Abstract
Due to their toxicity, persistence, and bioaccumulation, metals are important marine environment pollutants, especially in low renewal rate water such as the Mediterranean Sea, receiving a lot of untreated industrial waste. The impact of a phosphate fertilizer plant on the marine biota metal contamination was studied. Several types of organisms: crabs, mussels, patella and fish were collected from two areas of the Lebanese coast, one subjected to the impact of the plant and another away from it; samples were analyzed for Zn, U, Cr, V, Mn, Ni, Co, Cu, As, Cd and Pb by ICP-MS. Higher accumulation was in crabs, patella, and mussels. Fish accumulated principally Zn, Cu, and Cd; a difference was observed between species and tissues. Cytosol metal fractionation using size-exclusion LC-ICP-MS showed principally Pb, As, Co, and Mn in the low molecular weight fraction (<1.8 Da); Cd, Zn, and Cu in the metallothionein fraction (1.8–-18 k Da), and Ni in high molecular weight fraction (>20 kDa).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The very low renewal rate makes the Mediterranean Sea extremely sensitive to pollution. The decline of the Mediterranean threatens the health of many millions of people living on its shore and jeopardizes the long-term development of key economic sectors such as fishing and tourism (Haffner and Sommer 2008). This fact has led to initiatives to help the countries of the southern and eastern Mediterranean reduce the waste released into the sea. The key issue is the assessment of the present contamination linked to waste discharges from plants located at the seacoast.
Heavy metals are normal constituents of the marine environment at trace levels, but their concentrations have been continually increasing due to domestic, industrial, mining, and agricultural activities (Uysal and Emre 2011). Discharges of heavy metals into the marine environment can change both aquatic species diversity and ecosystems (Funes et al. 2006). Because of their persistence, toxicity, and bioaccumulation, heavy metals may cause a risk to human and global ecosystem (Funes et al. 2006; Geeraerts and Belpaire 2009). On the other hand, some aquatic organisms such as, in particular, fish but also seaweeds and molluscs can be used to assess heavy metal contamination in the marine environment, since they accumulate metals to concentrations many times higher than those present in water or sediment (Silva et al. 2006). Studies have shown that metals accumulate in various organs of fish at different levels (Fernandes et al. 2007; Uluturhan and Kucuksezgin 2007). Heavy metals often accumulate to a higher concentration in the active metabolite parts such as the liver, gill, and kidney (Canli and Atli 2003). In general, the accumulation of heavy metals in a tissue is mainly dependent from water pollution level, time of exposure, intrinsic factors (fish age, feeding habits, metabolisms), and other environmental factors (salinity, pH, water temperature) (Canli and Atli 2003). Mussels, crabs, and patella are excellent metal biomonitoring species available throughout the year with great affinity to pollution and important accumulation capacities (Nesto et al. 2007). Thus, metal concentration in their tissues reflects environmental levels.
A further insight into the toxicity, bioavailability, and environmental behavior of the metals can be obtained by the identification of the biomolecule targets involved in its metabolic pathways (Wallace et al. 2003; Vijver et al. 2004; Geffard et al. 2010). Indeed, the total accumulation of a metal in aquatic organisms may be insufficient to predict its toxicity since its various accumulated chemical forms are not biologically equally active (Vijver et al. 2004). For the toxicokinetics and toxicodynamics of metals, the cytosolic fraction was shown to be the most important (Perceval et al. 2006) since it contains both the metal-detoxified compounds (metallothionein-like proteins) and the metal proteins (Wallace et al. 2003; Perceval et al. 2006).
In the Mediterranean countries, most of the industries are located at the seaside due to the need for cooling and transportation (Fakhri et al. 2008). The Lebanese coastal marine zone is intensely affected by several sources of heavy metal contamination, which discharge directly in seawater without any pretreatment. The objective of this study was to probe, for the first time, the direct impact of a phosphate fertilizer plant on the contamination of edible aquatic resources by heavy metals. The accumulation of U, Cr, V, Mn, Ni, Co, Cu, As, Cd, and Pb was investigated in the gill, liver, and kidney of five common fish species, in crabs, mussels, and patella samples, in terms of total metal concentration and molecular mass fractionation.
Materials and methods
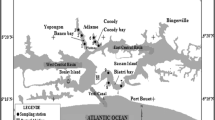
Sample collection and study area
Five commonly consumed fish species Diplodus sargus, Oblada melanura, Pagellus erythrinus, Siganus rivulatus, and Redcoat squirrelfish (Nylon); crabs (Portunus trituberculatus); mussels (Mytilus edulis sp.); and patella (Patella vulgata) were collected from two areas of the Lebanese coastal zone Selaata (34° 16′ 54.9″ N; 35° 38′ 19.37″ E), an area subjected to the impact of a phosphate fertilizer plant and Jbeil (34° 07′ 50.0″ N; 35° 36′ 28.78″ E), away from the direct industrial impact. The collected samples were transferred to the laboratory in an ice box, rinsed with deionized water, and kept frozen until dissection. All fish samples were dissected with sterile scissors to separate gills, liver, and kidney; muscles of crabs, mussels, and patella were removed for analysis. Tissues from individuals of the same species were pooled together, lyophilized, and stored (at 4 °C) until analysis.
Reagents
All the solutions were prepared with high-purity deionized water (resistivity ≤ 18.2 Ω cm) obtained from a Milli-Q Elix 3 water purification system (Millipore, Molsheim, France). All chemicals and reagents were of the highest grade available from each supplier. The hydrogen peroxide, H2O2 (30 %), used was AnalaR NORMAPUR, HNO3 (70 %, INSTRA-ANALYZED)—from Baker (Deventer, The Netherlands)—and the protein standards were purchased from Sigma-Aldrich (St. Quentin Fallavier, France). A SPEX CertiPrep (Metuchen, NJ) PlasmaCAL mono-element standards of U, Th, Cr, V, Mn, Ni, Co, Cu, As, Cd, and Pb were used to prepare standard working solutions by diluting the stock solution of 1000 μg.g−1 of each element with 2 % nitric acid. DORM-2: Dogfish muscle certified reference material from the National Research Council of Canada was used.
Multielemental analysis
Digestions were performed using a DigiPREP device (SCP Science, Courtaboeuf, France). One milliliter of hydrogen peroxide and 3 mL of conc. nitric acid were added to the weighed sample (50 mg) in a PE tube of 50 m; the cap was tightened and the tube placed in the instrument at 65 °C for 4 h. Digests were diluted to obtain a final concentration of 2 % HNO3 prior to total determination. Elemental analysis was conducted using an Agilent ICP-MS 7500ce (Agilent, Tokyo, Japan), fitted with an octopole reaction-collision cell. The operating conditions are given in Table 1. Single-element standard stock solutions PlasmaCAL (1000 ppm) were used for the calibration. The method has been validated by the analysis of a DORM-2 material.
Fractionation analysis
The cytosols were prepared from 50 mg of the gill, liver, and kidney tissues of the S. rivulatus species, diluted in 2 mL of 100 mM ammonium acetate buffer (pH 7.4). The samples were sonicated with an ultrasonic probe (Branson Ultrasonics Corporation, Danbury, USA). Metal determination was performed in the supernatant (cytosolic fraction) after centrifugation (Thermo, CR3i multifunction centrifuge) at 32,000g for 45 min at 4 °C. An Agilent 1100 (Agilent, Wilmington, DE) liquid chromatography equipped with a binary HPLC pump, an autosampler, and a diode array detector were used. Detection was performed by ICP-MS with an Agilent ICP-MS 7500ce (Agilent, Tokyo, Japan). A Superdex 200 HR size-exclusion column (GE Healthcare, Orsay, France) with a mass separation range between 10 and 600 kDa was used at the operating conditions shown in Table 1. The column was calibrated with commercial solutions of thyroglobulin (670 kDa), ferritin (474 kDa), metallothionein (6–7 kDa), carbonic anhydrase (29 kDa), albumin (66 kDa), and Vit E/cobalamin (1.3 kDa). One hundred microliters of fish cytosol was injected and fractionated isocratically at 0.7 mL.min.−1 of 100 mM ammonium acetate (pH 7.4). UV absorbance was monitored at 280 nm.
Results and discussion
The Selaata phosphate fertilizer plant is located at the coast 46 km north of Beirut. The fertilizers are derived from phosphate ores which—in addition to phosphate minerals—contain a wide range of metals such as Hg, Cd, As, Pb, Cu, Ni, Cr, and Zn (Al-Masri et al. 2004; Aoun et al. 2010). During the production process, part of the contaminants is transferred from ores to finished products: phosphoric acid and fertilizers. However, most of the contaminants are found in the effluents and mainly in the solid waste of the phosphate fertilizer industry, known as phosphogypsum (Al-Masri et al. 2004). Due to weathering processes, piles of phosphogypsum disposed without any treatment may lead to chemical contamination of the surrounding environment. In this study, fish belonging to five commonly consumed species and patella were caught all around the industrial area while crabs and mussels were collected from both sides (north and south) of the factory.
Metal contamination in marine organisms
Trace metal in marine environment can be divided into two classes: (i) those essential for effective function of biochemical processes (e.g., cobalt, copper, zinc, manganese) but toxic in excess and (2) those with no established biological functions and considered as toxic (e.g., cadmium, chromium, lead) (Kennish 2001). The concentration levels of Pb, Ni, Cu, V, Cr, Cd, As, Zn, Mn, Co, and U in the liver, gill, and kidney of the investigated species were shown in Fig. 1. Taking into consideration all the collected fish species from the two stations, the mean metal concentrations in the gill, liver, and kidney were, respectively, 2.53, 1.52, and 0.87 mg.kg−1 for V; 0.74, 0.88, and 1.04 mg.kg−1 for Cr; 14.76, 5.36, and 4.86 mg.kg−1 for Mn; 1.47, 2.76, and 1.77 mg.kg−1 for Ni; 0.88, 1.03, and 0.58 mg.kg−1 for Co; 3.32, 35.96, and 4.24 mg.kg−1 for Cu; 60.18, 164.15, and 109.67 mg.kg−1 for Zn; 4.85, 8.76, and 7.75 mg.kg−1 for As; 3.18, 21.43, and 5.50 mg.kg−1 for Cd; 3.51, 1.44, and 1.08 mg.kg−1 for Pb; and 0.34, 0.31, and 0.27 mg.kg−1 for U. The overall metal accumulation capability of the studied fish organs decreases in the order liver > gill > kidney. Several previous studies approved that the liver is a target organ for metal accumulation and detoxification and can be considered as an important sample for the water pollution monitoring (Kljakovic et al. 2002; Canli and Atli 2003).
Accumulation levels of a Zn, b Cu, c Mn, d Ni, e Cr, f Co, g Cd, h V, i As, j Pb, and k U in Diplodus sargus (1 indicates the gill, 2 the liver, and 3 the kidney), Pagellus erythrinus (4 indicates the gill, 5 the liver, and 6 the kidney), Siganus rivulatus (7 indicates the gill, 8 the liver, and 9 the kidney), Oblada melanura (10 indicates the gill, 11 the liver, and 12 the kidney), and Nylon (13 indicates the gill, 14 the liver, and 15 the kidney)
The mean concentrations of essential elements in fish organs follow the sequence: Zn > Cu > Mn >> Co and Zn > Cu − Mn > Co > in Selaata and Jbeil, respectively. The concentrations of the toxic elements Cd, As, Pb, Ni, Cr, and U varied in a broad range between 0.3 mg.kg−1 for U and 92.5 mg.kg−1 for Cd in the fish organs. Concentrations of Ni and Cr are higher in fish collected from Selaata than from Jbeil showing a contamination of the area by the fertilizer plant.
Results obtained for the molluscs are presented in Fig. 2. As for fish, the concentrations of the three essential elements Zn, Cu, and, Mn in the analyzed crabs, mussels, and patella samples were the highest among those in the studied elements. There are higher levels of Ni and Cr in the molluscs than in fish organs that were observed, especially in mussel samples (Fig. 2). Related to other toxic elements, the highest levels of Cd obtained in mussels, crabs, and patella were 3.2, 9.7, and 5.3 mg.kg−1 in Selaata. Similarly, as for fish organs, high accumulation of V and As was observed in both regions while the high level of lead 13.5 mg.kg−1 was determined in mussels from Jbeil; this region away from the direct impact of the plant is surrounded by several other industries.
Zinc
It is an essential trace element which becomes toxic in excessive amounts. Its concentrations in the studied fish organs varied between 44 and 360 mg.kg−1 dry. In several studies, high accumulations of Zn were observed in fish organs, which is in agreement with our results as Zn was the most abundant metal in the studied organs of the fish. The lowest levels of Zn were observed in the gills even if for fish seawater is the main source of Zn, and some researchers indicated that gills are an important organ of its accumulation (Fernandes et al. 2007; Yilmaz and Yilmaz 2007). The levels found in this study in gills of D. sargus and S. rivulatus were several times lower than in the previous report of Uysal and Emre 2011 (Tables 2 and 3), and high levels were observed in the liver organ; in fact, it was also reported that when the levels of Zn exceeded the requirement in the gills, the excess was excreted by the liver (Birungi et al. 2007), which is considered a detoxification organ. In this study, high accumulation of Zn was observed in the liver of S. rivulatus and Nylon and in the kidney of D. sargus collected from Selaata industrial area compared to that from the area of Jbeil highlighting the impact of the phosphate industry. No effect of the sampling location was observed for organs of O. melanura and P. erythrinus, although the concentrations found in the last species were slightly higher than in Turkish coastal water (Uluturhan and Kucuksezgin 2007; Dural et al. 2010). In fact, in addition to the water pollution level, metal concentrations are strongly dependent on fish species and other environmental factors (Canli and Atli 2003). Concentrations of Zn measured here in crabs, mussels, and patella were higher than those found in other areas (Table 4); Zn in patella samples were higher in our study even than in the contaminated area in Cravo studies (Table 4), which is an indicative of environmental pollution from industrial sources.
Copper
This essential element assuring healthy metabolism and growth of marine organisms becomes highly toxic at concentrations higher than the levels required for growth (Eisler 2000). In this study, the concentrations of Cu in liver tissues were significantly higher than in the gill and kidney; data obtained by previous studies show that even at low levels, Cu show a distinct affinity to the liver organ (Jezierska and Witeska 2006). However, the enrichment related to possible industrial contamination was observed for one species only: S. rivulatus with Cu values of 179 and 46 mg.kg−1 for Selaata and Jbeil, respectively. The values reported for P. erythrinus liver were in the same range as those cited by (Uluturhan and Kucuksezgin 2007; Dural et al. 2010) for the species sampled in Turkish coastal water. The levels of copper found in the gill were the same or slightly higher than those reported by (Uysal and Emre 2011) (Table 3) for D. sargus and S. rivulatus, respectively. Cu in crabs and mussels from Selaata were higher than those found in other areas such as Kuwait bay, Adriatic Sea, and Venice lagoon (Table 4), which is an indicative of environmental pollution from industrial sources.
Manganese
The toxicity of this element is considered to be low (Kennish 2001). The fish organs contained between 0.7 and 26.6 mg.kg−1 in the kidney of S. rivulatus in Selaata and in the gill of S. rivulatus in Jbeil, respectively. The privileged organ for Mn accumulation in fish was the gill. The Mn in crabs and mussels were significantly higher reaching for some locations more than 100 mg.kg−1. The concentration of Mn found in the gill of S. rivulatus and D. sargus were slightly higher than those reported by Uysal and Emre 2011 (Table 3). The values reported for P. erythrinus liver were in the same range as those found for the species sampled in Turkish coastal water (Uluturhan and Kucuksezgin 2007; Dural et al. 2010), which can be due to the fact that manganese levels in phosphogypsum are between 3.5 and 20 mg.kg−1, low compared to other metal concentrations (Davister 1998).
Nickel
Chronic Ni toxicity to marine organisms was recently evaluated and found to be strongly species dependent (DeForest and Schlekat 2013). Levels of Ni in fish organs were relatively low in comparison to those of other metals studied and ranged from 0.5 to 4.4 in Selaata and 1.1 to 5.1 mg.kg−1 in Selaata and Jbeil, respectively. Similar levels were observed for crabs, patella, and mussels with the exception of mussels sampled in the south from the Selaata plant where the concentration as high as 18.4 mg.kg−1 was found.
Chromium
Toxicological impact can result both from the action of Cr (VI) itself as an oxidizing agent and from Cr (III) which is capable to form complexes with various organic compounds and thus may inhibit several metalloenzyme systems. In the studied fish organs, concentrations of Cr were at the level of a few mg.kg−1; the highest value (4.2 mg.kg−1) was obtained except for the liver of the Nylon sampled in Selaata. The same levels were present in crabs, mussels, and patella with the highest concentrations observed in the north of the Selaata plant. The content obtained in P. erythrinus liver was in agreement with that observed (Dural et al. 2010) in Turkish coastal water.
Cobalt
It is generally considered to present no or very low toxicity. In this study, no significant differences were observed for Co levels in the Selaata and Jbeil sampling sites; the highest concentrations in fish organs and molluscs were at the same level: 3.7 mg.kg−1 in the gill of S. rivulatus of Jbeil and 3.4 mg.kg−1 in mussel from Selaata.
Cadmium
It is capable to produce chronic effects even at low levels (Taylor 1983). In the tissues, studied concentrations of Cd were generally below 10 mg.kg−1 for both fish and molluscs with the exceptionally high values observed for S. rivulatus liver in Selaata (92.5 mg.kg−1) and in Jbeil (29 mg.kg−1). It was shown that the liver is the primary target for Cd accumulation; the levels in Selaata were generally higher than in Jbeil. Cadmium levels observed in the liver of P. erythrinus were significantly higher than those reported in previous works on the same species sampled in Turkish coastal areas (Uluturhan and Kucuksezgin 2007; Dural et al. 2010); it was reported in previous studies that cadmium is taken by to the gills and distributed throughout the body and accumulates in the liver for detoxification (Uluturhan and Kucuksezgin 2007) which may explain this difference, showing a contamination of the area by the plant. Cd, in patella, were higher in our study than in others studies, even than in contaminated area in Cravo studies (Cravo and Bebianno 2005).
Vanadium
The pH is an important modulator of V toxicity. No clear link was established between the sampling location and vanadium levels with the exception of the organs of D. sargus where the concentrations in Selaata were significantly higher than in Jbeil. On the basis of the obtained data, the accumulation of vanadium seems to be strongly species dependent for both fish and molluscs.
Arsenic
Toxicity of this trace element is highly species dependent; however, in marine biota, most of arsenic is known to be present in the form of harmless organic species such as arsenobetaine. Similar to vanadium, the accumulation of As was found strongly species dependent with no clear correlation with the sampling location. Out of the fish organs studied, the livers contained the highest levels of As up to 14.6 mg.kg−1 in the liver of O. melanura from Jbeil. The concentrations found in molluscs were significantly higher reaching 44.8 mg.kg−1 for crabs.
Lead
It is a non-essential element which causes toxic effects (Garcia-Leston et al. 2010). Distribution of Pb was similar between the different organs of both P. erythrinus and O. melanura species, and a similar accumulation was observed in D. sargus species from both regions. The highest levels were generally observed in the gills, with the highest concentration of 10.2 mg.kg−1 found in the gill of Nylon from Jbeil. Lead levels observed in the liver of P. erythrinus were at the same level as those reported in the work of Uluturhan and Kucuksezgin 2007 and Dural et al. 2010 for the same species sampled in Turkish coastal areas. Previous studies have reported low levels of Pb in seawater, and fish bioaccumulates Pb proportionally to its concentrations in seawater, or Pb concentrations in phosphogypsum are low compared to the levels of other metals (0.5–17 mg.kg−1) (Davister 1998), which may explain in our study the low levels of Pb compared to those of other metals in fish organs.
Uranium
Uranium is a non-essential element, with documented chemical and radiological toxicity (Al Kaddissi et al. 2011). The levels observed in both fish and molluscs were generally low (with a maximum of 0.6 mg.kg−1 in the gill of S. rivulatus from Selaata) with no significant differences in the levels for the different organs and species studied. Levels of U in marine organisms from Selaata were higher than from Jbeil.
Difference of accumulation between species
When compared with other studies, the observed difference in distribution of the studied elements between organs of the different species in this study is in agreement with that in the previous studies on other fish species (Kljakovic et al. 2002; Yilmaz and Yilmaz 2007). Metal concentrations were strongly dependent on fish species which is reported to may be related to the differences in ecological needs, swimming behaviors, and the metabolic activities of the different species (Canli and Atli 2003). Few studies exist on metal levels in the chosen fish species; based on the obtained data, the S. rivulatus species has almost the highest levels of Co, Cu, Zn, Cd, Pb, and U; D. sargus had the highest levels of V and Ni; Nylon had the highest levels of Cr; and the highest levels of As and Mn were observed in the O. melanura and P. erythrinus species, respectively. The concentrations were higher in crabs: levels of 2715, 167, 158, and 52 mg.kg−1 were observed for Zn, Cu, Mn, and As, respectively. Accumulation in patella samples was lower; the highest concentration found was 841.5 mg.kg−1 for Zn while U had the lowest level of 0.2 mg.kg−1.
Difference of accumulations between locations
The comparison of data obtained for fish showed that metal concentrations varied between the sampling locations. For instance, significant Mn concentration was found in Jbeil, while those of Zn, Cu, and Cd were in Selaata. The highest metal concentrations in molluscs were generally found in Selaata, except for V, Ni, and Mn which were concentrated in Jbeil samples. The levels of heavy metals in patella collected from Jbeil and Selaata were comparable.
In general, the concentrations of metals in fish organs in Selaata were higher than those in Jbeil, which may be due to the impact of the phosphate fertilizer industry in this area. Phosphate loading activities and phosphogypsum storage are considered as the main sources of Zn, Cu, and Cd which can be leached to the surrounding environment (Al-Masri et al. 2004; Aoun et al. 2010). The high levels of some elements found in the species from Jbeil, such as Cd and Mn concentrations, may have anthropogenic origin. This area is highly populated with considerable agricultural and metallurgic industry activities.
Pearson test was performed to find the intercorrelation between the organs of the collected fish samples. The linear correlation between each pairs of organs based on the Pearson correlation factor (two-tailed sigma, p = 0.05) (Weiss 2008) showed significant linear relations between the gill, liver, and kidney for V and Cd in Selaata sample (p < 0.05), also between the liver and kidney for Cu and Cr (p = 0.023, p = 0.0001) and between gill–liver for U, As, and Pb (p = 0.04, p = 0.00, p = 0.012). Few significant correlations between organs were observed in the fish species collected from Jbeil, mainly between the gill, liver, and kidney for As; gill–kidney for U; and liver–kidney for Pb (Annexe 2).
The linear correlation between each pairs of elements based on the Pearson correlation factor (two-tailed sigma, p = 0.05) showed strong correlations between many elements in the three organs of fish samples collected from Selaata, especially in the liver (Annexe 2). For instance, Zn correlates positively with Ni, Cu, Cd, and U with Pb while strong correlations were observed between Ni, Cu, Co, and Cd. These correlations are absent in the case of Jbeil which allows to attribute the origin of these metals to the phosphate plant.
Speciation studies
For the assessment of toxicity, mobility, and bioavailability of the studied metals, their molecular size fractionation was investigated further to their total determination. The experiments were focused on the cytosolic fraction of the liver, gill, and kidney of the S. rivulatus, which contains the highest levels of metals. The species eluting from the size-exclusion column were divided into three metal–ligand pools: (i) high molecular weight fraction (HMW) (245–18 kDa), from 12 to 22 min; (ii) metallothionein-like protein fraction (MTLP) (18–1.8 kDa) in the range of 22–30 min; and (iii) lower molecular fraction (LMW) (<1.8 kDa) after 30 min as shown in Fig. 3. Most of the metals were bound to several species, e.g., Cd binds to different protein fractions, mainly to MTLP in the liver and gill and low molecular weight species in the kidney (Annexe 3). Surprisingly, only a small fraction of the cytosolic Cu was bound to MTLP in the liver, kidney, and gill of the Siganus rivulatus from both Jbeil and Selaata. As showed in Fig. 3, Cu was preferably bound to the LMW fraction, which increased with the concentration of Cu in the liver samples of Siganus rivulatus from Selaata. This highlights a high contamination and healthy effect. Indeed, ecotoxicological studies have shown that MTLP are detoxifier proteins whereas HMW and LMW would be biomarkers of harmful effects (Giguère et al. 2003).
Arsenic, cobalt, and lead were found predominantly in the low molecular fraction, whereas a significant fraction of nickel (up to 72 % depending on the organ) was bound to high molecular proteins.
Conclusion
Data have shown a heavy contamination of marine biota directly related to the phosphate plant activity in Selaata, especially for Zn, Cu, and Cd which are contained in the coproduct and industrial waste of the factory. The contamination is species dependent. The highest metal concentrations were found in the livers (organ of detoxification) of the studied fish species. The concentrations of Zn in all organs were the highest. The metals were relatively homogenously distributed among the pools of biological ligands which suggest that the levels of contamination exceed those at which molecular mechanisms are still active.
References
Al Kaddissi S, Legeay A, Gonzalez P et al (2011) Effects of uranium uptake on transcriptional responses, histological structures and survival rate of the crayfish Procambarus clarkii. Ecotoxicol Environ Saf 74:1800–1807
Al-Masri MS, Amin Y, Ibrahim S et al (2004) Distribution of some traces metals in Syrian phosphogypsum. Appl Geochem 19:747–753
Al-Mohanna SY, Subrahmanyam MNV (2001) Flux of heavy metal accumulation in various organs of the intertidal marine blue crab, Portunus pelagicus (L.) from the Kuwait coast after the Gulf War. Environ Int 27(4):321–326
Aoun M, El Samrani A, Lartiges BS et al (2010) Releases of phosphate fertilizer industry in the surrounding environment: investigation on heavy metals and polonium-210 in soil. J Environ Sci 22:1387–1397
Birungi Z, Masola B, Zaranyika MF et al (2007) Active biomonitoring of trace heavy metals using fish (Oreochromis niloticus) as bioindicator species. The case of Nakivubo wetland along Lake Victoria. Phys Chem Earth 32:1350–1358
Campanella L, Conti ME, Cubadda F et al (2001) Trace metals in seagrass, algae and molluscs from an uncontaminated area in the Mediterranean. Environ Pollut 111(1):117–126
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121(1):129–136
Cravo A, Bebianno MJ (2005) Bioaccumulation of metals in the soft tissue of Patella aspera: application of metal/shell weight indices. Estuar Coast Shelf Sci 65(3):571–586
Davister A (1998) Phosphogypsum: a waste (more or less harmful) or a resource
DeForest DK, Schlekat CE (2013) Species sensitivity distribution evaluation for chronic nickel toxicity to marine organisms. Integr Environ Assess Manag 9(4):580–589
Dural M, Genc E, Yemeniciog¢lu S (2010) Accumulation of some heavy metals seasonally in Hysterotylacium aduncum (Nematoda) and its host Red Sea Bream, Pagellus erythrinus (Sparidae) from Gulf of Iskenderun (North-Eastern Mediterranean). Bull Environ Contam Toxicol 84:125–131
Eisler R (2000) Metals. Lewis Publishers, New York
Fakhri M, Abi Saab MA, Romano JC (2008) The use of sediments to assess the impact of Selaata phosphate plant on Batroun coastal area (Lebanon, Levantine Basin). Leban Sci J 9:29–42
Fattorini D, Notti A, Di Mento R et al (2008) Seasonal, spatial and inter-annual variations of trace metals in mussels from the Adriatic sea: a regional gradient for arsenic and implications for monitoring the impact of off-shore activities. Chemosphere 72(10):1524–1533
Fernandes C, Fontainhas -Fernandes A, Peixoto F et al (2007) Bioaccumulation of heavy metals in Liza saliens from the Esmoriz-Paramos Coastal lagoon, Portugal. Ecotoxicol Environ Saf 66:426–431
Funes V, Alhama J, Navas JI et al (2006) Ecotoxicological effects of metal pollution in two mollusc species from the Spanish South Atlantic littoral. Environ Pollut 139:214–223
Garcia-Leston J, Mendez J, Pasaro E et al (2010) Genotoxic effects of lead: an updated review. Environ Int 36:623–636
Geeraerts C, Belpaire C (2009) The effects of contaminants in European eel. Ecotoxicology 19:239–266
Geffard A, Sartelet H, Garric J et al (2010) Subcellular compartmentalization of cadmium, nickel, and lead in Gammarus fossarum: comparison of methods. Chemosphere 78:822–829
Giguère A, Couillard Y, Campbell PGC et al (2003) Steady-state distribution of metals among metallothionein and other cytosolic ligands and links to cytotoxicity in bivalves living along a polymetallic gradient. Aquat Toxicol 64(2):185–200
Haffner C, Sommer C (2008) Elaboration of a Mediterranean hot spot investment programme (MeHSIP).
Jezierska B, Witeska M (2006) The metal uptake and accumulation in fish living in polluted waters. Soil and water pollution monitoring, protection and remediation 1: 3–23
Kennish JM (2001) Practical handbook of marine science. CRC Press, FL
Kljakovic G, Zvonaric T, Vrgoc N, Odzak N, Baric A (2002) Cadmium and lead in selected tissues of two commercially important fish species from the Adriatic Sea. Water Resour 36:5023–5028
Nesto N, Romano S, Moschino V et al (2007) Bioaccumulation and biomarker responses of trace metals and micro-organic pollutants in mussels and fish from the lagoon of Venice, Italy. Mar Pollut Bull 55:455–469
Perceval O, Couillard Y, Pinel-Alloul B et al (2006) Linking changes in subcellular cadmium distribution to growth and mortality rates in transplanted freshwater bivalves (Pyganodon grandis). Aquat Toxicol 79:87–98
Silva CAR, Smith BD, Rainbow PS (2006) Comparative biomonitors of coastal trace metal contamination in tropical South America. Mar Environ Res 61:439–455
Taylor D (1983) The significance of the accumulation of cadmium by aquatic organisms. Ecotoxicol Environ Saf 7(1):33–42
Uluturhan E, Kucuksezgin F (2007) Heavy metal contaminants in Red Pandora (Pagellus erythrinus) tissues from the Eastern Aegean Sea, Turkey. Water Res 41:1185–1192
Uysal K, Emre Y (2011) Bioaccumulation of copper, zinc, manganese, iron and magnesium in some economically important fish from the western shores of Antalya. Anadolu Univ J Sci Technol C Life Sci Biotechnol 1(1):95–102
Vijver M, Van Gestel CAM, Lanno RP et al (2004) Internal metal sequestration and its ecotoxicological relevance. Environ Sci Technol 38:4705–4712
Wallace WG, Lee BG, Luoma SN (2003) Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSFs) and biologically detoxified metal (BDM). Mar Ecol Prog 249:183–197
Weiss NA (2008) Elementary statistics, 8th edn. Pearson Addison-Wesley, Boston
Yilmaz AB, Yilmaz L (2007) Influences of sex and seasons on levels of heavy metals in tissues of green tiger shrimp (Penaeus semisulcatus de Hann, 1844). Food Chem 101:1664–1669
Acknowledgments
We would like to thank the Lebanese National Council of Scientific Research for their support to achieve this work and the laboratory of Analytic Bio-inorganic Chemistry and Environment LCABIE CNRS/UPPA, France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Aoun, M., Arnaudguilhem, C., El Samad, O. et al. Impact of a phosphate fertilizer plant on the contamination of marine biota by heavy elements. Environ Sci Pollut Res 22, 14940–14949 (2015). https://doi.org/10.1007/s11356-015-4691-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4691-4