Abstract
In order to reduce the costs, the recycle of spent TiO2-based SCR-DeNOx catalysts were employed as a potential catalytic support material for elemental mercury (Hg0) oxidation in simulated coal-fired flue gas. The catalytic mechanism for simultaneous removal of Hg0 and NO was also investigated. The catalysts were characterized by Brunauer-Emmett-Teller (BET), scanning electron microscope (SEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) method. Results indicated that spent TiO2-based SCR-DeNOx catalyst supported Ce-Mn mixed oxides catalyst (CeMn/SCR1) was highly active for Hg0 oxidation at low temperatures. The Ce1.00Mn/SCR1 performed the best catalytic activities, and approximately 92.80 % mercury oxidation efficiency was obtained at 150 °C. The inhibition effect of NH3 on Hg0 oxidation was confirmed in that NH3 consumed the surface oxygen. Moreover, H2O inhibited Hg0 oxidation while SO2 had a promotional effect with the aid of O2. The XPS results illustrated that the surface oxygen was responsible for Hg0 oxidation and NO conversion. Besides, the Hg0 oxidation and NO conversion were thought to be aided by synergistic effect between the manganese and cerium oxides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal-fired utility boilers are major anthropogenic sources of mercury emissions (Pirrone et al. 2010). Many countries and environmental researchers have paid considerable attention to mercury (Hg) because of its high volatility, long persistence, and strong biological accumulative properties (Brown et al. 1999). In 2011, the USA announced the Mercury and Air Toxics Standard (MATS) (He et al. 2014). In January 2013, 140 nations adopted the first legally binding international treaty to set enforceable limits on emissions of mercury and exclude, phase out, or restrict some products that contain mercury (Auzmendi-Murua et al. 2014).
Many technologies have been used for mercury control in coal combustion flue gas, including activated carbon injection (ACI), catalytic oxidation, photochemical oxidation, and existing air pollution control devices (APCDs) (Srivastava et al. 2006). Catalytic oxidation of elemental mercury (Hg0) could greatly enhance mercury capture efficiency using currently available pollution control devices such as wet scrubbers (Ji et al. 2008). Compared to activated carbon injection, combining Hg0 oxidation with wet flue gas desulfurization (WFGD) is considered as a promising method for mercury removal due to its lower cost. With extensive application in coal-fired power plants, selective catalytic reduction (SCR) technology could control NOx emission as well as exhibit the co-benefit of promoting Hg0 oxidation. Many studies have evaluated the performance of the SCR catalysts on Hg0 oxidation (Cao et al. 2008; Kamata et al. 2008; Pudasainee et al. 2010; Yan et al. 2011). However, the commercial V2O5-WO3(MoO3)/TiO2 catalyst has the disadvantages of high reaction temperature (573–673 K) (Zhibo et al. 2013). Moreover, the high concentration of dust in the flue gas reduces its performance and longevity at this temperature (Zhang et al. 2012). Accordingly, in order to avoid the cost for reheating the flue gas and the deactivation by high concentration of dust, it is desirable to develop a novel SCR catalyst with high catalytic activity at low temperatures. MnOx-based catalysts (He et al. 2013; Li et al. 2012; Qu et al. 2014; Wang et al. 2014; Wiatros-Motyka et al. 2013) have been proposed as economical low temperature SCR catalysts for NOx removal and Hg0 oxidation. It has been widely studied owing to their excellent capture activity, easy manufacturing, and low cost. Ji et al. (Ji et al. 2008) synthesized a novel MnOx/TiO2 catalyst and found NO conversion and Hg0 capture could achieve 97 and 90 %, respectively. Moreover, Li et al. (2010) reported that the Hg0 oxidation efficiency over MnOx/alumina and modified MnOx/alumina catalysts was more than 90 %. Currently, CeO2 has been widely studied as an oxygen provider for heterogeneous catalytic reactions due to its oxygen storage capability by storing or releasing O via the Ce4+/Ce3+ redox couple (Shen et al. 2014a; Shen et al. 2014b; Zhang et al. 2013). Significantly, cerium oxides provide oxygen to manganese oxides at low temperatures, which promoted the oxidation activity of manganese (He et al. 2014; Larachi et al. 2002). In particular, Ce-Mn mixed oxide was found to be an excellent low-temperature SCR catalyst (Li et al. 2012; Wu et al. 2008). Li et al. (2012) reported that TiO2 supported Mn-Ce mixed oxides (Mn-Ce/Ti) was highly active for Hg0 oxidation at low temperatures (150–250 °C) under both simulated flue gas and SCR flue gas. He et al. (2014) investigated Ti-pillared-clay supported MnOx-CeO2 mixed oxide as catalysts for Hg0 capture. The results found that the catalyst exhibited high Hg0 adsorbent and catalytic oxidant in the absence of HCl. Besides, the addition of CeO2 could significantly enhance the sulfur resistance and water resistance (Wang et al. 2014). Consequently, Mn-Ce mixed oxide has been recently developed as a SCR catalyst with extraordinarily high activity for NOx removal and Hg0 oxidation.
It is worth noting that the TiO2-based SCR-DeNOx catalysts (V2O5-WO3/TiO2) usually suffer some types of deactivation during the practical application, such as poisoning, fouling, thermal degradation, and vapor compound formation (Shang et al. 2012). As a result, TiO2-based SCR catalysts are usually in operation for 3 years until the catalytic performance is finally deteriorated. Subsequently, the spent TiO2-based SCR catalyst is either disposed in landfill or recycle. The recycle of catalyst is considered as the best approach due to its cost-saving and environment-friendly benefit. Some works have been done to overcome the drawback of SCR-DeNOx catalysts, including washing by water and acid solution, thermal regeneration, and reductive regeneration (Guo et al. 2008; Khodayari and Odenbrand 2001; Shang et al. 2012). Therefore, the novel development of spent TiO2-based SCR-DeNOx catalyst as a potential catalytic support material could present a more environmentally and financially sound option for simultaneous removal of Hg0 and NO in conventional coal-fired power plants.
This work attempts to reuse the spent TiO2-based SCR catalyst. The spent TiO2-based SCR-DeNOx catalyst was used as a support to synthesize CeMn/SCR1 catalysts. The Hg0 oxidation activities were tested at 100–300 °C in simulated coal-fired flue gas without HCl. The effects of Ce/Mn mass ratios, flue gas components including NH3, SO2, H2O, and O2 were evaluated. Besides, in order to further investigate the improvement of physicochemical properties by the modified Ce-Mn mixed oxide, Brunauer-Emmett-Teller (BET), scanning electron microscopy (SEM), X-ray diffractogram (XRD), and X-ray photoelectron spectroscopy (XPS) analyses were carried out and analyzed in detail. The catalytic mechanism for simultaneous removal of Hg0 and NO was investigated in the end.
Experimental
The preparation of catalysts
The spent TiO2-based SCR-DeNOx catalysts were obtained from power plant after exposure to exhaust gases in the SCR system for 3 years. Their DeNOx activity (about 30.00 %) was much less than fresh ones. The spent TiO2-based SCR catalysts were washed in deionized water to remove poisons such as ash, alkali metals, and salt molecules from the samples. Once washing was completed, the sample was dried in an oven at 105 °C for 12 h. Finally, the samples were crushed and sieved to 100–120 mesh. The samples are denoted as SCR1 catalysts and stored in desiccator.
The Ce/SCR1, Mn/SCR1, and CeMn/SCR1 samples were prepared using cerium nitrate and/or manganese nitrate aqueous solution and SCR1 catalysts by an impregnation method enhanced by ultrasound. Specifically as follows: first, a certain amount of cerium nitrate and/or manganese nitrate was dissolved in deionized water to form the solution. Then, the SCR1 catalyst was added to the solution with stirring in a proportion. Third, the mixture was exposed to an ultrasonic bath for 1 h. Excess water was evaporated while stirring and ultrasonic bath. The obtained solid was dried at 105 °C for 12 h and calcined at 500 °C for 3 h. Finally, the obtained catalysts were ground and sieved to 100–120 mesh. The catalysts were denoted as Mn/SCR1, Ce/SCR1, and CexMn/SCR1, where x represented the Ce/Mn mass ratio (0.25, 0.67, 1.00, 1.50, and 4.00). The mass loading of (CeO2 + MnOx)/SCR1 were 1 %. The mass loading of Ce/SCR1 or Mn/SCR1 were 1 % as well.
Catalyst characterization
The analysis of surface area, average pore size, and average pore volume was carried by Micromeritics Tristar ІІ 3020 analyzer (Micromeritics Instrument Crop, USA). All samples were degassed in vacuum at 180 °C for 5 h. The specific surface area was determined using the standard Brunauer-Emmett-Teller (BET) equation. The average pore diameter and average pore volume of the samples were obtained from the desorption branches of N2 adsorption isotherm and calculated by the Barrett-Joyner-Halenda (BJH) formula.
To further analyze the morphology and surface structure of the samples, SEM analysis was performed using a Hitachi S-4800 (Hitachi Limited, Japan). The separated areas for each sample were magnified to ×100,000.
The samples were characterized by XRD using a Rigaku rotaflex D/Max-C powder diffractometer (Rigaku, Japan) with Cu-Ka radiation (l = 1.5406 A°) in the 2θ range from 10° to 80° with 0.2o/min.
XPS experiments were performed on a K-Alpha 1063 X-ray photoelectron spectrometer (Thermo Fisher Scientific, UK). XPS spectra were obtained using an Al Ka X-ray source operated at 12 kV and 6 mA. The observed spectra were calibrated with the carbon 1s electron binding energy (BE) value of 284.6 eV.
Catalytic activity measurement
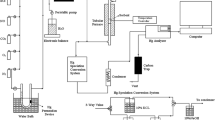
The experiments were performed in a fixed-bed quartz reactor (i.d. 20 mm), which was located inside a temperature-controlled tubular furnace, as shown in Fig. 1. An Hg0 permeation tube (VICI Metronics, USA) was used to generate a constant quantity of Hg0 vapor (70.00 μg/m3), which was supplied into the gas steam. Other simulated flue gas (SFG) components including 300 ppm SO2, 500 ppm NO, 500 ppm NH3 (when used), 12 % CO2, 8 % H2O vapor (when used), 5 % O2, and balanced gas N2 were supplied by gas cylinders and were precisely controlled by mass flow controllers (MFC). A total flow rate of 1 L/min were used during the experiments, which corresponded to a gas hourly space velocity (GHSV) of 100,000/h (the catalysts mass was 0.5 g). All the gas components were mixed and preheated to the desired temperature, and then passed through the fixed-bed quartz reactor. The feed water was exactly controlled by peristaltic pump and injected into the Teflon tube that wrapped with a temperature-controlled heating tape. Afterward, the water vapor was generated. One hundred milliliters per minute pure N2 took along the water vapor to mix with the flue gas. Besides, in order to avoid adsorption of Hg0 on the inner surface and condensation of water vapor, all Teflon tubes that Hg0 and water vapor passed through were heated up to 120 °C. A RA-915M mercury analyzer (LUMEX Ltd, Russia) was employed to measure the concentration of Hg0 while NO concentration was monitored by a flue gas analyzer (MGA5, Germany). It was verified that interferences on Hg0 measurement by flue gas components such as SO2, NH3, and water vapor were negligible. Even so, before proceeding to the mercury analyzer, acid gases, NH3, and water vapor were removed by the conditioning unit and the condenser. The conditioning unit converged to a 10 % NaOH solution in which gases such as NH3 and SO2 were captured. The H2O vapor condensed into liquid water through the condenser.
Six sets of experiments were conducted, and the experimental conditions are summarized in Table 1. In set I to set II, experiments aimed at finding the optimal catalyst and operating temperature. The Hg0 oxidation activities over SCR1, Mn/SCR1, Ce/SCR1, and CexMn/SCR1 samples were evaluated under SFG (500 ppm NO, 300 ppm SO2, 5 % O2, 12 % CO2, and 70.00 μg/m3 Hg0) for 2 h. In set III to set V, the effects of individual gas components on Hg0 oxidation and the reaction pathways were explored. The experiments were conducted on the optimal sample in the presence of individual flue gases at optimal operating temperature. In set VI, the optimal sample was tested in SCR atmosphere (500 ppm NO, 500 ppm NH3, 300 ppm SO2, 5 % O2, and 70.00 μg/m3 Hg0) at each selected reaction temperature from 100 to 300 °C for investigating the simultaneous removal of NOx and Hg0.
At the beginning of each test for catalytic activity, the gas stream bypassed the reactor and the inlet gas was sampled to ensure a stable NO (NOin) and Hg0 (Hg0 in) feed concentration. Then, the gas flow was switched to pass through the catalysts, and the analyzer measured the gas compositions in the outlet (noted as NOout and Hg0 out). Before each test, the catalysts were first saturated with the established Hg0 in under N2 atmosphere at room temperature to avoid possible bias because of Hg0 physical adsorption (Anchao et al. 2014; Li et al. 2012; Li et al. 2011). The result indicated that Hg0 physical adsorption capacity of samples were negligible at room temperature (less than 1.0 μg/g at room temperature). Therefore, the loss of Hg0 over the catalysts should be due to the oxidation of Hg0. Hg0 oxidation efficiency (ηHg) over the catalysts was quantified by Eq. (1):
Meanwhile, the NO conversion efficiency (ηNO) can be calculated using Eq. (2):
Results and discussion
Characterization of the catalyst
BET
The BET specific surface area, pore volumes, and average pore sizes of the samples are listed in Table 2. Compared to the SCR1 catalyst, the addition of Mn reduced the surface areas of catalysts. The reason might be that MnOx occupied a part of free pores of supports during the impregnation process. Note that the surface areas of the sample were increased after the addition of Ce. This result suggested that Ce could improve the specific surface areas of catalysts. However, the BET surface areas of all samples had no obvious change. Furthermore, pore volumes of Ce1.00Mn/SCR1 catalyst were lower than that of Mn/SCR1 and Ce/SCR1. It has been reported that a prominent synergy effect could be exhibited between CeO2 and MnOx (Singh et al. 2013). Therefore, it could be inferred that the loss of pore volumes might be caused by deposited Ce-Mn mixed-oxide, which penetrated into the pores of carriers.
SEM
The SEM images of the selective samples are shown in Fig. 2. The SEM images showed that crystal morphologies of the SCR1 catalyst were not significant influenced by the addition of metal oxide. From Fig. 2a, it could be seen that the surface of SCR1 catalyst was smooth and the size was uniform. For Ce/SCR1 or Mn/SCR1 catalyst, a few depositions of CeO2 or MnOx formed, while on the Ce1.00Mn/SCR1 catalyst surface (Fig. 2d), more depositions were observed. Since the BET surface areas had no significant change as demonstrated in Table 2, it can be concluded that the dispersion of CeO2 or MnOx on the support surface was excellent. However, a synergy effect between CeO2 and MnOx could result in particles interconnection, which promoted the formation of agglomerates.
XRD
The XRD patterns of spent SCR1, Ce/SCR1, Mn/SCR1, and Ce1.00Mn/SCR1 catalysts are shown in Fig. 3. It was found that the major reflections of XRD patterns belonged to anatase phase TiO2. The addition of CeO2 or/and MnOx did not result in significant change in the positions or the shapes of typical TiO2 diffractions. Besides, for all the samples, no obvious traces of CeO2 or/and MnOx were found in any of the catalysts during XRD analysis, which indicated that CeO2 or/and MnOx species were well dispersed throughout the support structure or their crystalline structures were too small to detect.
XPS
To gain more insight into the reaction pathways, an XPS analysis was performed to determine the chemical state and the relative proportion of the elements on the surface of the fresh and spent Ce1.00Mn/SCR1 catalyst. The spent Ce1.00Mn/SCR1 catalysts were carried out on the fix bed reaction system under SCR atmosphere at 150 °C. The XPS results are shown in Fig. 4. The O1s peaks could be fitted into two peaks referred to the lattice oxygen (Oβ) at 529.3–530.0 eV and the chemisorbed oxygen (Oα) at 531.3–531.9 eV (Wu et al. 2008). As shown in Fig. 4a, the Oβ ratio for the peak at 530.0 eV (calculated by Oβ/( Oα + Oβ)) increased from 61.45 to 62.61 % compared to that of the fresh catalyst, whereas the ratio of Oα (calculated by Oα/(Oα + Oβ)) decreased from 38.54 to 37.38 %. These observations suggested that both chemisorbed oxygen and lattice oxygen participated in Hg0 oxidation and NO conversion. The increase of the Oβ ratio can be explained by two factors. First, O2 in flue gas could regenerate the lattice oxygen which consumed in the reaction. Second, the consumption of chemisorbed oxygen increased the percentage of lattice oxygen.
The XPS spectra for Ce 3d are shown in Fig. 4b. XPS peaks denoted as u/v, u2/v2, and u3/v3 can be assigned to Ce4+ while u1/v1 correspond to Ce3+ (Wang et al. 2014). Both Ce3+ and Ce4+ existed in the fresh Ce1.00Mn/SCR1 catalyst. Based on the peak areas, the peaks corresponding to Ce4+ appeared to be dominant. After the reaction, the ratio of Ce4+/Ce3+ decreased from 2.70 to 1.54 compared to the fresh catalyst, implying a reduction of Ce4+ during the reaction. Furthermore, the presence of Ce3+ may result in a charge imbalance, which would lead to oxygen vacancies and unsaturated chemical bonds (Liu et al. 2012). This situation could generate additional chemisorbed oxygen or weakly adsorbed oxygen species on the surface of the catalyst, which is beneficial for Hg0 oxidation and the SCR reaction.
The XPS results of Mn 2p for the fresh and spent Ce1.00Mn/SCR1 catalyst are shown in Fig. 4c. For the fresh Ce1.00Mn/SCR1 catalyst, the peaks observed at 643.1 and 641.5 eV correspond to Mn4+ and Mn3+, respectively (Xu et al. 2012). The co-existence state of MnO2–Mn2O3 can promote the catalytic oxidation activity of catalyst (Xu et al. 2012). For the catalyst used at 150 °C, the ratio of Mn4+/Mn3+ decreased from 2.38 to 0.72. The result confirmed that reactions took place between the adsorbed active species and lattice oxygen (Wang et al. 2014). The lattice oxygen came from the valence state changes of MnOx. O2 in the flue gas would supply the metal oxide with oxygen to ensure that reaction can be continued. Therefore, it can be concluded that the lattice and chemisorbed oxygen on the catalyst surface are responsible for their superior performance. The reduction of Mn4+ to Mn3+ on the surface of the catalyst contributed to the Hg0 oxidation.
Performance of catalysts
Catalytic activity tests
The activity curves of the different catalysts under simulated flue gas are shown in Fig. 5. Apparently, with the addition of CeO2 or/and MnOx into SCR1, the catalytic activity was obviously enhanced, especially at low temperature. Notably, Eoxi over Ce1.00Mn/SCR1 catalyst was higher than that over Mn/SCR1 and Ce/SCR1 catalysts. This result demonstrated that there were intense interactions between manganese oxides and cerium oxides (Li et al. 2012). The interactions resulted in more surface oxygen, which is responsible for Hg0 oxidation.
The Hg0 oxidation of the Ce1.00Mn/SCR1 catalysts with different Ce/Mn mass ratios is shown in Fig. 6. According to the results of Ce1.00Mn/SCR1 catalysts, it can be seen that the ηHg first increased with the increasing of Ce/Mn mass ratios, then the ηHg decreased when the Ce/Mn mass ratios was 1.50. Remarkably, Ce1.00Mn/SCR1 catalyst showed the best activity, and its Hg0 oxidation efficiency was 92.80 % at 150 °C. These results indicated that Ce/Mn mass ratios influenced the activity of CexMn/SCR1 catalysts, which is related to the synergy between CeO2 and MnOx.
Effect of NH3 on Hg0 oxidation
Since NH3 is the reactant gas for selective catalytic reduction of NOx, it is necessary to study the effect of NH3 on Hg0 oxidation. Figure 7 shows the influence of NH3 on mercury oxidation over Ce1.00Mn/SCR1 catalysts. In this work, a significant inhibitive effect of NH3 on Hg0 oxidation over the catalysts was observed both in pure N2 atmosphere and in the presence of O2. NH3 (500 ppm) was added to pure N2 atmosphere and gas flow containing 5 % O2 balanced in N2, respectively. It was observed that the Hg0 oxidation efficiency decreased. In the presence of O2, Hg0 oxidation dropped to only 19.73 % over Ce1.00Mn/SCR1 catalyst, which, however, was still higher than that without O2. Some researchers believe that NH3 adsorption on catalyst most probably takes place according to the following steps (Qi et al. 2004; Zhou et al. 2013):
where O* are active surface oxygen of the catalyst. In this process, NH3 consumed the surface oxygen (including lattice oxygen and chemisorbed oxygen) that is responsible for Hg0 oxidation and NO conversation. Gas-phase O2 regenerated the lattice oxygen, and replenished the consumed chemisorbed oxygen. Thus, the presence of gas-phase O2 offsets part of the inhibitive effect of NH3. Interestingly, the coexistence of NO with NH3 can also mitigate such inhibition. ηHg was 34.79 % in N2 + 500 ppm NO + 500 ppm NH3 + 5 %O2, which was higher than that in N2 + 500 ppm NH3. In addition, the ηHg over Ce1.00Mn/SCR catalyst was also higher than that of SCR1 catalyst. It indicated the participation of CeO2 and MnOx had certain effects on reducing the inhibitive effect of NH3.
Effect of SO2 and H2O on Hg0 oxidation
It is very important to evaluate the effect of SO2 and H2O on Hg0 oxidation because SO2 and H2O unavoidably exist in flue gas and have significant impacts on the activities of catalysts. Figure 8 presents the effects of SO2 and H2O on Hg0 oxidation over Ce1.00Mn/SCR1 catalysts at 150 °C. It can be seen that Hg0 oxidation was not promoted by SO2 itself. Otherwise, Hg0 oxidation efficiency should not rely on the existence of O2 and should rise with the increase of SO2 concentration (Li et al. 2013). As shown in Fig. 8a, introducing 200 ppm SO2 into gas flow containing 5 % O2 increased Hg0 oxidation efficiency from 60.15 to 63.10 %, which indicated that low SO2 concentration promoted Hg0 oxidation with the aid of O2. However, Hg0 oxidation efficiency gradually decreased as SO2 concentration increased from 200 to 1000 ppm. As can be seen in Fig. 8a (inset), ηHg maintained at almost the same level throughout the entire experimental period with the aid of O2. However, without the aid of O2, the Hg0 oxidation efficiency was influenced with a drop of 34.56 %, and a continuous declining trend was observed as time went by. It demonstrated that part of adsorbed SO2 reacted with catalyst to form some new species that were responsible for the enhanced Hg0 oxidation. For metal-oxide-based SCR catalysts, the new species was generally believed to be SO3 (Casapu et al. 2009; Dunn et al. 1998; Fan et al. 2010; Svachula et al. 1993). Therefore, it was inferred that SO2 would be oxidized by the lattice oxygen, reducing the active oxidant sites. The reduced active sites could not be reactivated without O2, which resulted in the decrease of Hg0 oxidation efficiency as time went by. On the other hand, with the aid of O2, the reduced oxidant sites were reoxidized by O2. Both the reactivated sites and SO3 promoted Hg0 oxidation efficiency. A promotional effect of SO2 on Hg0 oxidation can be proposed as follows (Li et al. 2008a):
Effect of SO2 and H2O on Hg0 oxidation over Ce1.00Mn/SCR1 catalysts. a Effect of SO2 concentration on Hg0 oxidation (reaction condition: 70.00 μg/m3 Hg0, 0–1000 ppm SO2, 5 % O2, 500 mg of sample, 150 °C, 1000 mL/min total flow rate, and GHSV 100,000/h). b Effect of SO2 and H2O on Hg0 oxidation (reaction condition: 70.00 μg/m3 Hg0, 300 ppm SO2, 8 % H2O, 5 % O2, 500 mg of sample, 150 °C, 1000 mL/min total flow rate, and GHSV 100,000/h)
Figure 8b illustrates the activity performance of Ce1.00Mn/SCR1 catalysts in the system with SO2 and water vapor simultaneously. As is shown, when adding 8 % H2O into the stream, the Hg0 oxidation efficiency dropped sharply. Obviously, H2O had a significant inhibitive effect on Hg0 oxidation (Li et al. 2008b). The inhibitive effect of H2O possibly came from its competitive occupancy of the available active sites and displacement of adsorbed Hg0 (Tan et al. 2012). When both H2O and SO2 were added, the inhibition was less severe than that when H2O were added individually. This indicates that H2SO4 can form when SO2, O2, and H2O co-exist, which will likely affect the behavior of Hg0 on the surface of catalyst. After the H2O and SO2 were cut off, it was found that the catalytic activity gradually recovered but not returned to the original level yet. It implies that the addition of SO2 can reduce the H2O poison. However, the catalysts were deactivated by H2SO4 somehow.
Effect of O2
To obtain higher catalytic oxidation, the presence of O2 is normally necessary, especially for metal oxide catalysts (Anchao et al. 2014). Figure 9 shows the effect of O2 on catalytic activity of Ce1.00Mn/SCR1 catalysts. The results showed that the NO conversion and Hg0 oxidation efficiency in N2 + 2 % O2 was greater than that in pure N2 atmosphere. This observation indicated that O2 is beneficial to the catalytic activity of catalyst. Meanwhile, Hg0 oxidation might comprise some chemical reactions with the presence of O2 (Fan et al. 2012; Tao et al. 2012; Yang et al. 2011a; Yang et al. 2011b). Combined with the XPS results, it was possible that gas-phase O2 achieved the supplement of the lost lattice oxygen, which facilitated Hg0 oxidation and NO conversion.
Simultaneous removal of Hg0 and NO
The simultaneous removal of Hg0 and NO by Ce1.00Mn/SCR1 catalysts was also investigated and the results are illustrated in Fig. 10. As shown in the figure, 82.20 % NO conversion was obtained at 200 °C with a GHSV of 100,000/h. An obvious decrease of the Hg0 oxidation efficiency was found in the experiment compared with the NH3-free condition. Although simultaneously removal of Hg0 and NO was unsatisfactory, it is still encouraging because lower space velocity would result in higher Hg0 oxidation efficiency and NO conversion. Combined with the result of upper experiment, the problem that how to design and choose the best optimized experimental conditions for simultaneous removal of Hg0 and NO still needs further study.
The cooperation of Mn and Ce in the SCR catalysts
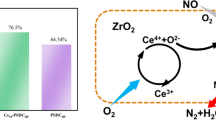
It was worthy noted that the reaction on the surface of Ce1.00Mn/SCR1 catalysts included both NO conversion and Hg0 oxidation. NO was reduced by NH3 through the catalysis of NH3-SCR while Hg0 was oxidized to Hg2+. The reaction observed in this work like previous literature (He et al. 2014; Singh et al. 2013; Xu et al. 2012) which is thought to be aided by the synergistic mechanism between the manganese and cerium oxides. They explained the synergistic mechanism as follows:
The co-existence of MnO2-Mn2O3 can improve the NO conversion and Hg0 oxidation. The lattice oxygen generated from manganese oxides are beneficial in promoting the oxygen cycle. Moreover, the additional oxygen generated is thought to be aided by the oxygen transfer between Mn2O3 and CeO2 (Eq. 8). Ce provided oxygen to Mn, in which why the valence and oxidation ability of Mn increased. Finally, the consumed lattice oxygen would be replenished by oxygen from the flue gas (Eq. 9).
Combining with our results, the lattice oxygen participated in the NO conversion and Hg0 oxidation. On one hand, the lattice oxygen generated via Eq. 10 is invariably responsible for the oxidation of NO to the intermediate NO2-containing species on the catalyst surface. They can further react with NH3 to form ammonium nitrite (NH2NO), which subsequently decomposes into N2 and H2O. The reaction pathway can be described by Eqs. 10–12 according to the previous study (Jin et al. 2010). On the other hand, Hg0 (ad) would react with lattice oxygen that replenished by oxygen from the flue gas to form HgO. The pathway could be explained by Eqs. 13 and 14 according to the previous research (Ji et al. 2008; Kong et al. 2011; Zhao et al. 2014).
where ⋯ ⋯ represent the catalyst surface.
Conclusions
The spent TiO2-based SCR-DeNOx catalyst was used as a support for Hg0 oxidation, which presents a more environmentally and financially sound option for simultaneous removal of Hg0 and NO in industrial application. Ce-Mn mixed oxide modified catalyst was highly active for Hg0 oxidation reaction at low temperature. The Ce1.00Mn/SCR1 catalyst exhibited high Hg0 oxidation efficiency (92.80 %) at 150 °C. The flue gas components were found to have significant effects on Hg oxidation efficiency. NH3 consumed the oxygen on the catalyst surface, hence inhibiting Hg0 oxidation. Promotional effects of SO2 on the Hg0 oxidation were observed, while the presence of H2O inhibited the Hg0 oxidation. Results also indicated that the combination of CeO2 and MnOx resulted in significant synergy for Hg0 oxidation and NO conversion.
References
Anchao Z, Wenwen Z, Jun S, Song H, Zhichao L, Jun X (2014) Cobalt manganese oxides modified titania catalysts for oxidation of elemental mercury at low flue gas temperature. Chem Eng J 236:29–38. doi:10.1016/j.cej.2013.09.060
Auzmendi-Murua I, Castillo Á, Bozzelli JW (2014) Mercury oxidation via chlorine, bromine, and iodine under atmospheric conditions: thermochemistry and kinetics. J Phy Chem A 118:2959–2975. doi:10.1021/jp412654s
Brown TD, Smith DN, Hargis RA, O’Dowd WJ (1999) Mercury measurement and its control: what we know, have learned, and need to further investigate. J Air Waste Manage Assoc 49:1–97. doi:10.1080/10473289.1999.10463906
Cao Y, Gao Z, Zhu J, Wang Q, Huang Y, Chiu C, Parker B, Chu P, Pan WP (2008) Impacts of halogen additions on mercury oxidation, in a slipstream selective catalyst reduction (SCR), reactor when burning sub-bituminous coal. Environ Sci Technol 42:256–261. doi:10.1021/es071281e
Casapu M, Kröcher O, Elsener M (2009) Screening of doped MnOx–CeO2 catalysts for low-temperature NO-SCR. Appl Catal B-Environ 88:413–419. doi:10.1016/j.apcatb.2008.10.014
Dunn JP, Koppula PR, Stenger HG, Wachs IE (1998) Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts. Appl Catal B-Environ 19:103–117. doi:10.1016/S0926-3373(98)00060-5
Fan X, Li C, Zeng G, Gao Z, Chen L, Zhang W, Gao H (2010) Removal of gas-phase element mercury by activated carbon fiber impregnated with CeO2. Energ Fuel 24:4250–4254. doi:10.1021/ef100377f
Fan X, Li C, Zeng G, Zhang X, Tao S, Lu P, Li S, Zhao Y (2012) The effects of Cu/HZSM-5 on combined removal of Hg0 and NO from flue gas. Fuel Process Technol 104:325–331. doi:10.1016/j.fuproc.2012.06.003
Guo Y, Liu Z, Liu Q, Huang Z (2008) Regeneration of a vanadium pentoxide supported activated coke catalyst-sorbent used in simultaneous sulfur dioxide and nitric oxide removal from flue gas: effect of ammonia. Catal Today 131:322–329. doi:10.1016/j.cattod.2007.10.032
He J, Reddy GK, Thiel SW, Smirniotis PG, Pinto NG (2013) Simultaneous removal of elemental mercury and NO from flue gas using CeO2 modified MnOx/TiO2 materials. Energ Fuel 27:4832–4839. doi:10.1021/ef400718n
He C, Shen B, Chen J, Cai J (2014) Adsorption and oxidation of elemental mercury over Ce-MnOx/Ti-PILCs. Environ Sci Technol 48:7891–7898. doi:10.1021/es5007719
Ji L, Sreekanth PM, Smirniotis PG, Thiel SW, Pinto NG (2008) Manganese oxide/titania materials for removal of NOx and elemental mercury from flue gas. Energ Fuel 22:2299–2306. doi:10.1021/ef700533q
Jin R, Liu Y, Wu Z, Wang H, Gu T (2010) Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: a comparative study. Chemosphere 78:1160–1166. doi:10.1016/j.chemosphere.2009.11.049
Kamata H, Ueno S, Naito T, Yukimura A (2008) Mercury oxidation over the V2O5(WO3)/TiO2 commercial SCR catalyst. Ind Eng Chem Res 47:8136–8141. doi:10.1021/ie800363g
Khodayari R, Odenbrand CUI (2001) Regeneration of commercial TiO2-V2O5-WO3 SCR catalysts used in bio fuel plants. Appl Catal B-Environ 30:87–99. doi:10.1016/S0926-3373(00)00227-7
Kong F, Qiu J, Liu H, Zhao R, Ai Z (2011) Catalytic oxidation of gas-phase elemental mercury by nano-Fe2O3. J Environ Sci 23:699–704. doi:10.1016/S1001-0742(10)60438-X
Larachi F, Pierre J, Adnot A, Bernis A (2002) Ce 3d XPS study of composite CexMn1-xO2-y wet oxidation catalysts. Appl Surf Sci 195:236–250. doi:10.1016/S0169-4332(02)00559-7
Li Y, Murphy P, Wua CY (2008a) Removal of elemental mercury from simulated coal-combustion flue gas using a SiO2-TiO2 nanocomposite. Fuel Process Technol 89:567–573. doi:10.1016/j.fuproc.2007.10.009
Li Y, Murphy PD, Wu CY, Powers KW, Bonzongo JCJ (2008b) Development of silica/vanadia/titania catalysts for removal of elemental mercury from coal-combustion flue gas. Environ Sci Technol 42:5304–5309. doi:10.1021/es8000272
Li JF, Yan NQ, Qu Z, Qiao SH, Yang SJ, Guo YF, Liu P, Jia JP (2010) Catalytic oxidation of elemental mercury over the modified catalyst Mn/alpha-Al2O3 at lower temperatures. Environ Sci Technol 44:426–431. doi:10.1021/es9021206
Li HL, Wu CY, Li Y, Zhang JY (2011) CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue Gas. Environ Sci Technol 45:7394–7400. doi:10.1021/es2007808
Li H, Wu CY, Li Y, Zhang J (2012) Superior activity of MnOx-CeO2/TiO2 catalyst for catalytic oxidation of elemental mercury at low flue gas temperatures. Appl Catal B-Environ 111–112:381–388. doi:10.1016/j.apcatb.2011.10.021
Li H, Wu CY, Li Y, Li L, Zhao Y, Zhang J (2013) Impact of SO2 on elemental mercury oxidation over CeO2–TiO2 catalyst. Chem Eng J 219:319–326. doi:10.1016/j.cej.2012.12.100
Liu CX, Chen L, Li JH, Ma L, Arandiyan H, Du Y, Xu JY, Hao JM (2012) Enhancement of activity and sulfur resistance of CeO2 supported on TiO2-SiO2 for the selective catalytic reduction of NO by NH3. Environ Sci Technol 46:6182–6189. doi:10.1021/es3001773
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher GB, Streets DG, Telmer K (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10:5951–5964. doi:10.5194/acp-10-5951-2010
Pudasainee D, Lee SJ, Lee SH, Kim JH, Jang HN, Cho SJ, Seo YC (2010) Effect of selective catalytic reactor on oxidation and enhanced removal of mercury in coal-fired power plants. Fuel 89:804–809. doi:10.1016/j.fuel.2009.06.022
Qi G, Yang RT, Chang R (2004) MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl Catal B-Environ 51:93–106. doi:10.1016/j.apcatb.2004.01.023
Qu L, Li C, Zeng G, Zhang M, Fu M, Ma J, Zhan F, Luo D (2014) Support modification for improving the performance of MnOx–CeOy/γ-Al2O3 in selective catalytic reduction of NO by NH3. Chem Eng J 242:76–85. doi:10.1016/j.cej.2013.12.076
Shang X, Hu G, He C, Zhao J, Zhang F, Xu Y, Zhang Y, Li J, Chen J (2012) Regeneration of full-scale commercial honeycomb monolith catalyst (V2O5–WO3/TiO2) used in coal-fired power plant. J Ind Eng Chem 18:513–519. doi:10.1016/j.jiec.2011.11.070
Shen B, Wang F, Liu T (2014a) Homogeneous MnOx-CeO2 pellets prepared by a one-step hydrolysis process for low-temperature NH3-SCR. Powder Technol 253:152–157. doi:10.1016/j.powtec.2013.11.015
Shen B, Wang Y, Wang F, Liu T (2014b) The effect of Ce-Zr on NH3-SCR activity over MnOx(0.6)/Ce0.5Zr0.5O2 at low temperature. Chem Eng J 236:171–180. doi:10.1016/j.cej.2013.09.085
Singh S, Nahil MA, Sun X, Wu C, Chen J, Shen B, Williams PT (2013) Novel application of cotton stalk as a waste derived catalyst in the low temperature SCR-deNOx process. Fuel 105:585–594. doi:10.1016/j.fuel.2012.09.010
Srivastava RK, Hutson N, Martin B, Princiotta F, Staudt J (2006) Control of mercury emissions from coal-fired in electric utility boilers. Environ Sci Technol 40:1385–1393. doi:10.1021/es062639u
Svachula J, Alemany LJ, Ferlazzo N, Forzatti P, Tronconi E, Bregani F (1993) Oxidation of sulfur dioxide to sulfur trioxide over honeycomb DeNoxing catalysts. Ind Eng Chem Res 32:826–834. doi:10.1021/ie00017a009
Tan Z, Su S, Qiu J, Kong F, Wang Z, Hao F, Xiang J (2012) Preparation and characterization of Fe2O3–SiO2 composite and its effect on elemental mercury removal. Chem Eng J 195–196:218–225. doi:10.1016/j.cej.2012.04.083
Tao S, Li C, Fan X, Zeng G, Lu P, Zhang X, Wen Q, Zhao W, Luo D, Fan C (2012) Activated coke impregnated with cerium chloride used for elemental mercury removal from simulated flue gas. Chem Eng J 210:547–556. doi:10.1016/j.cej.2012.09.028
Wang P, Su S, Xiang J, You H, Cao F, Sun L, Hu S, Zhang Y (2014) Catalytic oxidation of Hg0 by MnOx–CeO2/γ-Al2O3 catalyst at low temperatures. Chemosphere 101:49–54. doi:10.1016/j.chemosphere.2013.11.034
Wiatros-Motyka MM, C-g S, Stevens LA, Snape CE (2013) High capacity co-precipitated manganese oxides sorbents for oxidative mercury capture. Fuel 109:559–562. doi:10.1016/j.fuel.2013.03.019
Wu Z, Jin R, Liu Y, Wang H (2008) Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal Commun 9:2217–2220. doi:10.1016/j.catcom.2008.05.001
Xu H, Zhang Q, Qiu C, Lin T, Gong M, Chen Y (2012) Tungsten modified MnOx–CeO2/ZrO2 monolith catalysts for selective catalytic reduction of NOx with ammonia. Chem Eng Sci 76:120–128. doi:10.1016/j.ces.2012.04.012
Yan NQ, Chen WM, Chen J, Qu Z, Guo YF, Yang SJ, Jia JP (2011) Significance of RuO2 modified SCR catalyst for elemental mercury oxidation in coal-fired flue gas. Environ Sci Technol 45:5725–5730. doi:10.1021/es200223x
Yang S, Guo Y, Yan N, Wu D, He H, Xie J, Qu Z, Jia J (2011a) Remarkable effect of the incorporation of titanium on the catalytic activity and SO2 poisoning resistance of magnetic Mn–Fe spinel for elemental mercury capture. Appl Catal B-Environ 101:698–708. doi:10.1016/j.apcatb.2010.11.012
Yang SJ, Guo YF, Yan NQ, Wu DQ, He HP, Qu Z, Jia JP (2011b) Elemental mercury capture from flue gas by magnetic Mn-Fe spinel: effect of chemical heterogeneity. Ind Eng Chem Res 50:9650–9656. doi:10.1021/ie2009873
Zhang S, Li H, Zhong Q (2012) Promotional effect of F-doped V2O5-WO3/TiO2 catalyst for NH3-SCR of NO at low-temperature. Appl Catal a-Gen 435:156–162. doi:10.1016/j.apcata.2012.05.049
Zhang X, Shen B, Wang K, Chen J (2013) A contrastive study of the introduction of cobalt as a modifier for active components and supports of catalysts for NH3-SCR. J Ind Eng Chem 19:1272–1279. doi:10.1016/j.jiec.2012.12.028
Zhao B, Liu X, Zhou Z, Shao H, Wang C, Si J, Xu M (2014) Effect of molybdenum on mercury oxidized by V2O5–MoO3/TiO2 catalysts. Chem Eng J 253:508–517. doi:10.1016/j.cej.2014.05.071
Zhibo X, Chunmei L, Dongxu G, Xinli Z, Kuihua H (2013) Selective catalytic reduction of NOx with NH3 over iron–cerium mixed oxide catalyst: catalytic performance and characterization. J Chem Technol Biot 88:1258–1265. doi:10.1002/jctb.3966
Zhou J, Hou W, Qi P, Gao X, Luo Z, Cen K (2013) CeO2–TiO2 sorbents for the removal of elemental mercury from syngas. Environ Sci Technol 47:10056–10062. doi:10.1021/es401681y
Acknowledgments
This project was financially supported by the National Natural Science Foundation of China (51278177, 51478173).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Highlights
• The spent commercial SCR catalyst was used as a support for Hg0 oxidation.
• CeMn/SCR1 catalyst was highly active for Hg0 oxidation at low temperatures.
• The surface oxygen was responsible for Hg0 oxidation and NO conversion.
• Catalytic activity of Hg0 oxidation and NO conversion was measured.
• The catalytic mechanism of catalysts was also investigated.
Rights and permissions
About this article
Cite this article
Zhao, L., Li, C., Zhang, X. et al. Oxidation of elemental mercury by modified spent TiO2-based SCR-DeNOx catalysts in simulated coal-fired flue gas. Environ Sci Pollut Res 23, 1471–1481 (2016). https://doi.org/10.1007/s11356-015-5143-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5143-x