Abstract
Use of animal manure is a main source of veterinary pharmaceuticals (VPs) in soil and groundwater through a series of migration processes. The sorption–desorption and transport of four commonly used VPs including trimethoprim (TMP), sulfapyridine, sulfameter, and sulfadimethoxine were investigated in three soil layers taken from an agricultural field in Chongming Island China and two types of aqueous solution (0.01 M CaCl2 solution and wastewater treatment plant effluent). Results from sorption–desorption experiments showed that the sorption behavior of selected VPs conformed to the Freundlich isotherm equation. TMP exhibited higher distribution coefficients (K d = 6.73–9.21) than other sulfonamides (K d = 0.03–0.47), indicating a much stronger adsorption capacity of TMP. The percentage of desorption for TMP in a range of 8–12 % is not so high to be considered significant. Low pH (<pK a of tested VPs) and rich soil organic matter (e.g., 0–20 cm soil sample) had a positive impact on sorption of VPs. Slightly lower distribution coefficients were obtained for VPs in wastewater treatment plant (WWTP) effluent, which suggested that dissolved organic matter might affect their sorption behavior. Column studies indicated that the transport of VPs in the soil column was mainly influenced by sorption capacity. The weakly adsorbed sulfonamides had a high recovery rate (63.6–98.0 %) in the leachate, while the recovery rate of TMP was only 4.2–10.4 %. The sulfonamides and TMP exhibited stronger retaining capacity in 20–80 cm and 0–20 cm soil samples, respectively. The transport of VPs was slightly higher in the columns leached by WWTP effluent than by CaCl2 solution (0.01 M) due to their sorption interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of the pharmaceutical industry, veterinary pharmaceuticals (VPs) play a huge role in livestock production. However, the abuse of a large number of VPs has led to continuous discharge of these substances into the groundwater system via the soil, surface water, and other environments (Watanabe et al. 2008). The contamination of groundwater by VPs is growing in complexity due to continuous migration, although natural attenuation is effective for many VPs (Lapworth et al. 2012; Sacher et al. 2001). Lincomycin, sulfamethazine, sulfamethoxazole, and other VPs were found in 37 groundwater samples at the nanogram-per-liter level (Batt et al. 2006; Díaz-Cruz et al. 2008). There are multiple pathways that VPs enter into the groundwater (Lapworth et al. 2012). Animal manure widely used in agricultural field is a major source of VPs in groundwater (Clarke and Smith 2011). Veterinary pharmaceuticals, which enter the animals by means of feed and treatment, are only partially digested and adsorbed, and then the rest is excreted through the feces and urine.
It is reported that more than half of the antibiotics used in animals are excreted through the feces in the form of the parent drug (Addison 1984). Kroker (1983) demonstrated that more than 90 % of the pharmaceuticals would be discharged with animal feces and urine. Residual drugs in manure are redistributed in soil and groundwater by a series of migration processes. The persistence of VPs in the soil mainly depends on their stability, solid-phase binding capacity and solubility, etc. (Halling-Sørensen et al. 1998; Jjemba 2002). The environmental characteristics, soil texture, and physicochemical properties of VPs have impacts on their fate in the subsurface environment. Erythromycin and tylosin can be adsorbed onto many soil types, but their sorption is mainly related to temperature (Jjemba 2002). Thiele-Bruhn et al. (2004) documented a weak adsorption of five sulfonamides on mineral soil and high desorption from a soil clay fraction. Solubility, the octanol–water partition coefficient (K ow), and the n-octanol–water distribution ratio (D ow) of VPs play a role in their environmental behavior (Sedlak 2011; Wells 2006). In addition, dissolved organic matter (DOM) in water environment (e.g., wastewater effluent) can affect the adsorption and migration of VPs in soils (Boxall et al. 2002; Lertpaitoonpan et al. 2009). Laboratory studies on four pharmaceuticals (Rabølle and Spliid 2000) showed that weakly adsorbed metronidazole and olaquindox were more easily transported through soil columns than strongly adsorbed tylosin and oxytetracycline. Unold et al. (2009) investigated the transport of sulfadiazine in soil columns by isotope tracer and found that most of the 14C was detected near the soil surface. Therefore, further studies are needed to illustrate the underlying mechanisms for VPs sorption behavior in soil.
In this study, four VPs including trimethoprim (TMP), sulfapyridine (SPD), sulfameter (SMT), and sulfadimethoxine (SDM) were selected for the determination of the sorption, desorption, and transport processes in three depths of an agricultural soil using two types of aqueous solutions (0.01 M CaCl2 solution and waste water treatment plant effluent) to evaluate the effect of soil type and DOM. To better understand the sorption of selected VPs in soils, the influence of pH was also investigated.
Materials and methods
Chemicals
TMP, SPD, SMT, and SDM were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade acetonitrile and acetic acid were purchased from Merck (Darmstadt, Germany). Other chemicals used in this study were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). The water for the experiment was deionized water. The chemical and physical properties of the studied pharmaceuticals are summarized in Table 1. Stock solutions of the pharmaceuticals were prepared in acetonitrile and then stored at 4 °C in darkness.
Soil samples and wastewater treatment plant (WWTP) effluent
Soil samples were collected at depths of 0–20 cm, 20–80 cm, and 80–100 cm using soil auger in a typical agricultural area of Chongming Island, China. Soil samples were placed in a cool, dry, and ventilated room without special air (25 °C) to air-dry, and coarse debris was removed. The air-dried soil was passed through a 2-mm sieve and then stored at 4 °C in dark until use. Major properties of the soil samples are presented in Table 2.
The WWTP effluent was collected from a municipal WWTP located in Chongming Island, China. Wastewater samples were filtered through a 0.45-μm filter before use and stored at 4 °C. The properties of WWTP effluent are summarized in Table 3.
Batch sorption–desorption tests
During all sorption–desorption tests, the solid/water ratio was maintained at 1:2.5 (w/v). Two grams of soil (dry weight) and 4 mL 0.01 M CaCl2 solution or WWTP effluent were added in a 50-mL Teflon centrifuge tube. For the determination of the effect of pH on sorption of VPs in soils, 0.1 M KOH or 1.0 M HCl solutions was added to soil samples to adjust pH values of 4, 5, 6, 7, 8, 9, and 10. All samples were agitated on a horizontal auto-shaker at 200 rpm at 20 °C in the dark for 24 h. Then the soil slurries were spiked with 1 mL VPs stock solutions (0.5–50 mg L−1) to yield initial concentrations of 0.1, 0.5, 1, 5, and 10 mg L−1. The samples were shaken under the same conditions for 48 h to reach adsorption equilibrium. After shaking, the samples were centrifuged at 4,000 rpm for 15 min (16,000×g).
Samples were then shaken again under the same condition for 72-h rotor speed and radius to carry out desorption and centrifuged for 15 min (16,000×g) to obtain the desorption supernatant.. During sorption and desorption experiments, all supernatants were filtered through a 0.22-μm glass fiber filter for VPs analysis. Samples were performed in triplicate. Blank samples without the addition of the VPs and control samples without VPs and with no soil were conducted in order to determine either the possible release of VPs from the soil or their possible sorption onto the glass walls of the tubes.
Column study
The column studies were carried out in a Plexiglass column with a length of 25 cm and an inner diameter of 3 cm. The bottom of the column had two pieces of gauze (aperture size 0. 1 mm) and a layer of glass beads (diameter size 5 mm). The air-dried soil samples from a given soil layer were packed into the column, and the depth of soil was controlled in 20 cm. After the column was filled with solid phase, the soil columns were immersed in a beaker filled with water until the columns were saturated. The liquid was infiltrated into the soil via capillary action to saturate the soil and remove the air from the soil. Before the leaching tests, the VPs stock solution (2 mL, 50 mg L−1) was homogeneously spiked on the top of the soil in the column. Then the soil was covered with a layer of glass beads and then two pieces of gauze. One liter of 0.01 M CaCl2 solution or WWTP effluent was pumped down from the top of the column at a low rate of 0.5 mL min−1 by a peristaltic pump. Leachate was collected in 20-mL fractions. All leachate samples collected were filtered through a 0.22-μm glass fiber filter before VPs analysis. The soil columns were cut into 4-cm fractions after freezing for extraction and analysis.
Wastewater and soil samples extraction
The four VPs in wastewater sample were extracted from 500-mL water samples (pH = 5.5) by solid-phase extraction (SPE) using Oasis HLB cartridges (3 mL, 60 mg). Before the sample loading, the solid-phase adsorbents were sequentially pre-conditioned with 3 × 2 mL methanol and 3 × 2 mL deionized water (pH = 5.5). After the sample loading, the adsorbents were washed with 10 mL deionized water (pH = 5.5) and then dried for about 30 min. The VPs were eluted with 6 mL methanol. The eluent was evaporated to near dryness under a gentle nitrogen stream, and finally 1 mL of 0.4 % acetic acid/water (v/v) was added for VPs analysis.
The residual VPs in soil samples were extracted by ultrasonic solvent extraction and then SPE using Oasis HLB cartridges (3 mL, 60 mg) with the same extraction procedure for wastewater samples. Briefly, 5 g crushed soil was extracted with 10 mL of pH 4.0 methanol/water mixture (v/v = 1:1) by shaking and ultrasonication for 30 min. The slurries were centrifuged at 4,000 rpm for 10 min (16,000×g). The above procedure was repeated twice and the supernatants were combined and evaporated to about 10 mL. Then the extracts were diluted with deionized water to a volume of 100 mL for SPE.
Quantitative determination of VPs
The four VPs were determined by a Hitachi L-2000 (Hitachi Ltd, Kokubunji, Tokyo, Japan) HPLC equipped with a UV detector. The detection wavelength was 268 nm and the oven temperature was set at 33 °C. The LC column was Shimadzu Shim-pack VP-ODS C18 (250 mm × 4.6 mm, 5 μm). Analyses were performed at a flow rate of 1 mL min−1, with a sample injection volume of 100 μL. The mobile phases used were pure acetonitrile and 0.4 % acetic acid. The elution started with 4 % acetonitrile and then with a linear gradient from 4 to 30 % acetonitrile over the course of 5 min and to 35 % over the next 4 min. Then the gradient program linearly increased to 45 % acetonitrile in 2 min and finally returned to the initial conditions within 2 min. Before the next injection, the system was allowed to equilibrate for 7 min. The limits of detection were 50 μg L−1 for TMP and 10 μg L−1 for other VPs.
The precision of the analytical method was validated by observing the short-term and long-term relative standard deviation (RSD) under identical conditions. The RSD for intra- and inter-day precision were 1.4–6.3 % and 2.2–4.6 %. For each batch of six samples, a procedural blank, a spiked blank, a matrix spiking sample, and a matrix spiking duplicate were processed. No quantifiable analytes were detected in the blank samples. The recoveries of VPs were 97.7 ± 3.2 % for water samples and 84.5 ± 5.3 % for soil samples.
Data analyses
The sorption–desorption data of VPs were fitted by linear (Henry) sorption model and the Freundlich sorption model.
Linear (Henry) sorption model:
Where C s (mg kg−1) is the concentration of VPs sorbed in soil and C e (mg L−1) is the equilibrium concentration of VPs in solution. K d (L kg−1) is the linear distribution coefficient.
Freundlich sorption model:
Where K d (mg1−1/n L1/n kg−1) is the Freundlich sorption coefficient. The value of 1/n in the Freundlich model is close to 1, indicating linear adsorption mechanism.
The Freundlich sorption coefficient K f was calculated by Eq. (3).
The organic carbon-normalized sorption coefficient (K oc, L kg−1) was calculated according to Eq. (4).
Where f oc is the weight fraction of soil organic carbon.
Results and discussion
Sorption–desorption experiments
The concentrations of VPs sorbed in the soil (C s) increased with increasing equilibrium concentration in the solution (Fig. 1), indicating no obvious natural decomposition under the experimental conditions. The VPs absorbed to the soil (C s) increased with the increase of their concentration in equilibrium solution (C w) (Fig. 1). In the sorption experiments, the C w of sulfonamide ranged from 0.08 to 9.85 mg L−1, and C s ranged from 0.05 to 3.30 mg kg−1 in the tested soils. C w and C s of TMP were in the range of 0.03–2.68 mg L−1 and 0.16–19.75 mg kg−1, respectively. Other studies of sulfonamides in soils have shown that the linear (Henry) and the Freundlich adsorption isotherms were fitted better to the sorption data than the Langmuir isotherm (Boxall et al. 2002; Thiele-Bruhn 2003; Thiele-Bruhn et al. 2004), thus linear distribution coefficient (K d) and Freundlich coefficient (K f) are presented in Table 4. The values of 1/n were close to 1.0 for most of the soil samples, indicating that the linear relationship was slightly better than the nonlinear model in describing the sorption of VPs in soil at low concentrations. Three sulfonamides were found to be more weakly adsorbed onto all soil samples than TMP, which indicates a higher mobility of sulfonamides. The K d values ranged from 0.03 to 0.47 for sulfonamides and from 6.73 to 9.21 for TMP. Compared with our data, other studies (Boxall et al. 2002; Díaz-Cruz et al. 2003; Thiele-Bruhn et al. 2004; Tolls 2001) reported higher sulfonamide K d values ranging from 0.62 to 10. In addition, Gao and Pedersen (2005) found different sulfonamide K d values up to 22.2 in clay minerals. Generally, adsorption of compounds to soil is divided into physical and chemical. These absorption processes are attributable to intermolecular forces (e.g., Van der Waals force, dispersion force, and hydrogen bonging) and the formation of chelate or complex (Durán-Álvarez et al. 2012). TMP exhibited significantly stronger sorption capacity (one-way ANOVA, p < 0.01) than sulfonamides due to their different molecular structures (Table 1).
To compare the results obtained from different soil layers, K oc was calculated by using the percent organic carbon (Table 2) and K d (Table 4). All compounds showed higher K oc, but lower K d in the soil at 80–100 cm depth than those at other depths. The lower sorption affinity of compounds to the 80–100 cm soil may be due to its lower organic matter content relative to the upper-layer soil (Table 2). Chiou et al. (1983) proposed that hydrophobic organic substances were first adsorbed to soil organic matter (SOM). SOM consequently has a positive impact on sorption affinity of compounds. Another research (Chefetz et al. 2000) reported that the nature of SOM in the deeper soil layers is hydrophobic due to its relatively abundant aromatic and alkyl moieties. Thus, there may be a competitive sorption on VPs between hydrophilic organic substances and hydrophobic SOM. As a result, VPs exhibit stronger sorption capacity to SOM originated from the upper-layer soil.
K d values obtained from the test with WWTP effluent were slightly lower than those with 0.01 M CaCl2 solution, indicating that the tested compounds were adsorbed less onto soils in WWTP effluent. This may be due to presence of dissolved organic matter (DOM, 12.6 mg L−1) in WWTP effluent. Some previous studies (Kan and Tomson 1990; Seol and Lee 2000; Thiele-Bruhn 2003; Totsche and Kögel-Knabner 2004) reported that the DOM in WWTP effluent can affect sorption behavior of organic substances to soils because of competitive sorption or through the formation of complexes between VPs and DOM.
Since the sorption capacity of sulfonamides in the tested soils was less than 30 %, only studies of TMP desorption were carried out. Desorption isotherms of TMP in three soils in two solutions are presented in Fig. 1. The values of linear desorption distribution coefficient (K des) ranged from 12.5 to 15.0, and the desorption efficiency ranged from 8.0 to 12.4 %. According to the K des values of the different soil layers in the tested liquid, the adsorbed TMP on soils was more likely to be released from the 20–80 cm soil in WWTP effluent (K des = 12.7) and the 80–100 cm soil samples in 0.01 M CaCl2 solution (K des = 12.49), but less easily released from the 80–100 cm soil in WWTP effluent (K des = 14.56) and the 20–80 cm soil samples in 0.01 M CaCl2 solution (K des = 15.01). Based on the tested VPs sorption–desorption studies, the sorption tendency of VPs in soils can be predicted. Generally, VPs can be strongly adsorbed to soils, indicating a high stability and a low mobility in the soil environment. Therefore, there is a lower risk of these compounds to move to groundwater.
Effect of pH on sorption
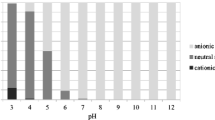
Since the pK a values of the selected VPs are often within the soil pH range, the VPs may deprotonate at high pH and protonate at low pH. The sorption affinity of these compounds is strongly affected by the pH of aqueous solution. The K d of the VPs were determined under different pH conditions using soil from the 0–20 cm layer (Fig. 2). The K d values of TMP increased with decreasing pH, similar to the result reported by Bekçi et al. (2006). Based on its pK a, TMP was in the organic cation form in acidic system (low pH) and neutral form in the neutral or basic system (pH ≥ 7). The highest sorption affinity of TMP was obtained in the range of pH 4 to 6. The dominant neutral form had little attraction for the electronegative soil surface above pH 6. Additionally, Bekçi et al. (2006) reported that protonated TMP has a competitive sorption with the hydrogen ions below pH 4.
Based on the K d values of three sulfonamides against pH, SPD exhibited stronger sorption at pH between 4 and 8, and subsequently decreased at pH greater than 8. The highest K d values of SMT and SDM were 0.27 and 0.48 at pH 6, respectively. Gao and Pedersen (2005) reported that sulfonamides have two ionizable functional groups (the anilinic amine and the amide moieties) with the change of soil pH. At pH value near the pK a of three sulfonamides (Table 1), the sulfonamides show more sorption to soil. This result was similar to other studies reported by Boxall et al. (2002) and Ter Laak et al. (2006). Because sulfonamides exist mostly as anions at high pH, sorption affinity is negatively impacted by the electrostatic repulsion between the negatively charged minerals and anionic sulfonamides, while cationic sulfonamides at low pH have an attraction to the negatively charged minerals surface through the electrostatic interactions (Gao and Pedersen 2005; Warisara et al. 2009).
Column experiments
Laboratory leaching experiments were performed to investigate the transport behavior of VPs in the soil samples using both 0.01 M CaCl2 solution and WWTP effluent. The soil columns were leached for about 4 days. Breakthrough curves of the compounds with 0.01 M CaCl2 solution and WWTP effluent in three columns are presented in Fig. 3. The results showed that the VPs were not detected or the VPs concentrations were very low in the early leaching, and then the VPs concentration gradually increased with an increase of pore volumes. At pore volumes of about 1–4, the concentrations of VPs reached a maximum and then gradually decreased. Almost no VPs were detected in leachate at about 12 pore volumes.
Obvious difference in the migration among veterinary drugs was observed in the same soil column. As shown in Fig. 3, the maximum TMP concentration of 0.12 mg L−1 in the leachate was significantly lower than that of the other three pharmaceuticals with SPD, SMT, and SDM of 0.47 mg L−1, 0.81 mg L−1, and 0.84 mg L−1, respectively (p < 0.05). The order of four VPs according to their leaching quantity was SMT > SDM > SPD > TMP. Leaching rates of the three sulfonamides (64–98 %) were different from those of TMP (4.2–10.4 %). Due to low sorption, sulfonamides were highly mobile in the soil columns, suggesting that they might be leached to groundwater. However, most of TMP was adsorbed and intercepted by soil. VPs residues extracted from soil fractions of 0–20 cm soil columns with 0.01 M CaCl2 and WWTP effluent are shown in Table 5. As shown in Table 5, TMP can be detected in almost all soil fractions and its maximal amounts were up to 26.8–32.8 μg in 8–12 cm soil fractions, while the sulfonamides were not found below the depth of 12 cm. The total residues for four VPs were in the order of TMP > SPD > SDM > SMT, which were in accordance with their sorption behavior.
The breakthrough curves obtained for the compounds were significantly different among three soil columns (one-way ANOVA, p < 0.05). Mobility of sulfonamides was the highest in the soil layer of 80–100 cm with leaching efficiency of 68–98 % of the total amount applied, while 10–37 % of the applied sulfonamides were retained in the soil layer of 20–80 cm. Previous studies reported that SOM content plays an important role in mobility for soils, but the results of mobility in various soils at different soil layers were not similar to their sorption capacities which mainly depend on SOM (Migliore et al. 1995). Soil porosity and other properties may also impact on VPs mobility. Further studies are needed to determine factors affecting VPs mobility in soil. In three soil columns, the transport behavior of TMP was different from the tested sulfonamides, which may result from different leaching speeds. Compared with the soil layers of 80–100 cm and 0–20 cm, TMP exhibited the highest mobility (10 %) in the soil layer of 20–80 cm, which could lead more TMP to migrate into groundwater.
The amount of VPs eluted from the soil layer of 0–20 cm in WWTP effluent was slightly higher than in 0.01 M CaCl2 solution (Fig. 3). The result is mainly attributed to lower sorption capacity in the presence of WWTP effluent. DOM from WWTP effluent may be competitively sorbed or the form of complexes between VPs and DOM. VPs residues extracted from the different soil layers presented in Table 5 also exhibited the enhanced transport with WWTP effluent. However, transport behavior of VPs seems to be more complicated due to varied natural conditions, hydraulic conditions (saturated and unsaturated), etc. in a natural subsurface environment. Therefore, more research on other factors affecting VPs transport is needed to comprehensively assess the fate of these compounds in the soil environment.
Conclusions
The results of this study showed that sorption–desorption and transport behaviors of VPs were closely related to properties of the compound, soil properties, pH, and type of aqueous solution. The order of both sorption and retention capacities of four VPs in soil layers of an agricultural soil was TMP > SPD > SDM > SMT. TMP was sorbed by soils more efficiently and was less mobile than the studied sulfonamides. Meanwhile, their migration in different soil layers may be also influenced by soil porosity and other properties, resulting in relatively complex migration behavior. Dissolved organic matter present in WWTP effluent reduced the extent of VPs sorption in soils and enhanced their leaching through soil columns. The solution pH is also an important factor in the sorption of VPs. Based on the results of soil column experiments, VPs can pose a risk of groundwater pollution via manure application to the land. However, groundwater pollution for the VPs is the result of the combined effect of many processes in the actual environment. Thus, to comprehensively assess the environmental impact of VPs contained in manures on agricultural soils, further research is needed on the degradation behavior and persistence of VPs, including the effect of relevant meteorological factors (such as temperature and rainfall).
References
Addison JB (1984) Antibiotics in sediments and run-off water from feedlots. Resid Rev 92:1–28
Batt AL, Snow DD, Aga DS (2006) Occurrence of sulfonamide antimicrobials in private water wells in Washington County, Idaho, USA. Chemosphere 64:1963–1971
Bekçi Z, Seki Y, Yurdakoç MK (2006) Equilibrium studies for trimethoprim adsorption on montmorillonite KSF. J Hazard Mater 133:233–242
Boxall ABA, Blackwell P, Cavallo R, Kay P, Tolls J (2002) The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicol Lett 131:19–28
Chefetz B, Chen Y, Clapp CE, Hatcher PG (2000) Characterization of organic matter in soils by thermochemolysis using tetramethylammonium hydroxide (TMAH). Soil Sci Soc Am J 64:583–589
Chiou CT, Porter PE, Schmedding DW (1983) Partition equilibriums of nonionic organic compounds between soil organic matter and water. Environ Sci Technol 17:227–231
Clarke BO, Smith SR (2011) Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ Int 37:226–247
Díaz-Cruz MS, García-Galán MJ, Barceló D (2008) Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography–quadrupole linear ion trap–mass spectrometry. J Chromatogr A 1193:50–59
Díaz-Cruz MS, López de Alda MJ, Barceló D (2003) Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. TrAC Trends Anal Chem 22:340–351
Durán-Álvarez JC, Prado-Pano B, Jiménez-Cisneros B (2012) Sorption and desorption of carbamazepine, naproxen and triclosan in a soil irrigated with raw wastewater: estimation of the sorption parameters by considering the initial mass of the compounds in the soil. Chemosphere 88:84–90
Gao J, Pedersen JA (2005) Adsorption of sulfonamide antimicrobial agents to clay minerals. Environ Sci Technol 39:9509–9516
Halling-Sørensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment—a review. Chemosphere 36:357–393
Ishihama Y, Nakamura M, Miwa T, Kajima T, Asakawa N (2002) A rapid method for pKa determination of drugs using pressure-assisted capillary electrophoresis with photodiode array detection in drug discovery. J Pharm Sci 91:933–942
Jjemba PK (2002) The potential impact of veterinary and human therapeutic agents in manure and biosolids on plants grown on arable land: a review. Agric Ecosyst Environ 93:267–278
Kan AT, Tomson MB (1990) Effect of pH concentration on the transport of naphthalene in saturated aquifer media. J Contam Hydrol 5:235–251
Kroker R (1983) Aspekte zur ausscheidung animikrobiell wirksamer subsanzen nach der chemoterapeutischen behandlung von nutztieren. Wissenschaft und Umwelt 4:305–308
Lapworth D, Baran N, Stuart M, Ward R (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303
Lertpaitoonpan W, Ong SK, Moorman TB (2009) Effect of organic carbon and pH on soil sorption of sulfamethazine. Chemosphere 76:558–564
Migliore L, Brambilla G, Cozzolino S, Gaudio L (1995) Effect on plants of sulphadimethoxine used in intensive farming (Panicum miliaceum, Pisum sativum and Zea mays). Agric Ecosyst Environ 52:103–110
Rabølle M, Spliid NH (2000) Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 40:715–722
Sacher F, Lange FT, Brauch H-J, Blankenhorn I (2001) Pharmaceuticals in groundwaters: analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J Chromatogr A 938:199–210
Sedlak DLPK (2011) Factors affecting the concentrations of pharmaceuticals released to the aquatic environment. J Contam Water Res Educ 7:56–64
Seol Y, Lee LS (2000) Effect of dissolved organic matter in treated effluents on sorption of atrazine and prometryn by soils. Soil Sci Soc Am J 64:1976–1983
ter Laak TL, Gebbink WA, Tolls J (2006) The effect of pH and ionic strength on the sorption of sulfachloropyridazine, tylosin, and oxytetracycline to soil. Environ Toxicol Chem 25:904–911
Thiele-Bruhn S (2003) Pharmaceutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sci 166:145–167
Thiele-Bruhn S, Seibicke T, Schulten H-R, Leinweber P (2004) Sorption of sulfonamide pharmaceutical antibiotics on whole soils and particle-size fractions. J Environ Qual 33:1331–1342
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35:3397–3406
Totsche KU, Kögel-Knabner I (2004) Mobile organic sorbent affected contaminant transport in soil. Vadose Zone J 3:352–367
Unold M, Kasteel R, Groeneweg J, Vereecken H (2009) Transport and transformation of sulfadiazine in soil columns packed with a silty loam and a loamy sand. J Contam Hydrol 103:38–47
Warisara L, Kee OS, Moorman TB (2009) Effect of organic carbon and pH on soil sorption of sulfamethazine. Chemosphere 76:558–564
Watanabe N, Harter TH, Bergamaschi BA (2008) Environmental occurrence and shallow ground water detection of the antibiotic monensin from dairy farms. J Environ Qual 37:S-78–S-85
Wells MJM (2006) Log DOW: key to understanding and regulating wastewater-derived contaminants. Environ Chem 3:439–449
Acknowledgments
This study was financed by the Natural Science Foundation of China (41072172, 51138009, 41101480), New Century Excellent Talents in University (NCET-11-0391), and the National Key Technologies R & D Program (2012BAJ25B04).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Laura McConnell
Rights and permissions
About this article
Cite this article
Zhang, YL., Lin, SS., Dai, CM. et al. Sorption–desorption and transport of trimethoprim and sulfonamide antibiotics in agricultural soil: effect of soil type, dissolved organic matter, and pH. Environ Sci Pollut Res 21, 5827–5835 (2014). https://doi.org/10.1007/s11356-014-2493-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2493-8