Abstract
The electrooxidation process, one of the advanced oxidation processes, is one of the effective treatment processes used in treating various industrial wastewaters. This study investigated the treatment of olive mill wastewater using the electrooxidation process. This study includes the effects of different experimental parameters on chemical oxygen demand and total phenol removal efficiencies in olive mill wastewater. Ti/IrO2/RuO2 mesh plates as anode material and Ti mesh plates as cathode material were used in the study. The effects of stirring rate, dilution factor, pH, type of support electrolyte, the concentration of support electrolyte, and current density on chemical oxygen demand and total phenol removal efficiencies were examined in the experiments using a batch reactor. The study found that the chemical oxygen demand and total phenol removal rates were 96.93% and 100% under optimum conditions, respectively. According to the treatment data obtained, it can be said that olive mill wastewater can be treated by the electrooxidation method and can be proposed as a pretreatment system before entering biological treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Domestic and industrial wastewater are among the main causes of water pollution, one of today’s most critical environmental problems. These wastewaters cause negative consequences for the living things that benefit from the receiving water environment and its environment, as they cause oxygen consumption by biodegrading as well as aesthetic pollution and bottom accumulations in the receiving water environment.
If no measures are taken, one of the industrial establishments that harm the environment is the olive oil industry (Barbera et al., 2013). Liquid (olive mill wastewater, OMW) and solid (pomace) waste originating from the olive oil industry are an important source of pollution in regions where olive production is intensive due to their composition, foul odors, high toxic organic load, and low pH values (Boubaker & Ridha, 2007). Due to the small-scale and scattered structures of the production enterprises, wastewater is directly discharged into the soil and groundwater. When released unconsciously, it damages the environment and the ecosystem it is located (McNamara et al., 2008). For all these reasons, the treatment and disposal of these wastes called OMW and pomace, without polluting the environment, is highly important for olive oil-producing countries.
The wastewaters originating from the olive oil industry and qualified as OMW are very difficult to treat with traditional methods with high organic matter, suspended solids, oil and grease content, and low molecular weight phenolic substances (Nogueira et al., 2016). In many countries worldwide, research on the treatment of OMW is being carried out. Until now, electrocoagulation (Adhoum & Monser, 2004; Ghahrchi et al., 2021; Ün et al., 2006), ultrasound, and advanced oxidation processes (Al-Bsoul et al., 2020; Görmez et al., 2020), photovoltaic electrocoagulation (Elkacmi et al., 2020), membrane processes (Bottino et al., 2020), biological treatment (Kul & Nuhoğlu, 2020; Tufaner, 2020), hybrid processes (Khani et al., 2020), advanced oxidation processes (Ekmekyapar Torun et al., 2020), etc. are used to prevent and reduce the polluting effect of OMW and to develop different treatment technologies. Many studies covering the processes have been completed (Kul et al., 2015). Among these methods, electrooxidation is based on the use of an insoluble anode material such as graphite (Chinarro et al., 2020), Ti/Ta/Pt/Ir (Giannis et al., 2007; Gotsi et al., 2005), Ti/RuO2 (Chatzisymeon et al., 2009a; Panizza & Cerisola, 2006; Papastefanakis et al., 2010; Un et al., 2008), BDD (Chatzisymeon et al., 2009b; Deligiorgis et al., 2008), Ti/Pt (Kul et al., 2015), and Ti/IrO2/Ta2O5 (Scialdone et al., 2009) to oxidize the organic material directly or indirectly. The direct or indirect electrooxidation mechanisms are shown in Fig. 1 (Kul et al., 2015).
In direct electrooxidation, pollutants are adsorbed on the anode surface first, and then an e-transfer takes place from the anode surface (Fıl et al., 2014; Kul et al., 2015). Direct electrooxidation occurs in two different ways, including electrochemical cycle and disintegration, and during the process, two oxide types of active oxygen are produced electrochemically at the anode surface (MOx). The first of these active oxygens is chemically adsorbed active oxygen given in Eq. (1) and is responsible for electrochemical cycles (MOx+1). The other is physically adsorbed oxygen (MOx(·OH)), responsible for electrochemical degradation given in Eq. (2) (Deng & Englehardt, 2007; Fıl et al., 2014; Kul et al., 2015).
where R is organic compounds and n is the amount of OH− adsorbed on the anode surface. Only a certain amount of organic material is broken down during electrochemical cycles, and sequential biological treatment may be required. However, the final product of the electrochemical transformation is CO2, which indicates that the water is fully treated (Fıl et al., 2014; Kul et al., 2015).
During the indirect electrooxidation process, agents such as Cl2, HOCl, OCl−, and H2O2 that affect the oxidation of organic materials are produced at the anode. While the treatment of OMW by electrooxidation is carried out by direct anodic oxidation of pollutants to a certain degree on the anode surface, it can be performed in indirect electrooxidation due to OCl− formed as a result of the anodic reaction of Cl2 present in OMW or added later. The indirect reactions of the electrooxidation process are shown in Eqs. (3)–(9). The OCl− formed in Eq. (7) is a strong oxidizer that can oxidize organic material (Bashir et al., 2014; Deng & Englehardt, 2007; Fıl et al., 2014; Kul et al., 2015).
Anodic reactions;
Solution reactions;
Cathodic reactions;
In this study, where Ti/IrO2/RuO2 screen plates were used as anode material and Ti screen plates as cathode material, the effects of the time, stirring speed, dilution ratio, pH, support electrolyte type, support electrolyte concentration, current density, and temperature parameters were determined on chemical oxygen demand (COD) and total phenol (TP) removal efficiencies.
2 Material and Methods
2.1 Wastewater
The OMW used in the study was obtained from local businesses in Balıkesir/Türkiye, and its characteristic features are shown in Table 1 (Kul et al., 2015).
2.2 Experimental Setup
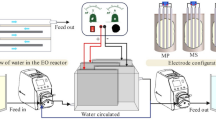
A jacketed glass reactor of 10 cm inner diameter and 16 cm depth was used for the electrooxidation of OMW. 5 Ti/IrO2/RuO2 sieve plates as anode material, and 5 Ti sieve plates as cathode material were connected parallel to each other (Galvano Technical Corporation, Türkiye). The total surface area of the plates was 2600 cm2, and the distance between the plates was set at 0.3 cm. The volume of wastewater used for the experiments was set to 800 mL. An adjustable direct current power supply (Quassar 150 Switch Mode) was used for the experiments. The wastewater was continuously mixed with the help of a magnetic stirrer. For analysis, samples were taken with the help of a peristaltic pump without interrupting the electric current, and pH and conductivity measurements were measured using a WTW ProfiLine 3310 Multimeter at the time of sampling with the help of a multimeter. The experimental system is detailed in Fig. 2 (Kul et al., 2015).
In studies, COD concentrations were determined spectrophotometrically in accordance with standard methods (APHA, 2005). TP concentrations were determined using the Folin Ciocalteu method (Atanassova et al., 2005; Folin & Ciocalteu, 1927; Kul et al., 2014). COD and TP removal efficiencies are calculated according to the equation given in Eq. (10).
where C0 is the initial concentrations of COD and TP (mg L−1), and Ct is the concentrations of COD and TP at any time t (mg L−1).
The energy consumption required for treating olive mill wastewater was calculated using Eq. (11).
where V is mean cell voltage (V), I is current (A), t is electrolysis duration (h), and ν is OMW volume (m3).
3 Results and Discussion
3.1 Effect of Time on COD, TOC, and TP Removal
The variation of wastewater content over time was investigated in the trials conducted with Ti/IrO2/RuO2 plates at 7.69 mA cm−2 current density, pH 4.5–4.7 (natural pH value of OMW), and 20 °C for 24 h. COD, TOC, and TP analyses were made in samples taken at different times, and the results are shown in Fig. 3.
As can be seen in Fig. 3, COD, TOC, and TP removal efficiencies were determined as 22.86%, 21.86%, and 57.37% at the end of the first 5 h, and as 41.57%, 37.77%, and 89.43% at the end of the 24 h, respectively. No change was observed in removal efficiencies after 5 h of contact time when the COD removal efficiencies were examined. Considering this situation, it was decided that the contact time should be 5 h in all other trials using Ti/IrO2/RuO2 plate to keep energy consumption to a minimum. These results showed that the COD value of the OMW was approximately equal to the TOC value, and the COD value decreased the TOC at the same rate. Since phenol is a more easily degradable substance, it has been removed at a high rate in electrooxidation. The phenol content of the wastewater constitutes approximately 5–10% of the COD, as seen in Table 1, and although the phenol removal efficiency was high, the COD and TOC removal remained low.
3.2 Effect of Stirring Speed on COD and TP Removal
During the experiments, the wastewater was continuously mixed with the help of a magnetic stirrer. Stirring speeds were selected as 100, 200, 300, 400, and 600 rpm in the experiments conducted at 7.69 mA cm−2 current density, pH 4.5, and 20 °C, and the removal efficiencies were determined. Obtained results and working conditions are shown in Fig. 4.
As shown in Fig. 4, the increase in stirring speed did not affect the removal efficiencies much, even causing a slight decrease. When the stirring speed was increased from 0 to 300 rpm, the COD removal efficiency decreased from 20 to 18% at the end of the 5 h, while the TP removal efficiency increased from 40 to 51%. When the stirring speed was increased from 300 to 600 rpm, it was observed that COD and TP removal efficiencies decreased by 1% and 7%, respectively. Increasing the stirring speeds accelerates the diffusion of organic pollutants to the anode surface. This causes an increase in removal efficiency for each parameter. However, the excessively increasing stirring speed decreases the diffusion effect on the anode surface and reduces the removal efficiency (Bayar et al., 2011; Kul et al., 2015). However, it was decided not to mix in the following experiments for the treatment of OMW, considering that the changes in removal efficiency are not too much and will reduce system costs. When stirring is not done, the gases formed in the anode and cathode in the reactor allow the reactor contents to be mixed. The turbulent effect of stirring at a very high speed also has a negative impact on natural stirring, as it prevents the gases formed from reaching the reactor surface.
3.3 Effect of Initial Concentration on COD and TP Removal
The experiments investigating the effect of initial concentration were conducted at 7.69 mA cm−2 current density, pH 4.5, and 20 °C without stirring. With the aid of dilution using distilled water, the initial concentrations of COD and TP were adjusted as 10, 20, 30, 40, and 50 g L−1. COD and TP analyses were made by diluting the appropriate amount for the samples taken over time. The data obtained at the end of the 5 h is given in Fig. 5.
As shown in Fig. 5, the decrease in initial concentrations increased the removal efficiencies. When the initial concentration was decreased from 30,000 to 10,000 mg L−1, the COD removal efficiency increased from 25 to 28%, and the TP removal efficiency increased from 41 to 57%. When the initial concentration was 50,000 mg L−1, COD and TP removal efficiencies were 20% and 40%, respectively. The reduction of initial concentrations by dilution reduces the existing organic pollution load and increases the removal efficiencies for COD and TP parameters (Kul et al., 2015; Piya-Areetham et al., 2006). For this reason, in the electrooxidation process using Ti/IrO2/RuO2 anode, it was decided to set the initial concentration as 10,000 mg L−1 in the following experiments. As seen in Fig. 5, it was determined that the wastewater used for TP removal contained approximately 5% TP. TP concentrations after dilution of wastewaters were determined as 500, 1000, 1500, 2000, and 2500 mg L−1 TP for 10,000, 20,000, 30,000, 40,000, and 50,000 mg L−1 COD concentrations, respectively. In this case, almost the same removal efficiencies were obtained at the initial TP of 1500, 2000, and 2500 mg L−1 in TP removal by the electrooxidation method, while the removal efficiency increased at 500 and 1000 mg L−1 TP concentrations. Although the COD removal efficiency is not much affected by the concentration, the increase in TP removal with decreasing concentration indicates that TP decomposition occurs faster than other organic substances.
3.4 Effect of Initial pH of Wastewater on COD and TP Removal
Experiments investigating the pH change were carried out at a current density of 7.69 mA cm−2 and 20 °C. No stirring was done during the experiment, and the initial concentration was adjusted to 10,000 mg L−1 by diluting the wastewater for the experiment. The change of removal efficiencies for Ti/IrO2/RuO2 anodes was investigated by choosing pH values as 2, 4, 4.6 (natural), 6, and 8. HNO3 and NaOH were used for pH adjustment. The COD and TP analyses of the samples taken at different times were performed, and the data obtained at the end of the 5-h experiment period are shown in Fig. 6.
When Fig. 6 is examined, it is seen that the change in the pH values of the wastewater causes some change in the removal efficiency. When the pH value was reduced to 2, COD and TP removal efficiencies were 30% and 100% at the end of the 5-h contact times, respectively. In the experiments conducted at natural pH, COD and TP removal efficiencies were 28% and 57%, respectively. When the pH value was increased to 8, COD and TP removal efficiencies were 17% and 100%, respectively. Although removal efficiencies increased at low pH values, because there was not much difference in removal efficiencies considering the costs of the chemical substance to be used, it was decided not to make pH adjustments in wastewater in other studies (Kul et al., 2015). When Fig. 6 is examined, it can be said that the initial pH of the wastewater does not affect the COD removal much, but the removal is relatively higher at low pH. In addition, pH affects TP removal more than COD. It can be said that TP removal is much higher at low pHs because phenol is an easily reduced substance and that agents such as CI− in the wastewater are oxidized at low pH and cause phenol to break down (Ozturk & Yilmaz, 2019).
3.5 Effect of Type of Supporting Electrolyte on COD and TP Removal
In the experiments where the support electrolyte type was examined, Na2SO4, NaNO3, KCl, and NaCl were selected as the electrolyte type. The experiments were carried out at a current density of 7.69 mA cm−2, the natural pH of the wastewater, and 20 °C. The initial COD concentration of OMW was set at 10,000 mg L−1. The effects of support electrolyte type on removal efficiencies for Ti/IrO2/RuO2 anodes were investigated. The results obtained for COD and TP removal efficiencies at the end of the 5 h of experiments are shown in Fig. 7.
As shown in Fig. 7, different support electrolyte types cause significant changes in removal efficiencies. COD and TP removal efficiencies were 28% and 57% in experiments with no support electrolyte, respectively. The COD and TP removal efficiencies were 25%, 32%, and 24%, 90% in the experiments using Na2SO4 and NaNO3, respectively. Removal efficiencies increased significantly in the experiments where KCl and NaCl were used as supporting electrolyte types. The COD and TP removal efficiencies were 98% and 100% in the experiments that used KCl. The removal efficiencies were 100% for COD and TP in the NaCl experiments (Kul et al., 2015). The main reason NaCl and KCl increase the removal of COD and TP is that the chlorine it contains is oxidized due to the reactions in water seen in Eq. (12) and Eq. (13), thereby reducing the organic matter.
Due to the high conductivity of wastewater, the supporting electrolyte must be used in very high concentrations to reduce energy consumption. For this reason, since the supporting electrolyte used did not change the conductivity of the wastewater much, it did not affect the energy consumption much (Bingül et al., 2021a). Considering the costs, it was decided to use NaCl as the supporting electrolyte in other studies of the electrooxidation process using Ti/IrO2/RuO2 anode since NaCl is cheaper and easily available.
3.6 Effect of Supporting Electrolyte Concentration on COD and TP Removal
In studies, the effect of supporting electrolyte concentration, 0.25 M, 0.5 M, 0.75 M, 1 M, and 1.25 M NaCl, was used as supporting electrolytes. The experiments were carried out at the 7.69 mA cm−2 of current density, pH 4.5, 20 °C, and COD concentration of 10,000 mg L−1. The COD and TP analyses of the samples taken at different times were made, and the data obtained at the end of the 5 h of experiments are shown in Fig. 8.
As seen in Fig. 8, although the increase in the support electrolyte concentration increases the removal efficiency up to a point in the experiments using Ti/IrO2/RuO2 anodes, adding more has no effect on the removal efficiency. TP removal was obtained as 100% for all support electrolyte concentrations in the experiments. When the support electrolyte concentrations of 0.25 M, 0.5 M, and 1.25 M were selected, the COD removal efficiencies were determined as 88%, 97%, and 96%, respectively. As the support electrolyte concentration increases, removal efficiency increases up to a point. After this point, the increase in the support electrolyte concentration does not increase the efficiency but decreases the energy consumption as it increases the conductivity of the wastewater (Kul et al., 2015; Ozturk & Yilmaz, 2019). However, chemical matter removal is also increasing. Therefore, the optimum dose should be selected.
3.7 Effect of Current Density on COD and TP Removal
In the experiments where the initial current density was examined, the electrolyte concentration was set as 0.5 M NaCl, pH 4.5, 20 °C, and wastewater COD concentration as 10,000 mg L−1. Current densities for the trials were selected as 2.5, 5, 7.69, 10, and 15 mA cm−2, respectively. COD and TP analyses of the samples taken at different times were made and the results obtained at the end of the 5 h of experiments are shown in Fig. 9.
As shown in Fig. 9, the increase in the current density in the trials using Ti/IrO2/RuO2 anodes resulted in a clear increase in the removal efficiencies. TP removal reached 100% at all current densities studied. The COD removal efficiencies for 2.5, 7.69, and 15 mA cm−2 were determined as 50%, 97%, and 100%, respectively. Current density is an important parameter for the electrooxidation process. While optimizing electrochemical treatment processes, the current density is one of the first parameters to be optimized. Because all the reactions occur with energy exchange, this optimization is more important in countries with high energy costs. In addition, it is seen that the increase in current intensity increases the reaction rate. While 100% efficiency is reached in 180 min for 15 mA cm−2, this time is around 240 min for 10 mA cm−2 and 300 min for 7.69 mA cm−2. The cost of electrical energy required to achieve 70% efficiency for COD removal is given in Table 2. The electricity cost is assumed to be $0.07318/kWh (Bingül et al., 2021b).
The removal efficiencies did not change much when it was above 7.69 mA cm−2. However, energy costs increased significantly. For this reason, in other studies, it was decided to use a current density of 7.69 mA cm−2 (Kul et al., 2015).
3.8 Effect of Temperature on COD and TP Removal
In the experiments where the effects of temperature were investigated, 0.5 M NaCl was used as the electrolyte concentration. The temperature values for the experiments were chosen as 10, 20, 30, 40, and 50 °C, respectively. In the experiments carried out at the natural pH value of the wastewater, a constant temperature liquid circulator was used to ensure that the temperature remained at the desired value during the 5 h of experiments. There was no mixing during the experiment, and the initial concentration was set to 10,000 mg L−1, and COD and TP were made in the samples taken. The experimental conditions and the removal efficiencies obtained at the end of the 5 h are shown in Fig. 10.
As shown in Fig. 10, in the experiments using Ti/IrO2/RuO2 anodes, the increase in temperature did not affect the removal efficiency much, even causing a slight decrease. TP removal was obtained 100% in all experiments at different temperatures. COD removals are realized as 89%, 97%, and 78% for 10, 20, and 50 °C, respectively. When the COD removal efficiencies are compared, the maximum removal efficiency has been obtained at 20 °C. It is recommended to perform the processes at this temperature in the electrooxidation process using Ti/rO2/RuO2 anode (Kul et al., 2015).
3.9 Energy Consumption Under Optimum Conditions
The experiments investigating the effect of temperature changes on COD and TP removal efficiencies were carried out at the 7.69 mA cm−2 of current density, pH 4.5 (natural), 10,000 mg L−1 of wastewater COD concentration, and supporting electrolyte 0.5 M NaCl. The energy consumption values obtained using Eq. (11) with the help of the data obtained from these experiments using the Ti/IrO2/RuO2 anode are shown in Fig. 11.
As shown in Fig. 11, the lowest energy consumption in studies using Ti/IrO2/RuO2 anode was 396.25 kWh m−3 at 10 °C. When the temperature was increased to 20 °C, the energy consumption was 462.5 kWh m−3. Energy consumptions were 538.75, 593.75, and 692.5 kWh m−3 at 30 °C, 40 °C, and 50 °C, respectively.
4 Conclusions
This study used the electrooxidation process to examine the treatability of organic pollutants from OMW obtained from a local olive oil production facility in Balıkesir. The effects of system parameters on removal efficiencies were determined. The performance of the system, in which all experiments were carried out under batch conditions, was calculated by measuring COD and TP parameters. In the electrooxidation process using Ti/IrO2/RuO2 anode, stirring speed, dilution factor, pH, support electrolyte concentration, support electrolyte type, current density, and temperature were selected as system parameters.
In the experiments using Ti/IrO2/RuO2 anode plates, the stirring speed was chosen as 0, 200, 300, 400, and 600 rpm. Since the increase in stirring speed up to a certain value accelerates the diffusion of organic pollutants to the anode surface, the removal efficiencies slightly increased. Still, the excessively increased stirring speed reduced the diffusion effect on the anode surface and reduced the removal efficiency somewhat. It can be concluded that there is no need for stirring in electrochemical systems where Ti/IrO2/RuO2 anode plates are used because the removal efficiencies at different stirring speeds show similar results and the additional costs of stirring.
The decrease in initial wastewater COD concentrations causes a reduction in the organic pollution load in OMW and a noticeable increase in removal efficiencies. The results show that treating OMW and domestic wastewater with a low pollution load is possible. In the experiments made in the different wastewater pHs, although removal efficiencies have increased at low pH values, it can be seen that pH adjustment is not necessary for electrooxidation processes using Ti/IrO2/RuO2 as an anode and there are not many differences in removal efficiencies.
According to the results obtained in the experiments in which the effect of the support electrolyte type was examined, it was determined that the COD and TP removal efficiencies increased significantly in the electrooxidation processes using NaCl and KCl. It is possible to conclude that using NaCl from these salt types may be more reasonable due to its cost. At different concentrations of NaCl, the results show that 0.5 M NaCl is the most suitable supporting electrolyte concentration.
In the electrooxidation study using Ti/IrO2/RuO2 anodes, the optimum conditions are obtained as stirring speed of 0 rpm, wastewater COD concentration of 10,000 mg L−1, pH 4.6 (natural), electrolyte concentration of 0.5 M NaCl, current density 7.69 MA cm−2, and temperature 20 °C. At these optimum conditions, removal efficiencies were determined as 97% and 100% for COD and TP, respectively. The energy consumption value under optimum conditions was calculated as 462.5 kWh m−3.
The electrooxidation method can be used in treating this wastewater, characterized as OMW, and originates from the olive oil industry, which is difficult to treat with traditional methods due to the high amounts of organic matter, suspended solids, and color and low molecular weight phenolic substances. The space requirement of the electrooxidation process is low and easy to operate. For this reason, it seems possible that OMW can be treated with the electrooxidation process, and the treated wastewater can be given to the municipal sewerage network.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Adhoum, N., & Monser, L. (2004). Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chemical Engineering and Processing: Process Intensification, 43(10), 1281–1287. https://doi.org/10.1016/j.cep.2003.12.001
Al-Bsoul, A., Al-Shannag, M., Tawalbeh, M., Al-Taani, A. A., Lafi, W. K., Al-Othman, A., & Alsheyab, M. (2020). Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Science of the Total Environment, 700, 134576. https://doi.org/10.1016/j.scitotenv.2019.134576
APHA. (2005). Standard methods for the examination of water and wastewater. American Public Health Association
Atanassova, D., Kefalas, P., Petrakis, C., Mantzavinos, D., Kalogerakis, N., & Psillakis, E. (2005). Sonochemical reduction of the antioxidant activity of olive mill wastewater. Environment International, 31(2), 281–287. https://doi.org/10.1016/j.envint.2004.10.004
Barbera, A., Maucieri, C., Cavallaro, V., Ioppolo, A., & Spagna, G. (2013). Effects of spreading olive mill wastewater on soil properties and crops, a review. Agricultural Water Management, 119, 43–53. https://doi.org/10.1016/j.agwat.2012.12.009
Bashir, M. J. K., Lim, J. W., Aziz, S. Q., & Amr, S. S. A. (2014). Electrochemical methods: Electrochemical oxidation process contribution in remediating complicated wastewaters. In H. A. Aziz & A. Mojiri (Eds.), Wastewater engineering: Advanced wastewater treatment systems (pp. 96–106). IJSR Publications.
Bayar, S., Yıldız, Y. Ş., Yılmaz, A. E., & İrdemez, Ş. (2011). The effect of stirring speed and current density on removal efficiency of poultry slaughterhouse wastewater by electrocoagulation method. Desalination, 280(1–3), 103–107. https://doi.org/10.1016/j.desal.2011.06.061
Bingül, Z., İrdemez, Ş, Kul, S., Ekmekyapar Torun, F., & Demircioğlu, N. (2021a). Investigation of organic and inorganic matters removal from tannery wastewater using iron plate electrode by electrocoagulation process. International Journal of Environmental Analytical Chemistry. https://doi.org/10.1080/03067319.2021.1953002
Bingül, Z., İrdemez, Ş, & Demircioğlu, N. (2021b). Effect of controlled and uncontrolled pH on tannery wastewater treatment by the electrocoagulation process. International Journal of Environmental Analytical Chemistry. https://doi.org/10.1080/03067319.2021.1925261
Bottino, A., Capannelli, G., Comite, A., Costa, C., Firpo, R., Jezowska, A., & Pagliero, M. (2020). Treatment of olive mill wastewater through integrated pressure-driven membrane processes. Membranes, 10(11), 334. https://doi.org/10.3390/membranes10110334
Boubaker, F., & Ridha, B. C. (2007). Anaerobic co-digestion of olive mill wastewater with olive mill solid waste in a tubular digester at mesophilic temperature. Bioresource Technology, 98(4), 769–774. https://doi.org/10.1016/j.biortech.2006.04.020
Chatzisymeon, E., Dimou, A., Mantzavinos, D., & Katsaounis, A. (2009). Electrochemical oxidation of model compounds and olive mill wastewater over DSA electrodes: 1. The case of Ti/IrO2 anode. Journal of Hazardous Materials, 167(1–3), 268–274. https://doi.org/10.1016/j.jhazmat.2008.12.117
Chatzisymeon, E., Xekoukoulotakis, N. P., Diamadopoulos, E., Katsaounis, A., & Mantzavinos, D. (2009). Boron-doped diamond anodic treatment of olive mill wastewaters: Statistical analysis, kinetic modeling and biodegradability. Water Research, 43(16), 3999–4009. https://doi.org/10.1016/j.watres.2009.04.007
Chinarro, E., Pérez Orosa, L., García-Alegre, M., & Guinea, D. (2020). Graphite electrodes for hydrogen generation in alkali electrolysis assisted by an organic waste water compound: Olive mill wastewater. Grupo Español Del Carbón, 55, 8–12.
Deligiorgis, A., Xekoukoulotakis, N. P., Diamadopoulos, E., & Mantzavinos, D. (2008). Electrochemical oxidation of table olive processing wastewater over boron-doped diamond electrodes: Treatment optimization by factorial design. Water Research, 42(4–5), 1229–1237. https://doi.org/10.1016/j.watres.2007.09.014
Deng, Y., & Englehardt, J. D. (2007). Electrochemical oxidation for landfill leachate treatment. Waste Management, 27(3), 380–388. https://doi.org/10.1016/j.wasman.2006.02.004
Ekmekyapar Torun, F., Cengiz, İ, & Kul, S. (2020). Zeytin Karasuyunun İleri Oksidasyon Prosesleri İle Arıtımının İncelenmesi. Journal of the Institute of Science and Technology, 10(3), 1597–1606. https://doi.org/10.21597/jist.687345
Elkacmi, R., Boudouch, O., Hasib, A., Bouzaid, M., & Bennajah, M. (2020). Photovoltaic electrocoagulation treatment of olive mill wastewater using an external-loop airlift reactor. Sustainable Chemistry and Pharmacy, 17, 100274. https://doi.org/10.1016/j.scp.2020.100274
Fıl, B. A., Boncukcuoğlu, R., Yilmaz, A. E., & Bayar, S. (2014). Electro-oxidation of pistachio processing industry wastewater using graphite anode. Clean-Soil Air Water, 42(9), 1232–1238. https://doi.org/10.1002/clen.201300560
Folin, O., & Ciocalteu, V. (1927). On tyrosine and tryptophane determinations in proteins. Journal of Biological Chemistry, 73(2), 627–650. https://doi.org/10.1016/S0021-9258(18)84277-6
Ghahrchi, M., Rezaee, A., & Adibzadeh, A. (2021). Study of kinetic models of olive oil mill wastewater treatment using electrocoagulation process. Desalination and Water Treatment, 211, 123–130. https://doi.org/10.5004/dwt.2021.26516
Giannis, A., Kalaitzakis, M., & Diamadopoulos, E. (2007). Electrochemical treatment of olive mill wastewater. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology, 82(7), 663–671. https://doi.org/10.1002/jctb.1725
Görmez, F., Görmez, Ö., Yabalak, E., & Gözmen, B. (2020). Application of the central composite design to mineralization of olive mill wastewater by the electro/FeII/persulfate oxidation method. SN Applied Sciences, 2(178), 1–11. https://doi.org/10.1007/s42452-020-1986-y
Gotsi, M., Kalogerakis, N., Psillakis, E., Samaras, P., & Mantzavinos, D. (2005). Electrochemical oxidation of olive oil mill wastewaters. Water Research, 39(17), 4177–4187. https://doi.org/10.1016/j.watres.2005.07.037
Khani, M. R., Mahdizadeh, H., Kannan, K., Kalankesh, L. R., Kamarehei, B., Baneshi, M. M., & Shahamat, Y. D. (2020). Olive mill wastewater (OMW) treatment by hybrid processes of electrocoagulation/catalytic ozonation and biodegradation. Environmental Engineering & Management Journal (EEMJ), 19(8), 1401–1410.
Kul, S., Boncukcuoğlu, R., Yilmaz, A. E., & Fil, B. A. (2015). Treatment of olive mill wastewater with electro-oxidation method. Journal of the Electrochemical Society, 162(8), G41.
Kul, S., & Nuhoğlu, A. (2020). Removal kinetics of olive-mill wastewater in a batch-operated aerobic bioreactor. Journal of Environmental Engineering, 146(3), 04019122.
Kul, S., Nuhoğlu, A., & Değermenci, N. (2014). Zeytin Karasuyunun Respirometrik Analizi. Journal of the Institute of Science and Technology, 4(3), 35–40.
McNamara, C. J., Anastasiou, C. C., O’Flaherty, V., & Mitchell, R. (2008). Bioremediation of olive mill wastewater. International Biodeterioration & Biodegradation, 61(2), 127–134. https://doi.org/10.1016/j.ibiod.2007.11.003
Nogueira, V., Lopes, I., Rocha-Santos, T., Gonçalves, F., Duarte, A., & Pereira, R. (2016). Photocatalytic treatment of olive oil mill wastewater using TiO2 and Fe2O3 nanomaterials. Water, Air, & Soil Pollution, 227, 88. https://doi.org/10.1007/s11270-016-2787-1
Ozturk, D., & Yilmaz, A. E. (2019). Treatment of slaughterhouse wastewater with the electrochemical oxidation process: Role of operating parameters on treatment efficiency and energy consumption. Journal of Water Process Engineering, 31, 100834.
Panizza, M., & Cerisola, G. (2006). Olive mill wastewater treatment by anodic oxidation with parallel plate electrodes. Water Research, 40(6), 1179–1184. https://doi.org/10.1016/j.watres.2006.01.020
Papastefanakis, N., Mantzavinos, D., & Katsaounis, A. (2010). DSA electrochemical treatment of olive mill wastewater on Ti/RuO2 anode. Journal of Applied Electrochemistry, 40(4), 729–737. https://doi.org/10.1007/s10800-009-0050-9
Piya-Areetham, P., Shenchunthichai, K., & Hunsom, M. (2006). Application of electrooxidation process for treating concentrated wastewater from distillery industry with a voluminous electrode. Water Research, 40(15), 2857–2864. https://doi.org/10.1016/j.watres.2006.05.025
Scialdone, O., Randazzo, S., Galia, A., & Filardo, G. (2009). Electrochemical oxidation of organics at metal oxide electrodes: The incineration of oxalic acid at IrO2–Ta2O5 (DSA-O2) anode. Electrochimica Acta, 54(4), 1210–1217. https://doi.org/10.1016/j.electacta.2008.08.064
Tufaner, F. (2020). Zeytin Karasuyunun Anaerobik Arıtılabilirliği ve Biyogaz Üretim Potansiyelinin Araştırılması. Bitlis Eren Üniversitesi Fen Bilimleri Dergisi, 9(4), 1766–1778. https://doi.org/10.17798/bitlisfen.676940
Un, U. T., Altay, U., Koparal, A. S., & Ogutveren, U. B. (2008). Complete treatment of olive mill wastewaters by electrooxidation. Chemical Engineering Journal, 139(3), 445–452. https://doi.org/10.1016/j.cej.2007.08.009
Ün, Ü. T., Uğur, S., Koparal, A., & Öğütveren, Ü. B. (2006). Electrocoagulation of olive mill wastewaters. Separation and Purification Technology, 52(1), 136–141. https://doi.org/10.1016/j.seppur.2006.03.029
Acknowledgements
The authors are grateful for Atatürk University Department of Environmental Engineering’s laboratory support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Sinan Kul, Recep Boncukcuoğlu, Fatma Ekmekyapar Torun, Züleyha Reçber, Onur Sözüdoğru, and Erdinç Aladağ. Sinan Kul wrote the first draft of the manuscript and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kul, S., Boncukcuoğlu, R., Ekmekyapar Torun, F. et al. Investigation of the Treatment of Olive Mill Wastewater by Electrooxidation. Water Air Soil Pollut 233, 421 (2022). https://doi.org/10.1007/s11270-022-05894-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05894-1