Abstract
Olive mill wastewater is considered as one of the most polluting effluents of the food industry and constitutes a source of important environmental problems. In this study, the removal of pollutants (chemical oxygen demand (COD), biochemical oxygen demand (BOD5), polyphenols, turbidity, color, total suspended solids (TSS), and oil and grease) from olive oil mill processing wastewater by peroxi-electrocoagulation/electrooxidation-electroflotation process with bipolar aluminum electrodes was evaluated using a pilot continuous reactor. In the electrochemical unit, aluminum (Al), stainless steel, and RuO2/Ti plates were used. The effects of pH, hydrogen peroxide doses, current density, NaCl concentrations, and reaction times were studied. Under optimal conditions of pH 4, current density of 40 mA/m2, 1000 mg/L H2O2, 1 g/L NaCl, and 30-min reaction time, the peroxi-electrochemical method yielded very effective removal of organic pollution from the olive mill wastewater diluted four times. The treatment process reduced COD by 96 %, BOD5 by 93.6 %, total, polyphenols by 94.4 %, color by 91.4 %, turbidity by 88.7, suspended solids by 97 % and oil and grease by 97.1 %. The biodegradability index (BOD5/COD) increased from 0.29 to 0.46. Therefore, the peroxi-electrocoagulation/electrooxidation-electroflotation process is considered as an effective and feasible process for pre-treating olive mill wastewater, making possible a post-treatment of the effluent in a biological system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extraction of oil from olive fruit is one of the important industries that create valuable product, although generate large amount of wastewater. Olive oil mill wastewater (OMW) is the liquid by-product obtained from mechanical olive processing. The quantity of generated wastewater, depending on the method used for the oil extraction, varies from (per 100 kg of olives) 40–60 l for pressing method to 80–100 l for three-phase centrifugation technique (Aparicio and Harwood 2013). OMW is one of the strongest industrial effluents, with high organic materials content, for example: chemical oxygen demand (COD) values in the range of 40–220 g/L, biochemical oxygen demand (BOD5) values in the range of 15 to 135 g/L, suspended solids (SS) from 6 to 69 g/L, and total phenols from 2 to 15 g/L. The pH of this wastewater usually ranges from 4.5 to 5.8 (Al-Malah et al. 2000; Crognale et al. 2006; Fadil et al. 2003; Khoufi et al. 2006). Therefore, the biological treatment process is lack to treating OMW. Various methods, such as centrifugation–ultrafiltration (Turano et al. 2002), composting and direct watering on fields (Paredes et al. 2002), chemical treatments (Atanassova et al. 2005; Hodaifa et al. 2013; Kallel et al. 2009; Nieto et al. 2011), flocculation (Sarika et al. 2005), and ultrafiltration have been studied for the treatment of this wastewater (Akdemir and Ozer 2009). Among the wide range of methods, electrochemical technology has been taken much interesting, and a variety of very effective techniques based on this technology, including electrocoagulation, electroflotation, and electrochemical oxidation, have been developed for the treatment of both low and high organic load wastewaters. These technologies are considered to be the most perspective methods for the purification and treatment of potable water and municipal wastewater. The mechanisms of reactions in the electrochemical process are considered adequately (Antropov 1972). Among the electrochemical technologies, electrocoagulation and electroflotation may be effective substitutions for conventional coagulation and flotation in a wastewater treatment process (Yilmaz et al. 2007). Another suitable technique for chemical treatment of wastewaters is the electrolysis generating by chemical oxidizing agents such as chlorine and/or hypochlorite and hydrogen peroxide (Panizza and Cerisola 2009). In the electrocoagulation cell, the electrochemical reactions with metal Al as electrodes are as follows:
The Al3+ ions formed hydrolyze to generate corresponding hydroxides and/or polyhydroxides in appropriate pH. It has been suggested that the Al hydroxides and polyhydroxides formed during the electrochemical dissolution have a strong affinity to capture the pollutants in the wastewater, causing more coagulation than conventional Al coagulants (Zaroual et al. 2006). Also, cathodic and anodic reactions produce tiny bubbles of hydrogen and oxygen gases, respectively. These tiny bubbles (average diameter of approximately 20 μm) can cause flotation of the generated flocs and the coagulated materials (Kotti et al. 2009). Thus, electroflotation may also play an important role in an electrocoagulation cell. The oxygen produced through the anodic reactions can prevent the anaerobic conditions of the wastewater. Moreover, hydrolysis and polymerization of Al3+ cause the formation of gelatinous charged hydroxo-cationic complexes, which are able to remove pollutants via adsorption and charge neutralization (Barash et al. 2009; Ge et al. 2004). In electrocoagulation process, metal hydroxide flocs are produced which have a large surface area. These valuable characteristics are very useful for removal of non-settleable pollutants by rapid adsorption of soluble organic compounds and trapping of colloidal particles. Then, these flocs are removed easily from effluent by sedimentation or flotation (Farhadi et al. 2012). Recently, the use of hydroxyl radicals in aqueous medium has been suggested to promote the oxidation of toxic pollutants (Dehghani et al. 2011; Roa-Morales et al. 2007). Huang et al. have reviewed different advanced oxidation processes and demonstrated that the methods based on hydrogen peroxide to promote the formation of hydroxyl radicals have many advantages, since these methods are efficient and less expensive (Huang et al. 1993). Additionally, the hydrogen peroxide methods have more efficient mass transfer properties than those involving other hydroxyl radical promoting species such as ozone (Peralta-Hornandez et al. 2005). H2O2 can be produced strong oxidative hydroxyl radical and the ·OH attacks organic compounds and thus causes chemical degradation of these compounds in a short period (Liou and Lu 2007). According to proposed mechanism by Miller and Valentine, the main reactions occurring at the electrode surface (S) are as follows (Miller and Valentine 1999; Plant and Jeff 1994):
Also, the hydrogen peroxide will form the hydroxyl radical at the cathode (Bard 2004):
Some of the Al3+ ions formed in the anode can be reduced at the cathode. Then, the aluminum will react in the solution as
A sequence reaction then take places between the hydroxyl radical and an organic compound R (Bard 2004):
Additionally, presence of chloride ions (Cl−) has benefits, particularly when using aluminum plates. It has been suggested that chloride ions are breakdown potential of aluminum through pitting corrosion. During the electrolysis of aluminum in anode, alumina film is usually formed in surface, and it causes increased power consumption and decreased process efficiency because it can inhibit the release of Al3+ ions as well as electron transfer. Therefore, the efficiency of the system can be significantly increased via the breakdown of the alumina film (Al2O3) (Gao et al. 2010).
This work aimed to study the treatment of OMW by peroxi-electrocoagulation/electrooxidation-electroflotation process by using bipolar aluminum electrodes under different operational conditions of pH, current density, detention time, NaCl concentration, and hydrogen peroxide concentration.
Materials and methods
Experimental setup
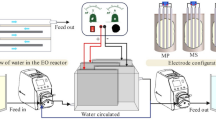
The experimental setup is schematically shown in Fig. 1. The plates consisted of nine pieces of Al and two pairs of RuO2/Ti and stainless steel. The bipolar electrochemical cell consisted of three aluminum plates placed between pairs of a RuO2/Ti anode and a stainless steel (SS) cathode, and only two pairs of plates were connected to the D.C. power supply with 0–60 V of electrical potential. Different amounts of H2O2 were added to the electrochemical process to enhance pollutant removal. The reactor was electrochemical and separation units with 5 and 7.5 l in volume, respectively. The effective surface area for each plate was 90 cm2 (9 cm × 10 cm), and the space between the plates was 8 mm. Aluminum was used as the sacrificial anode, rather than iron, because the residual ferrous ions are easily oxidized by air, which may increase the color of effluent. Scum was separated out using a flotation tank, and flotation was achieved by hydrogen gas bubbles generated from the cathode, allowing the top layer to be skimmed. The efficiency of the reactor was first tested at the different operational conditions of pH (3–9), current densities of 5–40 mA/cm2, NaCl concentrations of 0.5–3 g/L, H2O2 concentrations of 0–2000 mg/L, and detention times of 5–30 min. After determining the optimum operational conditions, the maximum efficiency of the reactor was tested under all optimum operational conditions obtained from past stages.
Samples
The olive mill wastewater was obtained from an olive oil production factory located in the Roodbar region, home of the olive growing and processing sector, in the northern part of Iran. All experiments were done on a 25 % solution of the wastewater. The main characteristics of OMW, diluted four times (25 % (v/v)), are presented in Table 1.
Analytical methods
All reagents used in the electrochemical oxidation experiments were of analytical grade. H2SO4 and NaOH were used to adjust pH during the experiment. pH was measured by a pH-meter (E520 Metrohm Herisau). Electric conductivity was assessed with a CRISON conductivity meter, GLP31 model. Turbidity (Nephelometric Turbidity Unit, NTU) was determined with a turbidity-meter Tecnal, model TB1000. Color was measured according to the Hongve and Akesson method (Hongve et al. 2004). The sample was first filtered through a membrane filter with the pore size of 0.45 μm. The color was determined by measuring sample absorbance at 410 nm with a CARY 1E VARIAN spectrophotometer. The absorbance of a reference solution with 100 mg/L Pt was used for conversion of the results to true color units (TCU). The polyphenol concentration was determined by measuring the sample’s absorbance at 278 nm, using a CARY 1E VARIAN spectrophotometer. Since the presence of residual H2O2 introduces a positive error in COD determination (Kang et al. 1999), in electrolysis experiments with H2O2, the pH of the samples was raised to above 10 with NaOH 6N prior to analysis. COD, suspended solids (SS), BOD5, oil and grease were measured in accordance with the standard methods (Association and Association 1976).
Results and discussion
Effect of initial pH on the performance of process
In order to evaluate the effect of pH on removal efficiencies of COD, polyphenols, color, and turbidity of OMW, experiments were carried out at different pH value between 3 and 9. Figure 2a demonstrates the removal efficiencies of OMW at different initial pH without changing other parameters during the degradation reaction ([H2O2] = 500 mg/L, [NaCl] = 1.5 g/L, CD = 20 mA/cm2, Time = 20 min). The pH is one of the most important parameters influencing the electrocoagulation process (Shen et al. 2003). Results showed that the greatest pollutant removal was achieved within the pH range of 4–6 and maximum COD, and phenol removal efficiencies were obtained at pH 4 (Fig. 2a). As it can be seen, at these pH values, COD and polyphenol removal efficiencies were achieved as 84.5 and 88.4 %, respectively. These results are in agreement with recent research, suggesting that the optimum pH for treatment of OMW wastewater by electrocoagulation is below 6 (Hanafi et al. 2010; Inan et al. 2004) and around 3–5 by Fenton and Fenton oxidation with zero-valent iron and also by hydrogen peroxide and peroxi-electrocoagulation (Ahmadi et al. 2005; Kallel et al. 2009; Rivas et al. 2001; Roa-Morales et al. 2007; Tekin et al. 2006). The maximum efficiencies for color and turbidity removal were observed at pH 5.2 and 6, respectively. The possible reason of this phenomenon was given from the observation of the solubility diagram of aluminum hydroxide (Holt et al. 2002). In acidic conditions, chemical dissolution corresponds to the oxidation of the aluminum plates with the simultaneous reduction of water to form hydrogen, according to Eq. (14) (Picard et al. 2000):
a Effect of current density on removal efficiency of COD, polyphenols, color, and turbidity (H2O2 500 mg/L, pH 4, reaction time 20 min, NaCl 1.5 g/L). b Effect of the amount of NaCl salt on removal efficiency of COD, polyphenols, color, and turbidity (H2O2 500 mg/L, pH 4, reaction time 20 min, CD 40 mA/cm2). c Effect of H2O2 concentration on removal efficiency of COD, polyphenols, color, and turbidity (NaCl 1 g/L, pH 4, reaction time 20 min, CD 40 mA/cm2)
On the other hand, the electrochemical processes that occur on the anode and cathode surfaces are represented by Eqs. (15)–(17). On the anode, oxygen evolution is completely occurred by aluminum dissolution (chemically or electrochemically), while main reactions occurring on the cathode is the hydrogen evolution. In both the chemical and the electrochemical processes, hydrogen is generated as follows:
As a result of the reactions (14) and (16), the formation of Al(OH)3(s) is expected. When the aluminum is dissolved (electrochemically and chemically), depending on the pH of the solution and the presence of other chemical species, it can form different species. At low Al concentrations (5 × 10−3 M), the following chemical species can be found in aqueous solution: Al3+, Al(OH)2+, Al(OH)3, Al13(OH)4 5+, and Al13(OH)32 7+. In the present study, in pH 4, the predominant species were Al3+ and Al(OH)3, which were present in equal amounts. However, at pH 4.5 and above, the predominant species is only Al(OH)3, which can be soluble, insoluble, or colloidal (Domınguez et al. 1998). Also in the acidic aqueous solution, H2O2 generate the maximum amount of ·OH and oxidize the organic compounds. This result is in agreement with the results obtained in other studies (Farhadi et al. 2012; Zazo et al. 2005).
Effect of current density on the performance of process
One of the most important parameters that can affect the pollutant removal or destruction efficiency in the electrochemical process is the current density. In this study, the current density mode was employed; therefore, the effect of the applied current density on the COD, polyphenols, color, and turbidity removal was investigated, as depicted in Fig. 2b. It was found that the removal of COD, polyphones, color, and turbidity was increased with increasing current density. When the current density was raised from 5 to 40 mA/cm2, the removal efficiencies of COD, polyphenols, color, and turbidity rose from 48.8, 60.1, 75.3 and 58.3 to 88.7, 89.9, 84.8 and 77.9 %, respectively. Current density was calculated as the applied current divided by the surface area of the studied electrode. According to Faraday’s law, the amount of aluminum dissolved electrochemically is proportional to the charge loadings. When 1 F (26.8 Ah) passes through the electric circuit, the hydrogen gas evolution will be equal to 0.0224 Nm3, which is much greater than the volume of gas released in traditional DAF. Consequently, increasing current density will give rise to an increase removal of pollutants. Furthermore, with increasing current density, the rate of bubble-production increases and the bubble size decreases; both of these trends are beneficial in terms of high pollutant-removal efficiency by H2 floatation.
Effect of Cl− addition in the performance of process
Figure 2c shows the effect of addition of Cl− ions as NaCl into the solution on the removal efficiencies of COD, polyphenols, color, and turbidity. The removal efficiencies were greatly enhanced by adding 1 g/L NaCl to the solution, obtaining removal efficiencies of 89.5 % of COD, 91.8 % of polyphenols, 84.7 % of color, and 80.2 % of turbidity. Table salt is usually used to increase the conductivity of the wastewater to be treated. In addition to its ionic contribution in carrying the electric charge, it was found that chloride ions could significantly reduce the adverse effect of other anions such as HCO3− and SO4 2− (Chen 2004). The addition of NaCl, which increases conductivity of the solution, would lead to the decrease in power consumption. In general, it is obvious that as the concentration of Cl− ion decreases, the COD removal also proportionately decreased. This implies that the oxidation of organics depends on active chlorine generated during electrolysis. In the presence of chloride ion, the strong catalytic effect of Cl− ion contributes to conversion of organic pollutants to CO2 and H2O (Serikawa et al. 2000). Also, the indirect electrooxidation involving various forms of chlorine was a predominant process in removing organic pollutants from chloride medium. The anodic reaction in electrocoagulation process may be discharge of chlorine as
At 25 °C and normal atmospheric pressure, the chlorine gas generated from anode can dissolve in water to the amount of 6.413 g/L (Lange 1973). If its solubility is exceeded nearby at the electrode surface, then bubbles may form and release. Above pH 3.3, the chlorine can migrate and diffuse to the bulk aqueous solution away from the electrode surface and establishes equilibrium between chlorine, hypochlorous acid, and hypochlorite ion. However, the removal efficiency decreases when extra NaCl is added to the solution. This demonstrates that surplus amount of Cl− in the solution is unfavorable to the coagulation of the pollutants. The main reason is that the Cl− ions in the solution containing Al(OH)3 forms some transitory compounds, such as Al(OH)2Cl, Al(OH)Cl2, and AlCl3. The transitory compounds finally dissolve in the solution with excess Cl−, as a form of AlCl4 − (Wang et al. 2009). Therefore, the amount of Al(OH)3 coagulants decreases, resulting in the decrease of the removal efficiency.
Effect of hydrogen peroxide (H2O2) on the performance of process
Figure 3a shows the effect of hydrogen peroxide dosage on the removal efficiencies of COD, polyphenols, color, and turbidity. Increasing H2O2 concentrations improved efficiency of organic matter degradation. Higher H2O2 doses generated more hydroxyl radicals which enhanced the COD removal efficiency. However, excessive amounts of oxidant had no or a slight unfavorable effect on performance possibly due to H2O2 induced radical scavenging (Rivas et al. 2001).
As the results showed, the maximum removal rate of COD, color, and turbidity were 93.2, 89.9, and 86 %, respectively, for optimum concentration of 1000 mg/L H2O2 after 20-min electrolysis time for current density of 40 mA/cm2 and pH 4. Also, the maximum removal rate of polyphenols was 94.6 % for optimum concentration of 2000 mg/L H2O2 after 20-min electrolysis time for current density of 40 mA/cm2 and pH 4. The pollutant removal can be attributed to the fact that the system undergoes reactions concurrently, electrocoagulation and also H2O2 process.
When the concentration of H2O2 increased from 200 to 1000 mg/L, the COD, color, and turbidity removal increased from 81, 77.5, and 77.4 to 93.2, 89.9, and 86 %, respectively. However, when the concentration of H2O2 increased from 1000 to 2000 mg/L, the COD, color, and turbidity removal decreased to 85.6, 87.5, and 83 % respectively, demonstrating that most of organic pollutants in the wastewater could be oxidized by hydroxyl radical. Although the ·OH was formed by H2O2, which could improve the oxidation ability of treatment along with increase of H2O2 concentration, the degressive trend demonstrated that the excessive H2O2 could also consumed ·OH and become the elimination reagent of hydroxyl radical (Hong et al. 2007).
Effect of operating time on the performance of process
The changes in the removal efficiencies of COD, polyphenols, color, and turbidity during the electrolysis experiment ([H2O2] = 1000 mg/L, [NaCl] = 1 g/L, CD = 40 mA/cm2, pH = 4) is shown in Fig. 3b. As shown in this figure, the percentage of pollutant removal depended immediately on the process duration. Therefore, for the 5-min reaction time, 46.5 % of COD and 33.8 % of polyphenols was removed. It can be observed that high removal efficiencies of COD and polyphenols were obtained for the initial stages of the process and after that a continuous decrease in efficiencies during the experiment was occurred. Consequently, in reaction time of 30 min, 96.2 % of COD and 94.3 % of polyphenols were removed.
As shown in Fig. 3b amusingly, concentration of turbidity and color is higher than initial concentration, at 5 min electrolysis, possibly due to the breaking down of large particles and the formation of a large number of smaller particles and colloids, and then decreased, reaching of 88.5 % removal rate in 30 min. Also, color intensity increased in 5 min, and then decreased, reaching of 91.4 % removal rate in 30 min. This may have also been due to the oxidative polymerization of phenols and tannins originally present in the olive mill wastewater that contributed to the increase of color in samples (Khoufi et al. 2007).
These results are in accordance with previous results obtained in other similar studies that performed to treating OMW by electrocoagulation, and in which it was found that reaction time of 25 min were sufficient to remove more than 90 and 95 % of polyphenols and color, respectively (Adhoum and Monser 2004). Also, the results of this study are in accordance with the results of another study, in which 72 and 80 % removal were achieved for polyphenols and color, respectively, by electrocoagulation process after 15-min reaction time (Hanafi et al. 2010).
Effect of optimum conditions on degradation and biodegradability enhancement of OMW
Determination of optimum condition is a crucial task in improving the overall efficiency of the degradation processes. For the determination of appropriate process for OMW treatment, main results of the optimum parameters in treatment of OMW study were summarized in Table 2. These results indicated that in the optimum conditions (H2O2concentration of 1000 mg/L, NaCl concentration of 1 g/L, pH 4, CD 40 mA/cm2, and reaction time of 30 min), peroxi-electrocoagulation lead to a great decrease in the COD, BOD5, polyphenols, color, turbidity, TSS, and oil and grease. As it can be observed, the rate of COD and BOD5 removal reached to 96 and 93.6 % in the initial COD and BOD5 concentrations of 28.5 and 8.3 g/L, respectively. Also, the removal of polyphenols, color, turbidity, TSS, and oil and grease reached to 94.4, 91.4, 88.7, 97, and 91.7 % in the initial polyphenols concentration of 2.15 g/L, color 1530 TCU, turbidity 320 NTU, TSS concentration of 13.8 g/L, and oil and grease concentration of 5.32 g/L, respectively. The reduction in COD, TSS, and oil and grease removal efficiencies were more than other parameters, suggesting that more H2O2 and/or time of electrocoagulation is required to oxidize the intermediates from degradation.
The biodegradability of a wastewater is usually evaluated in terms of the BOD5 to COD ratio (Wu et al. 2008). The biodegradability of raw and treated OMW was investigated at optimum conditions in order to showing the capability of using biological treatment for the post-treatment of OMW effluents. As shown in Table 2, the BOD5 to COD ratio of the OMW after passing the treatment step in peroxi-electrocoagulation reactor (under conditions of H2O2 = 1000 mg/L, NaCl = 1 g/L, pH = 4, CD = 40 mA/cm2, and reaction time = 30 min) increased from 0.29 to 0.46. This points out that the biodegradability of OMW effluent significantly improved after electrochemical treatment means that it was converted to a biodegradable waste. The enhancement of the biodegradability is related to degradation of the polyphenols and aromatic rings, and thus, the conversion of the simple molecules and more degradable intermediates. Since a wastewater having the BOD5 to COD ratio of 0.4 and higher is considered easily biodegradable (Ghezzar et al. 2009), it can be demonstrated from these results that the effluent from peroxi-electrocoagulation reactor in treating OMW is biodegradable enough to be easily post-treated in a biological reactor.
Energy consumption
One of the most important parameters that must be determined to assess a method of wastewater treatment is the operating cost. Electrocoagulation process is an energy intense process and its efficiency is typically evaluated in terms of specific energy consumption (SEC). This is defined as the amount of energy consumed per unit mass of organic load (e.g., COD) removed. Electrical energy consumption was calculated using the Eq. (19):
where E is the electrical energy in Wh, U is the applied voltage (V), I the current in ampere (A) and t EC is the electrocoagulation time (h). The electric energy consumption in the duration of electrocoagulation (kWh/kg COD removal and kWh/kg polyphenol removal) is shown in Fig. 4. As seen, the SEC increased from 2.35 kWh/kg COD removed and 42.93 kWh/kg polyphenol removed at 5 min increased to 6.82 kWh/kg COD removed and 92.33 kWh/kg polyphenol removed, with increasing time of electrocoagulation time up to 30 min.
Conclusions
The main objective of the present study was to evaluate the performance of peroxi-electrocoagulation/electrooxidation-electroflotation process by using bipolar aluminum electrodes for the removal of COD, BOD5, polyphenols, color, turbidity, TSS, and oil and grease from OMW wastewater diluted four times. These wastewater samples have toxic nature and high resistance toward degradation by classical biological processes. The effects of applied current, initial pH, H2O2 concentration, NaCl concentration, and reaction time on the treatment performance were investigated. Experimental results showed that in the optimum conditions (H2O2 = 1000 mg/L, NaCl = 1 g/L, pH = 4, CD = 40 mA/cm2, and reaction time = 30 min), peroxi-electro coagulation/electrooxidation-electroflotation could remove more than 96 % of COD, TSS, and oil and grease, more than 93 % of BOD5 and polyphenols, 88.7 % of turbidity, and 91.4 % of color present in olive mill wastewater due to the in situ electrogeneration of aluminum hydroxide, electrochemical oxidation, reaction with soluble aluminum species, and reaction with hydroxyl radicals. The peroxi-electrocoagulation/electrooxidation-electroflotation process with aluminum electrodes could also significantly improve the biodegradability of the OMW, making possible the post-treatment of the effluent in a bioreactor.
References
Adhoum N, Monser L (2004) Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem Eng Process Process Intensif 43:1281–1287
Ahmadi M, Vahabzadeh F, Bonakdarpour B, Mofarrah E, Mehranian M (2005) Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J Hazard Mater 123:187–195
Akdemir EO, Ozer A (2009) Investigation of two ultrafiltration membranes for treatment of olive oil mill wastewater. Desalination 249:660–666
Al-Malah K, Azzam MO, Abu-Lail NI (2000) Olive mills effluent (OME) wastewater post-treatment using activated clay. Sep Purif Technol 20:225–234
Antropov LI (1972) Theoretical electrochemistry. Mir
Aparicio R, Harwood J (2013) Handbook of olive oil: analysis and properties. Imprint: Springer
Association APH, Association AWW (1976) Standard methods for the examination of water and wastewater
Atanassova D, Kefalas P, Petrakis C, Mantzavinos D, Kalogerakis N, Psillakis E (2005) Sonochemical reduction of the antioxidant activity of olive mill wastewater. Environ Int 31:281–287
Barash A, Ozer K, Adin A, Milstein D, Gasith A (2009) Electroflocculation–constructed wetland hybrid for improved phosphate removal in effluent reuse, 4th Switch Scientific Meeting. Delft, The Netherlands
Bard AJ (2004) Electrogenerated chemiluminescence. CRC Press, US
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41
Crognale S, D’Annibale A, Federici F, Fenice M, Quaratino D, Petruccioli M (2006) Olive oil mill wastewater valorisation by fungi. J Chem Technol Biotechnol 81:1547–1555
Dehghani M, Jaafari J, Alghasi A, Porkar G (2011) Using medium pressure ultraviolet reactor for removing azo dyes in textile wastewater treatment plant. World Appl Sci J 12:797–802
Domınguez J, Botello-Pozos J, López-Ortega A, Ramırez M, Sandoval-Flores G, Rojas-Hernández A (1998) Study of pillar precursors [Ga (III)–Al (III), Ln (III)–Al (III), Zr (IV)] for hydrothermally stable pillared clays. Catal Today 43:69–77
Fadil K, Chahlaoui A, Ouahbi A, Zaid A, Borja R (2003) Aerobic biodegradation and detoxification of wastewaters from the olive oil industry. Int Biodeterior Biodegrad 51:37–41
Farhadi S, Aminzadeh B, Torabian A, Khatibikamal V, Alizadeh Fard M (2012) Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J Hazard Mater 219:35–42
Gao S, Du M, Tian J, Yang J, Yang J, Ma F, Nan J (2010) Effects of chloride ions on electro-coagulation-flotation process with aluminum electrodes for algae removal. J Hazard Mater 182:827–834
Ge J, Qu J, Lei P, Liu H (2004) New bipolar electrocoagulation–electroflotation process for the treatment of laundry wastewater. Sep Purif Technol 36:33–39
Ghezzar M, Abdelmalek F, Belhadj M, Benderdouche N, Addou A (2009) Enhancement of the bleaching and degradation of textile wastewaters by gliding arc discharge plasma in the presence of TiO2/ catalyst. J Hazard Mater 164:1266–1274
Hanafi F, Assobhei O, Mountadar M (2010) Detoxification and discoloration of Moroccan olive mill wastewater by electrocoagulation. J Hazard Mater 174:807–812
Hodaifa G, Ochando-Pulido J, Rodriguez-Vives S, Martinez-Ferez A (2013) Optimization of continuous reactor at pilot scale for olive-oil mill wastewater treatment by Fenton-like process. Chem Eng J 220:117–124
Holt PK, Barton GW, Wark M, Mitchell CA (2002) A quantitative comparison between chemical dosing and electrocoagulation. Colloids Surf A Physicochem Eng Asp 211:233–248
Hong S, Zhang H, Duttweiler CM, Lemley AT (2007) Degradation of methyltertiary-butyl ether (MTBE) by anodic Fenton treatment. J Hazard Mater 144:29–40
Hongve D, Riise G, Kristiansen JF (2004) Increased colour and organic acid concentrations in Norwegian forest lakes and drinking water—a result of increased precipitation? Aquat Sci 66:231–238
Huang C, Dong C, Tang Z (1993) Advanced chemical oxidation: its present role and potential future in hazardous waste treatment. Waste Manag 13:361–377
Inan H, Dimoglo A, Şimşek H, Karpuzcu M (2004) Olive oil mill wastewater treatment by means of electro-coagulation. Sep Purif Technol 36:23–31
Kallel M, Belaid C, Boussahel R, Ksibi M, Montiel A, Elleuch B (2009) Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J Hazard Mater 163:550–554
Kang YW, Cho M-J, Hwang K-Y (1999) Correction of hydrogen peroxide interference on standard chemical oxygen demand test. Water Res 33:1247–1251
Khoufi S, Aloui F, Sayadi S (2006) Treatment of olive oil mill wastewater by combined process electro-Fenton reaction and anaerobic digestion. Water Res 40:2007–2016
Khoufi S, Feki F, Sayadi S (2007) Detoxification of olive mill wastewater by electrocoagulation and sedimentation processes. J Hazard Mater 142:58–67
Kotti M, Dammak N, Ksentini I, Ben Mansour L (2009) Effects of impurities on oxygen transfer rate in the electroflotation process. Indian J Chem Technol 16:513–518
Lange NA (1973) Handbook of chemistry, llth edn. McGraw-Hill Company, UK
Liou M-J, Lu M-C (2007) Catalytic degradation of nitroaromatic explosives with Fenton’s reagent. J Mol Catal A Chem 277:155–163
Miller CM, Valentine RL (1999) Mechanistic studies of surface catalyzed H2O2 decomposition and contaminant degradation in the presence of sand. Water Res 33:2805–2816
Nieto LM, Hodaifa G, Rodríguez S, Giménez JA, Ochando J (2011) Degradation of organic matter in olive-oil mill wastewater through homogeneous Fenton-like reaction. Chem Eng J 173:503–510
Panizza M, Cerisola G (2009) Electro-Fenton degradation of synthetic dyes. Water Res 43:339–344
Paredes C, Bernal M, Cegarra J, Roig A (2002) Bio-degradation of olive mill wastewater sludge by its co-composting with agricultural wastes. Bioresour Technol 85:1–8
Peralta-Hornandez J, Mejia S, Codinez LA, Meas-Vong Y (2005) Fenton and electrochemical approaches for water purification technologies
Picard T, Cathalifaud-Feuillade G, Mazet M, Vandensteendam C (2000) Cathodic dissolution in the electrocoagulation process using aluminium electrodes. J Environ Monit 2:77–80
Plant L, Jeff M (1994) Hydrogen peroxide: a potent force to destroy organics in wastewater. Chemical Engineering. Environ Eng 101:16–20
Rivas FJ, Beltrán FJ, Gimeno O, Frades J (2001) Treatment of olive oil mill wastewater by Fenton’s reagent. J Agric Food Chem 49:1873–1880
Roa-Morales G, Campos-Medina E, Aguilera-Cotero J, Bilyeu B, Barrera-Díaz C (2007) Aluminum electrocoagulation with peroxide applied to wastewater from pasta and cookie processing. Sep Purif Technol 54:124–129
Sarika R, Kalogerakis N, Mantzavinos D (2005) Treatment of olive mill effluents: part II. Complete removal of solids by direct flocculation with poly-electrolytes. Environ Int 31:297–304
Serikawa R, Isaka M, Su Q, Usui T, Nishimura T, Sato H, Hamada S (2000) Wet electrolytic oxidation of organic pollutants in wastewater treatment. J Appl Electrochem 30:875–883
Shen F, Chen X, Gao P, Chen G (2003) Electrochemical removal of fluoride ions from industrial wastewater. Chem Eng Sci 58:987–993
Tekin H, Bilkay O, Ataberk SS, Balta TH, Ceribasi IH, Sanin FD, Dilek FB, Yetis U (2006) Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J Hazard Mater 136:258–265
Turano E, Curcio S, De Paola MG, Calabrò V, Iorio G (2002) An integrated centrifugation–ultrafiltration system in the treatment of olive mill wastewater. J Membr Sci 209:519–531
Wang C-T, Chou W-L, Kuo Y-M (2009) Removal of COD from laundry wastewater by electrocoagulation/electroflotation. J Hazard Mater 164:81–86
Wu J, Doan H, Upreti S (2008) Decolorization of aqueous textile reactive dye by ozone. Chem Eng J 142:156–160
Yilmaz AE, Boncukcuoğlu R, Kocakerim MM (2007) A quantitative comparison between electrocoagulation and chemical coagulation for boron removal from boron-containing solution. J Hazard Mater 149:475–481
Zaroual Z, Azzi M, Saib N, Chaînet E (2006) Contribution to the study of electrocoagulation mechanism in basic textile effluent. J Hazard Mater 131:73–78
Zazo J, Casas J, Mohedano A, Gilarranz M, Rodriguez J (2005) Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environ Sci Technol 39:9295–9302
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Esfandyari, Y., Mahdavi, Y., Seyedsalehi, M. et al. Degradation and biodegradability improvement of the olive mill wastewater by peroxi-electrocoagulation/electrooxidation-electroflotation process with bipolar aluminum electrodes. Environ Sci Pollut Res 22, 6288–6297 (2015). https://doi.org/10.1007/s11356-014-3832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3832-5