Abstract
Profenofos (PF) is one of the heavily used organophosphorus pesticides (OPPs) of which its contamination is ubiquitous in an agricultural area. This study aims to acquire and characterize PF-degrading bacterial cultures from contaminated soil. OPP degradation by the novel isolates was then investigated. The experiment was performed at the initial PF concentration of 20 mg/L. The result showed that the enriched consortium comprised three predominant PF-degrading strains designated as PF1, PF2, and PF3. The isolates (PF1, PF2, and PF3) were characterized as Pseudomonas plecoglossicida, Pseudomonas aeruginosa, and P. aeruginosa, respectively. A consortium and all isolates could utilize PF as a sole carbon source with PF removal of more than 90 % via a hydrolysis process. The bacterial growth and PF degradation rates followed the first-order kinetic reaction with the rates of 0.4 to 2.7/h and 0.15 to 1.96/h, respectively. Additional carbon supplement deteriorated PF biodegradation. The enriched cultures were also capable for degrading chlorpyrifos and dicrotophos pesticides (33–73 % removal). The results indicated that the consortium and isolates are efficient for PF and other OPP degradation and have potential for PF remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticide (OPP) has been developed for agricultural purpose for years. One third of pesticides used globally belong to OPP because they are efficient and inexpensive (Kanekar et al. 2004; Singh and Walker 2006; Cycon et al. 2009). This results in OPP contamination in environmental media including soil, groundwater, and surface water (Roverdatti 2001; Kanekar et al. 2004). Among pesticides under OPP, profenofos (O-4-bromo-2-chlorophenyl O-ethyl S-propyl phosphorothioate), C11H15BrClO3PS, is one of OPPs broadly used in many countries, such as Thailand, Vietnam, and India (Swarnam and Velmurugan 2013; Toan et al. 2013). Profenofos (PF) is normally used for pest control in cotton, fruit, chili, and vegetable cultivation. Intensive use of PF leads to its contamination in the environment.
Bioremediation is one of the effective environmental treatment technologies (Siripattanakul et al. 2009). The technique has been successfully applied for OPP remediation (Xu et al. 2008; Cycon et al. 2009). The technique involved enrichment of pesticide-degrading microbial cultures and the utilization of the enriched cultures for removing pesticides later on. So far, there are only a few publications on enrichment and isolation of PF-degrading bacteria such as the enrichment of the degrading cultures (Malghani and Chatterjee 2009; Malghani et al. 2009; Salunkhe et al. 2013). Also, there is limited information on PF biodegradation kinetics and potential biodegradation pathway.
This study aimed to enrich and identify indigenous PF-degrading cultures from heavily PF-contaminated soil. Degradation of PF by an enriched consortium and predominant isolated strains was subsequently performed. The possibility of additional carbon supplement on PF degradation was carried out. This work also determined a potential PF degradation pathway using detection of degradative intermediate metabolites. The PF-enriched cultures could later on be applied for treatment of PF-contaminated sites. The basic knowledge of the degradation kinetics and pathway could be used to ensure the optimal treatment operation.
Materials and methods
Chemicals
Commercial-grade OPPs including Profenofos 500EC (50 % w/v EC, Syngenta Crop Protection Co., Bangkok, Thailand) were used in the degradation assay experiment. PF, chlorpyrifos (CF), and dicrotophos (DP) (analytical standard grade, Supelco, Sigma Chemical Co., Singapore) were used for OPP analysis. All other chemicals for bacterial medium preparation and OPP analysis were of analytical and HPLC grades, respectively, purchased from local chemical distributers.
Enrichment and isolation of PF-degrading cultures
PF-degrading bacteria were enriched from PF-contaminated chili farm soil (Ubon Ratchathani, Thailand). The culture enrichment and isolation procedures were as follows. Air-dried soil sample (20 g) from the site was mixed in the PF-containing basal salt medium (100 mL), incubated on a rotary shaker at 100 rpm and 30 ± 2 °C for 2 weeks. The soil suspension (10 mL) was used as the inoculum and inoculated into the PF-containing fresh medium (100 mL). Then, the re-cultivation was conducted for four times repeatedly. The enriched consortium was subcultured into the PF-containing medium for every 2 weeks to obtain a stable PF-degrading consortium. The enriched consortium was purified by spreading and streaking-plate techniques. All plates were incubated at 30 ± 2 °C for 14 days. Single colonies were obtained after several streaking-plate cultivations.
Formulation of PF-containing basal salt medium included KH2PO4 of 3.0 g/L, NH4Cl of 1 g/L, NaCl of 0.5 g/L, MgSO4 of 0.25 g/L, and PF of 20 mg/L (in 10 mM of phosphate buffer solution, pH 7.0). All chemicals except PF were sterilized by autoclave, while PF was filtered sterilely. Agar of 2.0 % (w/v) was added in agar medium preparation.
Identification of PF-degrading isolates
Colony and cell morphology of the isolated cultures were preliminarily observed using traditional microbiological methods. The isolates were classified by a biochemical test (API identification system) by the Bioscience Department, Thailand Institute of Scientific and Technology Research (TISTR), and 16S ribosomal RNA (rRNA) sequence analysis. Each bacterial culture was cultivated overnight. The genomic DNA from each culture was extracted using a standard boiling method. The 16S rRNA gene fragment was amplified from the genomic DNA by the polymerase chain reaction (PCR) using the bacterium-specific primers: a 63f-forward primer (5′CAGGCCTAACACATGCAAGTC3′) and a 1,387r-reverse primer (5′GGGCGGWGTGTACAAGGC3′) (Marchesi et al. 1998). A 25-μL PCR reaction mixture was prepared according to the manufacturer’s protocol (Fermentas, USA).
The 16S rRNA amplification was performed in a thermal cycler (PerkinElmer model-2400, USA) with the following conditions: 94 °C (3 min), followed by 30 cycles of 95 °C (1 min), 55 °C (1 min), and 72 °C (1.5 min), with a final extension of 72 °C (5 min). The PCR product was then cloned into pGEM-T Easy vector (Promega, USA) and transformed into competent Escherichia coli DH5α cells. The plasmid DNA was then isolated using the QIAprep Spin Miniprep kit (Qiagen, the Netherlands) and sequenced (Macrogen, Korea). The partial 16S rRNA gene sequence of the isolates was analyzed using the nucleotide BLAST (BLASTN) algorithm of the National Center for Biotechnology Information (NCBI).
PF biodegradation assay
Duplicate batch experiments of PF biodegradation by the consortium and the isolated cultures were conducted. The experiments with and without additional carbon supplement were performed. The consortium and isolates were shaken in the 200-mL medium with the initial PF concentration of 20 mg/L on a rotary shaker at 100 rpm and 30 ± 2 °C for 4 days. For the experiment with additional carbon sources, sodium succinate (C4H4O4Na2 · 6H2O), sodium acetate (C2H2O2Na), and glucose (C6H12O6 · H2O) of 500 mg carbon per liter were added. The consortium was chosen for the experiment with additional carbon supplement. PF concentration and cell number (viable plate count) measurements were performed daily. Abiotic control test (without bacterial cultures) was also performed. The PF residual was analyzed using a gas chromatograph with a mass selective detector (GC-MSD). The bacterial growth and PF degradation kinetic rates were then calculated.
The PF biodegradation assays at PF concentrations of 20–120 mg/L were conducted to identify PF intermediates and the potential PF degradation pathway. The experiment was performed similarly to an earlier assay with different PF concentrations. The PF degradation intermediate peak was monitored using GC-MSD along with PF detection.
Biodegradation assay of selected OPPs
Degradation assay of other selected OPPs was performed. CF and DP which were the insecticides widely used in vegetable cultivation were chosen. This is because these pesticides possibly co-contaminate in the same area. The isolates were inoculated in a liquid medium at 20 mg/L of the insecticides on a rotary shaker at 100 rpm and 30 ± 2 °C for 6 days.
PF, PF intermediate metabolite, and other OPP analysis
PF concentration was extracted using a Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) extraction technique. The sample of 10 mL was placed into a 50-mL disposable polypropylene centrifuge tube with 10 mL of acetonitrile and acetic acid of 0.1 % mixture. The centrifuge tube was capped and shaken for 1 min. After that, NaCl of 1 g and anhydrous MgSO4 of 4 g were added. The tube was then shaken vigorously for 1 min and centrifuged at 5,000 rpm for 5 min. The supernatant (acetonitrile extract) was transferred into a solid-phase extraction (SPE) tube (QuEChERS D-SPE, Agilent, USA) to clean up the residue. The tube was capped and mixed in a vortex mixer for 1 min and then centrifuged at 5,000 rpm for 3 min. The cleaned sample was transferred to a GC vial.
PF concentration was measured using a GC-MSD (Agilent 6890 N, Agilent, USA) with a DB-5 column (30.0-m length, 0.25-mm internal diameter (i.d.), and 0.25-μm film thickness). One microliter of the cleaned sample was injected into GC-MSD. The GC conditions were splitless injection, injection port temperature of 220 °C, and helium gas (carrier gas) flow of 2 mL/min. The GC temperature program was started at 90 °C, increased to 220 °C at a rate of 20 °C/min and retained for 1 min, increased to 280 °C at a rate of 10 °C 1/min and retained for 3 min, and increased to 300 °C. PF peak was detected at 9.07 min. Degradation intermediate peak was monitored along with PF detection.
For CF and DP analysis, the samples were extracted using the method for PF analysis as mentioned in the earlier paragraph. The samples were measured using a gas chromatograph (Agilent 6890 N series) with a flame photometric detector and a DB-1701 column (30.0-m length, 0.25-mm i.d., and 0.25-μm film thickness). One microliter of the cleaned sample was injected to the GC. The GC conditions were splitless injection, injection port temperature of 220 °C, and helium gas (carrier gas) flow of 0.75 mL/min. The GC temperature program was started at 80 °C and retained for 1 min, increased to 195 °C at a rate of 12 °C/min, increased to 210 °C at a rate of 2 °C/min and retained for 3 min, increased to 225 °C at a rate of 15 °C/min and retained for 2 min, and increased to 275 °C at a rate of 40 °C/min and retained for 10 min. The peak retention times of DP and CF were 11.7 and 18.0 min, respectively.
Results and discussion
Enrichment, isolation, and identification of PF-degrading bacteria
The PF-degrading consortium (referred to as MIX hereafter) was enriched in aerobic condition at pH of 7 and temperature of 30 °C. The consortium plated onto the medium agar comprised numerous types of bacterial colonies. The soil used in this study was rich in PF-tolerant cultures. During the isolation process, only three isolates were well and continuously grown in PF-containing medium. This indicates that the pure isolates were PF-degrading bacteria (namely PF1, PF2, and PF3).
All isolates are short rod with negative in gram staining. Colony form and elevation of all isolates are circular and raised. Colony margin of PF1 and PF3 is entire, while the margin of PF2 is undulate. The difference of colony sizes was also found. The colony sizes of PF1, PF2, and PF3 on basal salt medium agar with PF of 20 mg/L at 24 h are 2.0, 1.5, and 1.0 mm, respectively. Biochemical characteristics of the three isolates were listed in Table 1. The biochemical test of the isolates PF2 and PF3 showed 99.9 % characteristics of Pseudomonas aeruginosa, while the test result was a species belong to the Pseudomonas genus for the isolate PF1.
Therefore, they were then identified by 16S rRNA analysis. PF1 was then identified by 16S rRNA analysis as Pseudomonas plecoglossicida (Genetic Sequence Data Bank (GenBank) accession number KJ143902) with similarity of 99 % to P. plecoglossicida strain SR7 (GenBank accession number KC634234), P. plecoglossicida strain TCCC11291 (GenBank accession number FJ393302), and P. plecoglossicida strain C-B8A (GenBank accession number KJ806458). PF2 was characterized as P. aeruginosa (GenBank accession number KJ143903) with similarity of 100 % to P. aeruginosa strain LS6 (GenBank accession number KJ620776), P. aeruginosa strain RP28 (GenBank accession number KJ631608), and P. aeruginosa strain PAE 03 (GenBank accession number KJ612071). For PF3, the isolate was identified as P. aeruginosa (GenBank accession number KJ143904) with similarity of 99 % to P. aeruginosa strain PAE 03 (GenBank accession number KJ612071), P. aeruginosa strain RP28 (GenBank accession number KJ631608), and P. aeruginosa strain R20 (GenBank accession number KJ631606).
The consortium and three isolates reported in this work could utilize PF as a sole carbon source. This is similar to the previous studies which reported the OPP utilization as a sole carbon source (Cycon et al. 2009). Previously, Malghani and Chatterjee (2009) isolated P. aeruginosa strain OW from the PF-contaminated soil in China. Also, Fulekar and Geetha (2008) reported that P. aeruginosa could remove CF. In present study, two more strains that belong to P. aeruginosa (PF2 and PF3) isolated from the contaminated chili farm soil in Thailand were proposed for PF degradation. The result suggested that P. aeruginosa may conserve OPP-degrading microbial enzymes resulting in the widely discovered P. aeruginosa strains able to degrade OPPs. On the other hand, although the role of P. plecoglossicida as a pyrethroid pesticide-degrading bacterium has been previously reported (Boricha and Fulekar 2009), there is no published report on OPP biodegradation by this microbial species. To the best of our knowledge, this is the first finding on OPP degradation by P. plecoglossicida (strain PF1).
Bacterial growth and PF degradation
The growth of MIX, PF1, PF2, and PF3 measured by viable cell counting on basal salt medium agar containing PF is shown in Fig. 1. While the growth of MIX reached the stationary phase after 2 days and increased from 7.1 to 7.6 log CFU/mL after 4 days, the cell number of each isolate could quickly increase to 14.0–16.5 log CFU/mL within 4 days. Based on the growth kinetic rates presented in Table 2, the pure isolates (at the growth rates of 1.9–2.7/h) perforated approximately five to six times higher than MIX (0.4/h).
This result indicates that even though both consortium and isolated cultures were able to survive and reproduce in the medium containing PF, the growth of each isolate was enhanced when grown independently, the pure isolates likely to better survive in the PF-contaminated environment. This may be due to the competition between the cultures in the consortium. This is similar to the work by Salunkhe et al. (2013). The rapid growth rate of pure cultures (Bacillus subtilis strains) isolated from grapevines or grape rhizosphere during profenofos biodegradation was reported.
The reduction of PF concentration during the biodegradation test is shown in Fig. 1. For all tests, the PF concentration continuously decreased in the first 3 days, and the concentration dropped gradually thereafter. After the 4-day experiment, MIX, PF1, PF2, and PF3 removed PF for 90.0–95.3 % (Table 2). In the abiotic control test (no cell), the PF concentration decreased less than 5.0 % suggesting that PF removal by physical or chemical reactions was minimal. The PF degradation by all cultures followed the first-order kinetics (Table 2). The consortium and isolates utilized PF at the rate of 3.0–19.6 mg/L/day (Table 2). Apparently, the pure isolates performed better than the consortium. The treatment result correlated well to the bacterial growth result. MIX grew slower resulting in lower PF treatment rate compared to those by the pure isolates.
The PF degradations by the consortium and cultures (more than 90 %) were comparable to those by P. aeruginosa strain OW, Pseudomonas putida strain W, and Burkholderia gladioli strain Y, previously isolated (Malghani and Chatterjee 2009; Malghani et al. 2009). The result indicated that the newly enriched consortium and three isolated cultures have potential application for the PF bioremediation. Generally, the consortium has been shown to be more suitable for bioremediation compared to pure cultures in practice. This is because their biodiversity can enhance environmental survival and increase the number of catabolic pathways available for contaminant biodegradation (Alvey and Crowley 1996; Smith et al. 2005). In this case, however, the pure cultures were acclimated in the medium and no environmental stresses in the tested condition leading to better growth and performance presented by the isolated cultures.
Bacterial growth and PF degradation with additional carbon supplement
The experiment in this section was to determine the potential of additional carbon sources for the enhancement of PF biodegradation. Three additional carbon sources including sodium succinate, sodium acetate, and glucose were chosen (Xie et al. 2009).
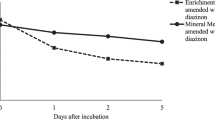
Microbial cell numbers quickly rose from 6.8 to 18.7 log CFU/mL after 48 h (Fig. 2). Microbial cell growth followed the first kinetic reaction at the rates of 5.97, 4.38, and 5.26/h with the supplementation of sodium succinate, sodium acetate, and glucose, respectively. The result suggested that the additional carbon source obviously accelerated the growth rate (10–14 times). This result related well to previous works (Jianlong et al. 2002; Grant and Betts 2004; Xie et al. 2009). The test with sodium succinate and glucose supplements had a higher growth rate than that of the test with sodium acetate. Additional carbon sources affected the growth rates to different extents since carbon structure was specific to each microorganism (Xie et al. 2009).
PF degradation by MIX under the presence of additional carbon sources is presented in Fig. 2. After the 48-h tests, the PF degradation was only 10–30 %. The result in this study indicated that the additional carbon sources partially inhibited PF biodegradation. MIX preferred to use the additional carbons for cell metabolism. Moreover, it was noticed that the different carbon sources affected PF degradation differently. The test with glucose had the lowest PF degradation performance (approximately 10 %). Some bacterial strains preferred short-chain fatty acids including sodium succinate and sodium acetate for xenobiotic co-metabolism rather than simple carbohydrate (glucose) (Xie et al. 2009). It could be said that the enriched cultures in this study could use PF for their metabolism. However, PF is a toxic substance. When additional non-toxic carbon is present, the enriched cultures switched to use the non-toxic carbon as a substrate. Otherwise, the result presented in this subsection was conducted using MIX. To have a better clarification of additional carbon source influence, the experiments using PF1, PF2, and PF3 separately were recommended for future investigation.
PF intermediate metabolite detection
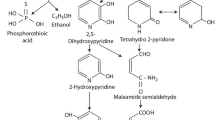
In the control (no cell) test, an intermediate product of PF, 4-bromo-2-chlorophenol, (BCP) (Fig. 3) was detected while PF concentration slightly decreased (less than 5 %). This indicated that PF reacted with water and produced BCP via an abiotic hydrolysis process. After continued monitoring for 14 days, BCP concentration detected was quite stable (data not shown).
For PF biodegradation assay, the PF intermediate metabolite detection from tests at the initial PF concentrations of 20–120 mg/L was performed. From the test with the initial PF concentration of 20 mg/L, BCP (which was presented in the microbial medium as mentioned in the earlier section) disappeared along with PF biodegradation. This revealed that the enriched cultures could simultaneously degrade PF and BCP.
During the PF degradation at the concentration of 40 mg/L and higher, BCP was accumulated in the early stage of the test (the first 3 h). In the later period (after 6 h), it was noticed that 3-methoxy phenol (3-MP) appeared and replaced BCP. The structure of 3-MP is presented in Fig. 3. This potentially can be that BCP degraded to 3-MP (Fig. 4). Additionally, 2,4-di-tert-butyl phenol (2,4-DTBP) during the degradation test was found. It was known that 2,4-DTBP could be used for pesticide production (Schwetlick et al. 1991).
For PF degradation intermediate detection, BCP production via an abiotic hydrolysis process was well correlated to the study by Zamy et al. (2004). It was reported that OPPs including PF could hydrolyze in dilute OPP solution. The result suggested that abiotic hydrolysis slightly contributes to PF degradation (less than 5 %). Since BCP is more stable than PF, BCP concentration remained constant for a longer period. During the PF degradation, BCP was accumulated similar to the result described elsewhere (Malghani et al. 2009). The result suggested that PF degraded via enzymatic organophosphorus hydrolysis. Organophosphorus hydrolase has been known as a typical bacterial enzyme for a wide range of organophosphorus pesticide degradation (Kanekar et al. 2004). The appearance of 3-MP is different from the proposed PF degradation in soil by the Food and Agriculture Organization of the United Nations (FAO) (FAO 2012). FAO expected that BCP potentially degraded to 3-chloro-4-methoxyphenol in a sterile soil (Fig. 4). Currently, no complete PF biodegradation pathway was published. There were degradation pathways of some organophosphorus pesticides, such as CF and DP (Singh and Walker 2006). Compared to CF which is a phosphothioate pesticide (liked PF), a similar trend of degradation pathway was found. CF is initially hydrolyzed at a phosphorus compound branch to 3,5,6-trichloropyridinol (TCP), and TCP is subsequently broken at the chlorine branch to be chlorodihydro-2 pyridone (Singh and Walker 2006). In this study, it was found that PF is primarily hydrolyzed at the phosphorus compound branch to BCP, and BCP is further broken at the chlorine and bromine branches to 3-MP. This could state that abiotic and biotic processes may give different PF degradation pathways. The cultures enriched in this study were promising for PF degradation since they could degrade both PF and its intermediate product (BCP). The potential PF degradation pathway was proposed (Fig. 4). The complete PF biodegradation pathway is recommended for future investigation.
Selected OPP degradation
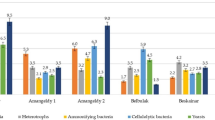
In practice, farmers apply a couple types of pesticides simultaneously for better pest control. For instance, in Thailand, farmers concurrently apply PF, CF, and DP for vegetable cultivation. Based on this information, degradation of CF and DP by the enriched cultures was performed for investigating the treatment potential. The degradation result is presented in Fig. 5. After the 4-day experiment, the degradation performance by PF1, PF2, and PF3 was similar. When the microbial cells of 106 CFU/mL and the initial pesticide concentration of 20 mg/L were tested, PF, CF, and DP reduced to 75–82 %, 57–73 %, and 33–47 %, respectively (Fig. 5).
Among three OPPs tested, PF and CF structure is similar. They are chemicals under the phosphorothioate group, while DP is a chemical belonging to the phosphate group (Fig. 6). PF is an S-alkyl phosphorothioate subgroup, whereas CF is an O-alkyl phosphorothioate subgroup. It was observed that the pesticide degradation performances were different. This may be from the pesticide structure. PF degradation performance is highest because the cultures were acclimated for PF. The chemical structure of CF is more similar to PF compared to that of DP resulting in high CF performance.
From an enzymatic degradation point of view, four enzymes including phosphatase, esterase, hydrolase and oxygenese were involved in the OPP biodegradation pathway (Kanekar et al. 2004). Singh and Walker (2006) summarized CF and DP biodegradation pathways. Like PF, CF primarily degrades via hydrolysis process. DP degradation is more complicated. DP transforms to monocrotophos via methylation and continuously degrades via hydrolysis process (Singh and Walker 2006). This could be the reason why DP was degraded less than PF and CF. However, based on the result in this section, the enriched cultures were a potential for PF and other OPP remediation. For future study, the influence of environmental conditions on PF degradation should be performed. Also, the full PF degradation pathway by isolates should be conducted for insight investigation.
Conclusions
Novel PF-degrading consortium and three isolated cultures including P. plecoglossicida strain PF1, P. aeruginosa strain PF2, and P. aeruginosa strain PF3 were enriched. The newly isolated PF-degrading bacteria utilized PF as a sole carbon source. All cultures were capable of PF insecticide degradation with PF removal of more than 90 % within 4 days. The cultures could degrade PF, PF intermediate, and other OPPs. For future study, the influence of environmental conditions on PF degradation should be performed. Also, the full PF degradation pathway by the isolates should be conducted for insight investigation.
References
Alvey S, Crowley DE (1996) Survival and activity of an atrazine-mineralizing bacterial consortium in rhizosphere soil. Environ Sci Technol 30:1596–1603
Boricha H, Fulekar MH (2009) Pseudomonas plecoglossicida as a novel organism for the bioremediation of cypermethrin. Biol Med 1:1–10
Cycon M, Wojcik M, Piotrowska-Seget Z (2009) Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Psesudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere 76:494–501
FAO (2012) Profenofos 171. http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation08/Profenofos.pdf
Fulekar MH, Geetha M (2008) Bioremediation of chlorpyrifos by Pseudomonas aeruginosa using scale up technique. J Appl Biosci 12:657–660
Grant RJ, Betts WB (2004) Mineral and carbon usage of two synthetic pyrethroid degrading bacterial isolates. J Appl Microbiol 97:656–662
Jianlong W, Xiangchun Q, Liping H, Yi Q, Hegemann W (2002) Kinetics of co-metabolism of quinoline and glucose by Burkholderia pickettii. Process Biochem 37:831–836
Kanekar PP, Bhadbhade BJ, Deshpande NM, Sarnaik SS (2004) Biodegradation of organophosphorus pesticides. Proc Indian Nat Sci Acad B 70:57–70
Malghani S, Chatterjee N (2009) Isolation and characterization of a PF degrading bacterium. J Environ Sci 21:1591–1597
Malghani S, Chatterjee N, Yu HX, Luo Z (2009) Isolation and identification of profenofos degrading bacteria. Braz J Microbiol 40:893–900
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Dymock D, Wade WG (1998) Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799
Roverdatti MG (2001) Monitoring of organochlrine and organophosphorus pesticdes in the water of the Reconquista River. Water Res 35:3457–3461
Salunkhe VP, Sawant IS, Banerjee K, Rajguru YR, Wadkar PN, Oulkar DP, Naik DG, Sawant SD (2013) Biodegradation of profenofos by Bacillus subtilis isolated from grapevines (Vitis vinifera). J Agric Food Chem 61:7195–7202
Schwetlick K, Pionteck J, Winkler A, Hfihner U, Kroschwitz H, Habicher WD (1991) Organophosphorus antioxidants: part X mechanism of antioxidant action of aryl phosphites and phosphonites at higher temperatures. Polym Degrad Stab 31:219–228
Singh BK, Walker A (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30:428–471
Siripattanakul S, Wirojanagud W, McEvoy JM, Limpiyakorn T, Khan E (2009) Atrazine degradation by stable mixed cultures enriched from agricultural soil and their characterization. J Appl Microbiol 106:986–992
Smith D, Alvey S, Crowley DE (2005) Cooperative catabolic pathways within an atrazine-degrading enrichment culture isolated from soil. FEMS Microbiol Ecol 53:265–273
Swarnam TP, Velmurugan A (2013) Pesticide residues in vegetable samples from the Andaman Islands, India. Environ Monit Assess 185:6119–6127
Toan PV, Sebesvari Z, Bläsing M, Rosendahl I, Renaud FG (2013) Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Sci Total Environ 452–453:28–39
Xie S, Liu J, Li L, Qiao C (2009) Biodegradation of malathion by Acinetobacter johnsonii MA19 and optimization of cometabolism substrates. J Environ Sci 21:76–82
Xu G, Zheng W, Li Y, Wang S, Zhang J, Yan Y (2008) Biodegradation of chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol by a newly isolated Paracoccus sp. strain TRP. Int Biodeterior Biodegrad 62:51–56
Zamy C, Mazellier P, Legube B (2004) Phototransformation of selected organophosphorus pesticides indilute aqueous solutions. Water Res 38:2305–2314
Acknowledgments
This material is based upon work supported by Ubon Ratchathani University, Office of the National Research Council of Thailand, and Farm Engineering and Automatic Control Technology Research Group, Khon Kaen University. This work is also conducted under a research program granted by the Center of Excellence on Hazardous Substance Management.
Conflict of interest
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of grant providers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Siripattanakul-Ratpukdi, S., Vangnai, A.S., Sangthean, P. et al. Profenofos insecticide degradation by novel microbial consortium and isolates enriched from contaminated chili farm soil. Environ Sci Pollut Res 22, 320–328 (2015). https://doi.org/10.1007/s11356-014-3354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3354-1