producing microbes. The strategy of using a short HRT of 3 days for elimination of slow growing methanogens proved decisive as it lead to improvement of H2 production (Kim et al. 2004). This shows that microbial dynamics inside a bioreactor is quite sensitive toward change in HRTs. In another study, lowering the HRT from 8 to 6 h led to improvement of H2 production. Moreover, propionate concentration of the soluble metabolite decreased with decrease in HRT (Zhang et al. 2006). The problem with shorter HRTs is the risk of cell washout which is also known as bleeding of the reactor. The mean cell residence time in a CSTR is the same as HRT; this limits H2 production in shorted HRT. Recently, it has been reported that there is self-granulation or flocculation even in CSTR which resulted in greater mean cell residence time. This decoupled mean cell residence time from HRT leading to higher biomass concentrations at low HRTs. For maintaining higher biomass, concentrations within the reactor immobilized/granulated type of reactors are more efficient. Hydrogen production by using immobilized cell has several advantages as compared to suspended cells (Azbar et al. 2009). Cell wash out problem in suspended cells reactor is overcome by using immobilized whole cells. The majority of whole cell immobilization techniques are based on adsorption or entrapment phenomenon. The demerits associated with gel entrapment of cell are degradation of the gel matrix on prolonged operation and limitations of nutrient and metabolite mass transfer. On the contrary, natural adsorption of cells on matrix is a simple and inexpensive technique. Such technique illustrates minimum internal mass transfer resistance and relatively cheaper to implement (Rattanapan et al. 2011). Different reactor systems are used for H2 and methane production (Fig. 4). Different reactor configurations show their effect on HRT. During methanogenesis, the HRT should be kept 2-fold greater than the generation time of the slowest growing microbes (Dohanyos and Zabranska 2001). The HRT should be held for suitable duration so that the dead zones get eliminated and it would also help in promoting an efficient syntrophic amongst the microorganisms present in the mixed culture.
Effect of inoculum on biohythane production
Most of the dark fermentative H2 productions have been reported on using simple sugars/soluble fermentable sugars. The advent of concept of “organic waste to energy” has driven the concept of development of a mixed microbial consortium that would harbor a symbiotically associated different group of bacteria. Preparation of a proper enriched inoculum is very important for achieving desired product. A single group of bacteria might not have all the hydrolytic enzymes required for hydrolysis of complex organic compounds like cellulose. The characteristic bacteria of enriched mixed consortium would have the ability to produce hydrolytic enzymes. These hydrolytic enzymes thus help in solubilization of complex carbohydrates present in the organic waste. The soluble fermentable sugars could be then utilized for hydrogen production. The natural microbial flora consists of different types of microbes such as H2-producing bacteria, H2-consuming bacteria, methanogenic microorganisms, and acetoclastic electrogens. To select H2-producing microbes amongst the mixed microbial population is regarded as enrichment of culture. In enrichment process, artificial selection pressure was applied that would selectively promote H2-producing bacteria and eliminate non-H2 producers.
Enriched mixed culture development
When large-scale production of H2 production is considered, use of mixed cultures is recommended. The sole reason behind it could be the fact that there is no prerequisite of medium sterilization during operation, thereby decreasing overall cost. Moreover, many wastewaters could be used as feedstock for H2 production (Valdez-Vazquez et al. 2005). The potential H2-producing microorganisms are present in various natural and manmade habitats. They are found in prominence in sewage sludge, anaerobically digested sludge, acclimated sludge, compost, animal manure, hot springs, oceanic sediments, and soil (Chen et al. 2002; Sparling et al. 1997; Ueno et al. 1995; Lin and Lay 2005). The major disadvantage of working with mixed consortia is the chances of dominance of non-H2 producing microorganisms, methanogens, H2 consuming microorganisms, homoacetogens, and lactic acid-producing bacteria. Dominance of these microbes thus could lead to decrease in yields. The need of the hour is to develop strategy to enrich the mixed consortia with H2-producing microbes. Such enrichment process provides the selection pressure which eventually leads to dominance of H2-producing microorganisms (Fig. 5). The ecological niche where the naturally habituating microbes are present provides all the necessary growth conditions. Mimicking those conditions in laboratory is a challenge. Selection of inoculum from such habitat is critical. The decrease in startup time and overall efficiency could be improved by preparing an efficient seed culture (Hawkes et al. 2002).
Heat shock treatment
Exposure to high temperature for short period of time and then cooling it to ambient temperature is regarded as heat shock treatment (HST). It facilitates suppression of non-spore-forming bacteria and allows growth of spore-forming bacteria (Ueno et al. 1996). The methanogenic Archaea and nonsporulating bacteria could not form spores to survive through the adverse condition created by high temperatures, whereas H2-producing microbes such as Bacillus and Clostridium sp. produce spores in response to HST (Lay et al. 2004). Therefore, the final outcome of HST is a mixed consortium enriched with H2-producing microbes, whereas methanogens get eliminated. The parameters governing HST depend upon temperatures ranges (80–104 °C) and time of exposure (15–120 min). During HST, the vegetative cells of non-spore-forming microorganisms get killed. These vegetative cells might encompass H2 consumers, methanogens, non-H2 producers, etc., but in this process, it would also kill H2-producing microbes which cannot form spores such as Enterobacter sp., Citrobacter sp., Bacillus coagulans sp. etc. (Watanabe et al. 1997). Thus, HST is preferentially suitable for Clostridial species as they have the spore-forming ability.
Acid treatment
The pH range (6.8–7.2) has been considered most favorable for methanogenesis. The acidogenic H2 producers grow over a wide range of pH. Methanogenic activity can be effectively suppressed if low pH harboring growth conditions were maintained. Treatment of naïve inoculum with acid effectively represses the growth of H2-consuming microbes. Moreover, harsh/adverse condition such as low pH promotes endospores formation in spore-forming H2-producing bacteria. During acid pretreatment, pH 3 or low was found suitable (keeping time of exposure 24 h). For pH adjustment, HCl and orthophosphoric acid are employed. Neutralization of pretreated inoculum can be done using NaOH. Formation of salts such as NaCl, Na2PO4 during neutralization could also play crucial role in influencing the microbial profile. Microbes labile to osmotic changes created by accumulated salts might also get repressed during acid pretreatments.
Load shock treatment
Another physical pretreatment employed to enrichment of inoculum is load shock treatment (Fang et al. 2002). In this process, the seed culture is subjected to an environment where the volumetric organic load is changed rapidly. Since no physical or chemical treatment was applied, this technique proved to be more effective than HST due to the presence of higher diversity of microbes. Moreover, rapid change in volumetric organic load could also lead to accumulation of organic acids thereby resulting in decrease of pH from 5.5 to 4.6. Thus, LST could eventually eliminate methanogens (Fang et al. 2002). When compared to other pretreatment processes viz. to base, acid, chemical (BESA), and HST methods, it was reported that LST proved more effective in enriching thermophilic H2-producing seeds (O-Thong et al. 2009).
Chemical treatment
Many chemicals have been explored as suppressor of methanogens and non-H2 producers. Iodopropane, acetylene, and 2-bromoethanesulfonic acid (BESA) are few well-known chemical agents used for pretreatment.
Treatment with BESA
The mode of action of BESA suggests that it is a structural analog of coenzyme-M required by methanogens to produce methane. Moreover, BESA is chemically inert and do not disturb H2-producing acidogens (Zhu and Beland 2006). In addition, there are some reports regarding side effects of BESA; it was found that BESA could hamper acetate-producing process. In long-term operation, supplementation of feed with BESA is not feasible, and there is high chance of development of BESA-resistant mutants (Sparling et al. 1997).
Idopropane treatment for enrichment
The mode of action of iodopropane is more contrasting when compare to BESA. Idopropane acts as a corrinoid antagonist which prevents functioning of B12 enzymes as a methyl group carrier (Kenealy and Zeikus 1981). Vitamin B12-associated enzymes plays a vital role in cellular metabolism of bacteria. Gram-negative microbes are more susceptible to iodopropane when compared to Gram-positive. Idopropane being hydrophobic in nature, easily enters through the outer cell membrane.
Use of gaseous acetylene for seed culture enrichment
Acetylene is another chemical inhibitor which causes a nonspecific inhibition of methanogenesis (Sprott et al. 1982). On exposure to acetylene, the methanogenic species lose their ability to maintain a transmembrane pH gradient. As the trans-membrane pH gradient is disrupted, a decline in ATP synthesis and methanogenesis could be observed (Sprott et al. 1982). Acetylene also shows its inhibitory effects on H2-producing eubacterial belonging to genus Enterobacter sp. but it does not harm the Clostridial species and other H2-producing microbes (Valdez-Vazquez et al. 2002). The major advantages of using acetylene for pretreatment of seed culture are as follows: (a) The process is economically cheap as production cost of acetylene is cheap, (b) it does not accumulate in solid or liquid culture, (c) no lag time is seen with seed culture treated with acetylene, and (d) its more rapid than other physical and chemical methods. In some reports, 1 % v/v acetylene was used to treat mesophilic seed culture so as to induce H2 production from model paper mill waste in batch reactors.
Other treatment
Alkaline pretreatment
Alkaline pretreatment was also explored for suppression of methanogens. Subjection of seed culture to extreme alkaline condition (pH 8.5–12) by using NaOH has shown the suppression of growth of methanogens (Cheong and Hansen 2006). If the efficacy of alkaline pretreatment is compared with heat shock treatment, it was found that HST completely eliminated methanogenic activity, whereas alkaline pretreatment led to partial suppression of methanogens. Thus, lower yields were reported when alkaline treatment has been used for H2 production (Mu et al. 2007).
Oxygen stress
Being obligate, non-spore-forming anaerobes, methanogens when exposed to oxygen eventually lead to its death. Conversely, spore-forming Clostridia could survive such stress. Moreover, facultative H2-producing anaerobe faces no problem when exposed to oxygen. Therefore, forced aeration of seed culture could eliminate methanogens. However, with this method of seed preparation, a lower H2 production rate is reported compared to HST-treated seed. Other miscellaneous treatments were also explored such as freezing and thawing, infrared radiation treatment, and mild sonication. Application of infrared pretreatment to seed inoculum also inhibits bioactivity of H2 consumers (Fan et al. 2006).
Role of alkanity of the medium on biohythane production
It is well known that hydrogen production takes place in a favorable pH range of 6.5 to 5.2. As the fermentation proceeds, pH of the system drops below the favorable range. If this drop of pH is compensated, then a marked improvement in hydrogen production could be achieved. The buffering capacity of the media is also regarded as alkalinity (Mohan et al. 2007). Its strength (buffer capacity) is governed by the presence of divalent ions like Ca2+, Mg2+, or other ions such as phosphates, carbonates, and citrates. In an interesting study, Venkat et al. 2007 showed the importance of alkalinity in negating the effected of accumulated organic acids. It showed that a balanced pH level could be achieved inside the reactor which thereby led to improvement in hydrogen production. On variation of organic rate from 2.4 kg COD m−3 day−1, the alkalinity concentration varied from a maximum of 2900 mg L−1 to a minimum of 300 mg L−1. During stable hydrogen production, the operation alkalinity values varied from a maximum of 1600 mg L−1 to a minimum of 125 mg L−1. Continuous H2 production using CSTR was studied where instead of using controlled pH regime, a buffered system was used (Shi et al. 2010). A significant influence of alkalinity was observed on hydrogen production. In this case, maximum biogas production rate was observed when the alkalinity was increased in the range 500 to 1000 mg L−1. Thus, for a stable hydrogen production, range of alkanity must be optimized. Once hydrogen production is over, the VFAs produced by the system are then subjected to biomethanation process. In such cases, the ratio between VFAs to alkalinity should be in the range of 0.1–0.25. Typically, the bicarbonate alkalinity in biomethanation ranges from 1000 to 5000 mg L−1 as CaCO3 (Shi et al. 2010).
Feedstock for biohythane production
For commercial production of biohydrogen, cheap feedstock/raw material should be used. Most of the studies on biohydrogen are based on utilization of simple sugars such as glucose, sucrose, maltose, and lactose. These simple sugars are expensive, and usages of such raw material are not economically viable. To address this issue, production of biohydrogen using different organic wastes as substrate is a cheap and promising approach. There is a relatively high abundance of complex sugars (polysaccharides) in nature. Most of these polymeric sugars (cellulose, hemicellulose, amylase, etc.) are inaccessible to microorganisms. In order to tap the energy bound in these polymeric sugars, a detailed research is required targeting the pretreatment and saccharification techniques. Biohydrogen could be considered as renewable and cheap when its production is based on low value renewable resources. Many high COD-containing wastes have been explored for biohydrogen production. This includes organic fractions of municipal solid wastes, food wastes, distillery wastes, cheese whey, etc. Organic fractions of municipal solid wastes are widely available renewable resource rich in polysaccharides and proteins (Noike and Mizuno 2000). Recently, usage of municipal solid waste for H2 production showed promising results. However, the yield with raw sewage sludge was still considerably low (i.e., 0.16 mg of H2 g−1 of dried solids (DS). Various pretreatment methods such as ultrasonic treatment, acidification, sterilization, and freezing-and-thawing were employed for improvement of H2 yield. Boiled sludge (heat treatment) leads to solubilization of nutrients present in raw sludge. Usage of boiled sewage sludge gave 15.64 mL of H2 g−1 DS. Pretreatment techniques such as sterilization and freezing-and-thawing gave H2 yield of 47 mL of H2 g−1of DS (Noike and Mizuno 2000). On other hand, food wastes also have a great environmental threat. It contains about 90 % volatile-suspended solids. High organic content makes them suitable feedstock for microbial fermentation. The institutional food wastes used for thermophilic hydrogen production gave 81 mL H2 g−1 VSS as compared to 63 mL H2 g−1 VSS by mesophilic dark fermentation (Wang et al. 2003). Dairy industry effluents have with high biological oxygen demand (BOD) and chemical oxygen demand (COD) which makes them hazardous for environment if discharged untreated. On using dairy wastewater, the maximum hydrogen production of 5.2 mL H2 g−1 COD was observed (Orhon et al. 1993). Distillery or alcoholic beverage industry wastewaters are rich in biodegradable organic material, such as sugars, hemicelluloses, dextrin, resins, and organic acids. These wastewaters have high chemical oxygen demand (COD) (80–160 g L−1). Many reports were available on biohydrogen production using distillery wastewater (Pant and Adholeya 2007; Mohan et al. 2011). In anaerobic sequencing batch biofilm reactor, maximum hydrogen production of 156.7 L H2 kg−1 COD was observed (Mohan et al. 2011). Few effluents are discharged at very high temperature, e.g., palm oil mill effluent (POME). These effluents have high organic content and could be a potential substrate for thermophilic dark fermentative H2 production. On an average 0.9–1.5 m3, POME is generated from 1 t of palm oil being produced. Using UASB reactor, hydrogen production rate of 4.4 L g−1 POME day−1 was observed (O-Thong et al. 2009). In recent years, lignocellulosic biomass as feedstock for biohydrogen production has gained importance (Song et al. 2014; Zhang et al. 2015). In one such study, corn stalk has been directly used for biohydrogen production by Clostridium sp. with maximum hydrogen yield of 96 L kg−1 (Song et al. 2014). Recently, use of thermophiles for direct conversion of cellulosic biomass to biohydrogen has shown higher yield as compared to mesophiles (Cao et al. 2014). Various reports were available on two stage biohythane concept (Table 3). A wide range of substrates viz. algal biomass, corn silage, and vine industry effluent have been explored as feedstock. Food waste had shown promise as substrate for biohythane production with highest yield of H2 and CH4 of 65 L kg−1 VS and 546 L kg−1 VS, respectively (Wang and Zhao 2009).Use of skim latex serum (SLS) generated during coagulation of skim latex as feedstock for biohythane process showed highest yield of H2 and CH4 of 2.25 ± 0.09 L L−1 SLS and 6.41 ± 0.52 L L−1 SLS, respectively (Kongjan et al. 2014).
IIT Kharagpur studied two-stage biohythane process using starchy wastewater. The volumetric H2 produced during first stage was found to be 12 L L−1 day−1. Furthermore, volumetric CH4 evolved in per day was 1.15 L L−1 from the spent medium of the dark fermentation process (unpublished data). The total gaseous energy recovery for two stage process was found to be 53.6 %. From single-stage H2 production, gaseous energy recovery was only 28 %. Thus, the integration of two processes could facilitate commercialization of the technology.
Conclusion
A vivid range of microorganisms are involved in dark fermentative H2 production. Facultative H2 producer has distinct metabolic characteristics in terms of H2 production when compared to obligate anaerobes. Moreover, thermophilic obligate anaerobes have shown greater promise toward high yield of H2 production. In second-stage biomethanation process, many methanogenic microorganisms play critical role. Interaction of methanogens with acetogens plays a crucial role in the performance of the reactor. Various techniques have been explored to enrich potential H2-producing microorganisms in seed culture physical pretreatment, and chemical pretreatments such as heat shock, load shock, acid, alkali, iodopropane, and acetylene are well-known techniques. Enriched mixed cultures have been explored to utilize complex organic residues for H2 production. Many challenges pertaining to implementation of pretreatment process, reactor configuration, and maintenance of suitable physicochemical parameters in scaled up reactors need to be addressed. Waste management, waste disposal, socio-economical issues, etc. must be studied for using organic waste as feedstock for biohythane process. Organic wastes such as food industry waste, starchy wastewater, and distillery effluents were few promising feedstock explored for fermentative H2 production. Thus, coupling of acetogenic H2 production with methanogenesis might help in improving the total gaseous energy recovery. With advent of fuel cells, hydrogen and methane could be efficiently converted to electricity in near future. Thus, the goal of “organic waste to energy” could become reality in the near future.
References
Asakawa S (2003) Methanoculleus bourgensis, Methanoculleus olentangyi and Methanoculleus oldenburgensis are subjective synonyms. Int J Syst Evol Microbiol 53:1551–1552
Azbar N, Çetinkaya Dokgöz FT, Keskin T, Korkmaz KS (2009) Syed HM (2009) Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int J Hydrog Energy 34:7441–7447
Baghchehsaree B, Nakhla G, Karamanev D, Argyrios M (2010) Fermentative hydrogen production by diverse microflora. Int J Hydrog Energy 35:5021–5027
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979a) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979b) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Beaty PS, McInerney MJ (1989) Effects of organic acid anions on the growth and metabolism of syntrophomonas wolfei in pure culture and in defined consortia. Appl Environ Microbiol 55:977–983
Benemann JR (1996) Hydrogen biotechnology: progress and prospects. Nat Biotechnol 14:1101–1103
Benemann JR (1997) Feasibility analysis of photobiological hydrogen production. Int J Hydrog Energy 22:979–987
Biavati B, Vasta M, Ferry JG (1988) Isolation and characterization of “Methanosphaera cuniculi” sp. nov. Appl Environ Microbiol 54:768–771
Blackwood AC, Ledingham GA, Neish AC (1956) Dissimilation of glucose at controlled pH values by pigmented and non-pigmented strains of escherichia coli. J Bacteriol 72:497–499
Bleicher K, Zellner G, Winter J (1989) Growth of methanogens on cyclopentanol/CO2 and specificity of alcohol dehydrogenase. FEMS Microbiol Lett 59:307–312
Boone DR, Johnson RL, Liu Y (1989) Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl Environ Microbiol 55:1735–1741
Bryant MP, Boone DR (1987) Emended Description of strain MST(DSM 800T), the type strain of methanosarcina barkeri. Int J Syst Bacteriol 37:169–170
Buitrón G, Kumar G, Martinez-Arce A, Moreno G (2014) Hydrogen and methane production via a two-stage processes (H 2-SBR+ CH 4-UASB) using tequila vinasses. Int J Hydrog Energy 39(33):19249–19255
Calli B (2008) Dark fermentative H2 production from xylose and lactose—effects of on-line pH control. Int J Hydrog Energy 33:522–530
Cao GL, Zhao L, Wang AJ, Wang ZY, Ren NQ (2014) Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnol Biofuels 7:82
Chen CC, Lin CY, Lin MC (2002) Acid–base enrichment enhances anaerobic hydrogen production process. Appl Microbiol Biotechnol 58:224–228
Cheng J, Liu Y, Lin R, Xia A, Zhou J, Cen K (2014) Cogeneration of hydrogen and methane from the pretreated biomass of algae bloom in taihu lake. Int J of Hydrog Energy 39(33):18793–18802
Cheong DY, Hansen CL (2006) Bacterial stress enrichment enhances anaerobic hydrogen production in cattle manure sludge. Appl Microbiol Biotechnol 72:635–643
Chu CF, Li YY, Xu KQ, Ebie Y, Inamori Y, Kong HN (2008) A pH-and temperature-phased two-stage process for hydrogen and methane production from food waste. Int J Hydrog Energy 33:4739–4746
Conrad R, Phelps TJ, Zeikus JG (1985) Gas metabolism evidence in support of the juxtaposition of hydrogen-producing and methanogenic bacteria in sewage sludge and lake sediments. Appl Environ Microbiol 50:595–601
Cord-Ruwisch R, Seitz H-J, Conrad R (1988) The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch Microbiol 149:350–357
Das D, Verziroglu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrog Energy 6:13–28
Das D, Khanna N, Veziroglu TN (2008) Recent developments in biological hydrogen production processes. Chem Indus Chem Eng Q 14:57–67. doi:10.2298/CICEQ0802057D
Ermler U (2005) On the mechanism of methyl-coenzyme M reductase. Dalton Trans 21:3451–3458
Fabiano B, Perego P (2002) Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Intl J Hydrog Energy 27:149–156
Fan YT, Zhang YH, Zhang SF, Hou HW, Ren BZ (2006) Efficient conversion of wheat straw wastes into biohydrogen gas by cow dung compost. Biores Technol 97:500–505
Fang HH, Liu H, Zhang T (2002) Characterization of a hydrogen-producing granular sludge. Biotechnol Bioeng 78:44–52
Finkelstein M, McMillan JD, Davison BH, Evans B (2004) For fuels and chemicals. Appl Biochem Biotechnol 113(116):653
Friedrich MW (2005) Methyl‐coenzyme M reductase genes: unique functional markers for methanogenic and anaerobic methane‐oxidizing archaea. Methods Enzymol 397:428–442
Grabarse W, Mahlert F, Duin EC, Goubeaud M, Shima S, Thauer RK, Lamzin V, Ermler U (2001) On the mechanism of biological methane formation: structural evidence for conformational changes in methyl-coenzyme M reductase upon substrate binding. J Mol Biol 309(1):315–330
Guo YC, Da Y, Bai YX, Li YH, Fan YT, Hou YW (2014) Co-producing hydrogen and methane from higher concentration of corn stalk by combining hydrogen fermentation and anaerobic digestion. Int J Hydrog Energy 39:14204–14211
Hawkes FR, Hussy I, Kyazze G, Dinsdale R, Hawkes DL (2007) Continuous dark fermentative hydrogen production by mesophilic microflora: principles and progress. Int J Hydrog Energy 32:172–184
Hippe H, Caspari D, Fiebig K, Gottschalk G (1979) Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by methanosarcina barkeri. Proc Natl Acad Sci 76:494–498
Hung CH, Chang YT, Chang YJ (2011) Roles of microorganisms other than Clostridium and Enterobacter in anaerobic fermentative biohydrogen production systems–a review. Bioresour Technol 102:8437–8444
Huser BA, Wuhrmann K, Zehnder A (1982) Methanothrix soehngenii gen. nov. sp. nov., a new acetotrophic non-hydrogen-oxidizing methane bacterium. Arch Microbiol 132:1–9
Intanoo P, Rangsanvigit P, Malakul P, Chavadej S (2014) Optimization of separate hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactor (UASB) system under thermophilic operation. Bioresour Technol 173:256–265
Jo JH, Lee DS, Park JM (2008) The effects of pH on carbon material and energy balances in hydrogen-producing Clostridium tyrobutyricum JM1. Bioresour Technol 99:8485–8491
Jung G (2002) Hydrogen production by a new chemoheterotrophic bacterium Citrobacter sp. Y19. Int J Hydrog Energy 27:601–610
Kadar Z, de Vrije T, van Noorden GE, Budde MAW et al (2004) Yields from glucose, xylose, and paper sludge hydrolysate during hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl Biochem Biotechnol 114:497–508
Kalia VC, Purohit HJ (2008) Microbial diversity and genomics in aid of bioenergy. J Ind Microbiol Biotechnol 35:403–419
Keskin T, Giusti L, Azbar N (2012) Continuous biohydrogen production in immobilized biofilm system versus suspended cell culture. Int J Hydrog Energy 37:1418–1424
Khanal SK, Chen WH, Sung LLS (2004) Biological hydrogen production: effects of pH and intermediate products. Int J Hydrog Energy 29:1123–1131
Khanna N, Das D (2013) Biohydrogen production by dark fermentation. Wiley Interdisciplinary Rev Energ Environ 2:401–421
Kim SH, Han SK, Shin HS (2004) Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int J Hydrog Energy 29:1607–1616
King PW, Posewitz MC, Ghirardi ML, Seibert M (2006) Functional studies of [FeFe] hydrogenase maturation in an escherichia coli biosynthetic system. J Bacteriol 188:2163–2172
Knappe J, Sawers G (1990) A radical-chemical route to acetyl-CoA: the anaerobically induced pyruvate formate-lyase system of escherichia coli. FEMS Microbiol Lett 75:383–98
Kongjan P, O-Thong S, Angelidaki I (2011) Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour Technol 102:4028–4035
Kongjan P, Jariyaboon R, Sompong O (2014) Anaerobic digestion of skim latex serum (SLS) for hydrogen and methane production using a two-stage process in a series of up-flow anaerobic sludge blanket (UASB) reactor. Int J Hydrog Energy 39(33):19343–19348
Konig H, Stetter KO (1982) Isolation and characterization of methanolobus tindarius; sp. nov., a coccoid methanogen growing only on methanol and methylamines. Zbl Bakt Hyg AbtOrig C3:478–490
Korres NE, Singh A, Nizami AS, Murphy JD (2010) Is grass biomethane a sustainable transport biofuel? Biofuel Bioprod Biorefining 4:310–325
Kotay SM, Das D (2007) Microbial hydrogen production with bacillus coagulans IIT-BT S1 isolated from anaerobic sewage sludge. Bioresour Technol 98:1183–1190
Kristjansson JK, Schonheit P, Thauer RK (1982) Different Ks values for hydrogen of methanogenic bacteria and sulfate reducing bacteria: an explanation for the apparent inhibition of methanogenesis by sulfate. Arch Microbiol 131:278–282
Kumar N, Das D (2000) Enhancement of hydrogen production by Enterobacter cloacae IIT-BT 08. Process Biochem 35:589–593
Kurr M, Huber R, Konig H, Jannasch HW, Fricke H, Trincone A, Kristjansson JK, Stetter KO (1991) Methanopyrus kandleri, gen. And sp. nov. Represents a novel group of hyperthermophilic methanogens, growing at 110 °C. Arch Microbiol 156:239–247
Lateef SA, Beneragama N, Yamashiro T, Iwasaki M, Umetsu K (2014) Batch anaerobic co-digestion of cow manure and waste milk in two-stage process for hydrogen and methane productions. Bioprocess Biosyst Eng 37(3):355–363
Lauerer G, Kristjansson JK, Langworthy TA, König H, Stetter KO (1986) Methanothermus sociabilis sp. nov., a second species within the Methanothermaceae growing at 97°C. Syst Appl Microbiol 8:100–105
Lay JJ, Fan KS, Chang J, Ku CH (2004) Influence of chemical nature of organic wastes on their conversion to hydrogen by heat-shock digested sludge. Int J Hydrog Energy 28:1361–1367
Lee YE, Jain MK, Lee C, Zeikus JG (1993) Taxonomic distinction of saccharolytic thermophilic anaerobes: description of thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; reclassification of Thermoanaerobium brockii, Clostridium. Int J Syst Bacteriol 43:41–51
Lee KS, Lo YC, Lin PJ, Chang JS (2006) Improving biohydrogen production in a carrier-induced granular sludge bed by altering physical configuration and agitation pattern of the bioreactor. Int J Hydrog Energy 31:1648–1657
Leonhartsberger S, Korsa I, Bock I (2002) The molecular biology of formate metabolism in enterobacteria. J Mol Microbiol Biotechnol 4(3):269–276
Levin D, Islam R, Cicek N, Sparling R (2006) Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Int J Hydrog Energy 31:1496–1503
Lin CY, Lay CH (2005) A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. Int J Hydrog Energy 30:285–292
Lovley DR, Phillips EJ (1987) Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol 53:2636–2641
Luo G, Xie L, Zou Z, Wang W, Zhou Q, Shim H (2010) Anaerobic treatment of cassava stillage for hydrogen and methane production in continuously stirred tank reactor (CSTR) under high organic loading rate OLR. Int J Hydrog Energy 35:11733–11737
Maeda T, Sanchez-Torres V, Wood TK (2007) Escherichia coli hydrogenase 3 is a reversible enzyme possessing hydrogen uptake and synthesis activities. Appl Microbiol Biotechnol 76:1035–1042
Mariakakis I, Meyer C, Steinmetz H (2012) Fermentative Hydrogen Production by Molasses; Effect of Hydraulic Retention Time, Organic Loading Rate and Microbial Dynamics. in: Manic, D., (Ed.), Hydrogen Energy - Challenges and Perspectives, In Tech, ISBN 978-953-51-0812-2
McInerney MJ, Bryant MP, Hespell RB, Costerton JW (1981) Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol 41:1029–1039
Menon NK, Robbins J, Wendt JC, Shanmugam KT, Przybyla AE (1991) Mutational analysis and characterization of the escherichia coli hya operon, which encodes [NiFe] hydrogenase1. J Bacteriol 173:4851–4861
Miller TL, Wolin MJ (1985) Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol 141:116–122
Miyake M, Sekine M, Vasilieva LG, Nakada E, Wakayama T, Asada Y, Miyake J ( 1998) Improvement of bacterial light-dependent hydrogen production by altering the photosynthetic pigment ratio, in: Zaborsky, O.R., Benemann, J.R., Matsunaga, T., Miyake, J., Pietro, A.S. (Eds.), BioHydrogen. Springer US, pp. 81–6
Mizuno O, Dinsdale R, Hawkes FR, Hawkes DL, Noike T (2000) Enhancement of hydrogen production from glucose by nitrogen gas sparging. Biores Technol 73:59–65
Mohan SV, Babu L, Sarma PN (2007) Anaerobic biohydrogen production from dairy wastewater treatment in sequencing batch reactor (AnSBR): effect of organic loading rate. Enzyme Microb Technol 41(4):506–515
Mohan SV, Agarwal L, Mohanakrishna G, Srikanth S, Kapley A, Purohit HJ, Sarma PN (2011) Firmicutes with iron dependent hydrogenase drive hydrogen production in anaerobic bioreactor using distillery wastewater. Int J Hydrog Energy 36:8234–8242
Moreno F, Muñoz M, Arroyo J, Magén O, Monné C, Suelves I (2012) Efficiency and emissions in a vehicle spark ignition engine fueled with hydrogen and methane blends. Int J Hydrog Energy 37:11495–11503
Mu Y, Yu HQ, Wang G (2007) Evaluation of three methods for enriching hydrogen producing cultures from anaerobic sludge. Enzyme Microb Technol 40:947–953
Nandi R, Sengupta S (1998) Microbial production of hydrogen: an overview. Crit Rev Microbiol 24:61–84
Nkemka VN, Gilroyed B, Yanke J, Gruninger R, Vedres D, McAllister T, Hao X (2015) Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour Technol. doi:10.1016/j.biortech.2015.02.100
Noike T, Mizuno O (2000) Hydrogen fermentation of organic municipal wastes. Water Sci Technol 42:155–162
Orhon D, Gorgun E, Germirli F, Artan N (1993) Biological treatability of dairy wastewaters. Water Res 27:625–33
O-Thong S, Mamimin C, Prasertsan P (2009) Effect of temperature and initial pH on biohydrogen production from palm oil mill effluent: long-term evaluation and microbial community analysis. Electron J Biotechnol 14(5):1–12
Pandu K, Joseph S (2012) Comparisons and limitations of biohydrogen production processes: a review. Int J Adv Eng Technol 2:342–356
Pant D, Adholeya A (2007) Biological approaches for treatment of distillery wastewater: a review. Bioresour Technol 98:2321–2334
Penfold DW, Sargent F, Macaskie LE (2006) Inactivation of the Escherichia coli K-12 twin-arginine translocation system promotes increased hydrogen production. FEMS Microbiol Lett 262:135–137
Platen H, Schink B (1987) Methanogenic degradation of acetone by an enrichment culture. Arch Microbiol 149:136–141
Rainey FA, Donnison AM, Janssen PH, Saul D et al (1994) Description of Caldicellulosiruptor saccharolyticus gen. nov., sp. nov: an obligate anaerobic, extremely thermophilic, cellulolytic bacterium. FEMS Microbiol Lett 120:263–266
Raskin L, Stromley JM, Rittmann BE, Stahl DA (1994) Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl Environ Microbiol 60:1232–1240
Rattanapan A, Limtong S, Phisalaphong M (2011) Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl Energy 88(12):4400–4404
Robinson JA, Tiedje JM (1982) Kinetics of hydrogen consumption by rumen fluid, anaerobic digestor sludge, and sediment. Appl Environ Microbiol 44:1374–1384
Robinson JA, Tiedje JM (1984a) Competition between sulfate-reducing and methanogenic bacteria for H2 under resting and growing conditions. Arch Microbiol 137:26–32
Robinson JA, Tiedje JM (1984b) Competition between sulfate-reducing and methanogenic bacteria for H2 under resting and growing conditions. Arch Microbiol 137:26–32
Rouviere PE, Wolfe RS (1988) Novel biochemistry of methanogenesis. J Biol Chem 263:7913–7916
Roy S, Ghosh S, Das D (2012) Improvement of hydrogen production with thermophilic mixed culture from rice spent wash of distillery industry. Int J Hydrog Energy 37:15867–15874
Roy S, Vishnuvardhan M, Das D (2014) Continuous hydrogen production in packed bed reactor. Appl Energy 136:51–58
Shi XY, Jin DW, Sun QY, Li WW (2010) Optimization of conditions for hydrogen production from brewery wastewater by anaerobic sludge using desirability function approach. Renew Energy 35(7):1493–1498
Soetaert W, Vandamme EJ (2009) Biofuels. John Wiley & Sons Ltd., Great Britain, pp 1–8
Song ZX, Li XH, Li WW, Bai YX, Fan YT, Hou HW (2014) Direct bioconversion of raw corn stalk to hydrogen by a new strain Clostridium sp. FS3. Bioresour Technol 157:91–97
Sparling R, Risbey D, Poggi-Varaldo HM (1997) Hydrogen production from inhibited anaerobic composters. Int J Hydrog Energy 22:563–566
Sprott GD, Jarrell KF, Shaw KM, Knowles R (1982) Acetylene as an inhibitor of methanogenic bacteria. J Gen Microbiol 128:2453–2462
Stephenson M, Stickland LH (1932) Hydrogenlyases. J Biochem 26:712–724
Stickland LH (1929) The bacterial decomposition of formic acid. Biochem J 23:1187–1198
Taguchi F, Mizukami N, Hasegawa K, Saito-Taki T, Morimoto M (1994) Effect of amylase accumulation on hydrogen production by Clostridium beijerinckii, strain AM21B. J Ferment Bioeng 77:565–567
Tanisho S (1998) Effect of CO2 removal on hydrogen production by fermentation. Int J Hydrog Energy 23:559–563
Tanisho S, Suzuki Y, Wakao N (1987) Fermentative hydrogen evolution by Enterobacter aerogenes strain E.82005. Int J Hydrog Energy 12:623–627
Tanisho S, Kamiya N, Wakao N (1989) Hydrogen evolution of E. aerogenes depending on culture pH: mechanism of hydrogen evolution from NADH by means of membrane bound hydrogenase. Biochim Biophys Acta 973:1–6
Teplyakov VV, Levlev AL, Durgaryan SG (1985) The gas permeability of copolymers. Polym Sci USSR 27:919–926
Thauer RK, Morris JG (1984) Metabolism of chemotrophic anaerobes: old views and new aspects. p. 123–168. In Kelly, D.-P., Carr, N.-G. (ed.) The Microbe 1984, Part II, Prokaryotes and Eukaryotes. Soc Gen Microbiol Symb 36 Cambridge University Press
Tran KT, Maeda T, Wood TK (2014) Metabolic engineering of escherichia coli to enhance hydrogen production from glycerol. Appl Microbiol Biotech 98:4757–4770
Ueno Y, Kawai T, Sato S, Otsuka S, Morimoto M (1995) Biological production of hydrogen from cellulose by natural anaerobic microflora. J Ferment Bioeng 79:395–397
Ueno Y, Otsuka S, Morimoto M (1996) Hydrogen production from industrial wastewater by anaerobic microflora in chemostat culture. J Ferment Bioeng 2:194–197
Valdez-Vazquez I, Rios-Leal E, Esparza-Garcia F, Cecchi F, Poggi-Varaldo HM (2005) Semi-continuous solid substrate anaerobic reactors for H2 production from organic waste: Mesophilic versus thermophilic regime. Int J Hydrog Energy 30:1383–1391
Vatsala T (1992) Hydrogen production from (cane-molasses) stillage by Citrobacter freundii and its use in improving methanogenesis. Int J Hydrog Energy 17:923–927
Vindis P (2009) The impact of mesophilic and thermophilic anaerobic digestion on biogas production. J Achiev Mater Manuf Eng 36:192–198
Wang X, Zhao Y (2009) A bench scale study of fermentative hydrogen and methane production from food waste in integrated two-stage process. Int J Hydrog Energy 34:245–254
Wang CC, Chang C, Chu CP, Lee DJ et al (2003) Producing hydrogen from wastewater sludge by Clostridium bifermentans. J Biotechnol 102:83–92
Watanabe H, Kitamura T, Ochi S, Ozaki M (1997) Inactivation of pathogenic bacteria under mesophilic and thermophilic conditions. Water Sci Technol 36:25–32
Wei H, Fu Y, Magnusson L, Baker JO, Maness, PC, Xu Q, Ding SY (2014) Comparison of transcriptional profiles of Clostridium thermocellum grown on cellobiose and pretreated yellow poplar using RNA-Seq.Frontiers in Microbio 5
Weizmann C, Rosenfeld B (1937) The activation of the butanol-acetone fermentation of carbohydrates by Clostridium acetobutylicum. Biochem J 31:619–639
Wieczorek N, Kucuker MA, Kuchta K (2014) Fermentative hydrogen and methane production from microalgal biomass (chlorella vulgaris) in a two-stage combined process. Appl Energy 132:108–117
Wiegant WM, Hennink M, Lettinga G (1986) Separation of the propionate degradation to improve the efficiency of thermophilic anaerobic treatment of acidified wastewaters. Water Res 20:517–524
Wiegel J, Ljungdahl LG (1981) Thermoanaerobacter ethanolicus gen. nov., spec. nov., a new, extreme thermophilic, anaerobic bacterium. Arch Microbiol 128:343–348
Wildgruber G, Thomm M, Konig H, Ober K, Richiuto T, Stetter KO (1982) Methanoplanus limicola, a plate-shaped methanogen representing a novel family, the Methanoplanaceae. Arch Microbiol 132:31–36
Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H (2005) Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl Environ Microbiol 71:6762–6768
Zeidan AA, van Niel EWJ (2010) A quantitative analysis of hydrogen production efficiency of the extreme thermophile Caldicellulosiruptor owensensis OLT. Int J Hydrog Energy 35:1128–1137
Zhang ZP, Show KY, Tay JH, Liang DT, Lee DJ, Jiang WJ (2006) Effect of hydraulic retention time on biohydrogen production and anaerobic microbial community. Process Biochem 41:2118–2123
Zhang JN, Li YH, Zheng HQ, Fan YT, Hou HW (2015) Direct degradation of cellulosic biomass to bio-hydrogen from a newly isolated strain clostridium sartagoforme FZ11. Bioresour Technol 192:60–67
Zhu H, Beland M (2006) Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int J Hydrog Energy 31:1980–1988
Zinder SH, Cardwell SC, Anguish T (1984) Methanogenesis in a thermophilic (58°C) anaerobic digestor: Methanothrix sp. As an important aceticlastic methanogen. Appl Environ Microbiol 47(4):796–807
Acknowledgments
Author is thankful to the Council for Scientific and Industrial Research (CSIR), Department of Biotechnology (DBT), Ministry of New and Renewable Energy (MNRE), Defence Research & Development Organisation (DRDO), and Department of Science and Technology (DST), Government of India for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This is to certify that the work described has not been published before, that it is not under consideration for publication anywhere else, and that its publication has been approved by all coauthors.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Roy, S., Das, D. Biohythane production from organic wastes: present state of art. Environ Sci Pollut Res 23, 9391–9410 (2016). https://doi.org/10.1007/s11356-015-5469-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5469-4