Abstract
Purpose
As irrigation with untreated wastewater often leads to an accumulation of contaminants in soils, nowadays, treated wastewater is increasingly used for irrigation. Here, we investigated whether and to which degree irrigation with treated wastewater might cause desorption of antibiotics from three soils (Leptosol, Phaeozem, Vertisol) and a sediment (Endhó reservoir) of the Mezquital Valley, Mexico, that were exposed to untreated wastewater in the past.
Materials and methods
We performed sequential batch desorption experiments with treated wastewater, artificial wastewater with anionic surfactants, and artificial wastewater without surfactants.
Results and discussion
We observed no desorption, but net sorption of ciprofloxacin in contact with treated wastewater containing 3.475 μg L−1 of ciprofloxacin. Sulfamethoxazole was desorbed from the Leptosol, the Phaeozem, and the Vertisol in a similar degree with and without surfactant, but not from sediment, where no sulfamethoxazole was detected. In contact with treated wastewater containing 1.045 μg L−1 of sulfamethoxazole, it was desorbed from the Leptosol and the Phaeozem with low clay, Fe oxide, and organic matter contents, whereas the Vertisol and sediment showed a net sulfamethoxazole sorption. Desorption could be described with a bi-phasic kinetic desorption model, with most sulfamethoxazole being desorbed via a rate-limited process from poorly accessible binding sites, where it had been accumulated during the long-term irrigation in the past.
Conclusions
We conclude that a potential release of pharmaceuticals as a result from changes in wastewater irrigation is soil specific: Leptosols and Phaeozems of the Mezquital Valley might act as long-term sources of the sulfonamide sulfamethoxazole, though not of the fluoroquinolone ciprofloxacin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The continuously growing world population and associated rising water demand increase water scarcity in both quantitative and qualitative senses (FAO 2011; UNDP 2006). The situation has become critical in the last decades especially in arid and semi-arid regions (FAO 2007). It is estimated that more than 40 % of the world’s population will face water stress or scarcity within the next 50 years (WHO 2006). This has led to many efforts of implementing sustainable management of the water resources like wastewater reuse (Chen et al. 2013; Vo et al. 2014). As almost 70 % of the water taken from natural sources like rivers and groundwater is destined to agriculture globally (UNESCO 2009; FAO 2012), irrigation represents a good target for water reuse. Irrigation of crop fields with untreated wastewater is an ancient practice (Asano and Levine 1996), and nowadays, more than 200 million farmers use wastewater for food production worldwide (Raschid-Sally and Jayakody 2008), especially in many impoverished countries (Pedrero et al. 2010; Qadir et al. 2010).
Wastewater irrigation can be attractive for farmers because it has an added value due to its content of nutrients needed by the crops. Nevertheless, the high load of pollutants and pathogens in wastewater represents a serious health risk for the farmers and the final consumers (Fatta-Kassinos et al. 2011; Hanjra et al. 2012). To reduce this risk, it is recommended that wastewater is treated before being used for irrigation (FAO 1992). The conventional wastewater treatment reduces particularly the dissolved organic carbon (Katsoyiannis and Samara 2007) and pathogen concentrations (Jiménez et al. 2010) in wastewater, yet the removal efficiency for some contaminants like antibiotics is not always satisfactory (Deblonde et al. 2011). In addition, even when the treated wastewater contains only small concentrations of pharmaceuticals, there may be an ongoing release of these contaminants from soils and sediments that were irrigated with untreated wastewater for prolonged periods of time in the past. Such a release or desorption might be enhanced by surfactants, which are widely used household detergents (Edser 2006) and have been found in conventional activated sludge treatment plants effluent (González et al. 2007; Gomez et al. 2011). Their amphiphilic properties allow them to interact with organic hydrophobic compounds and with polar solvents as well, increasing their concentration in aqueous solutions. A slightly enhanced mobility of enrofloxacin by the anionic surfactant sodium dodecylbenzene sulfonate was observed (Yu et al. 2012), but little is known yet, however, on the role of surfactants for pharmaceutical desorption, nor on the degree of such release of pharmaceuticals from soils of different soil orders.
The Mezquital Valley has received untreated wastewater from Mexico City for irrigation purposes for more than a century. Due to the large volume of wastewater discharged from the Mexico City Metropolitan Area, the Mezquital Valley currently is the largest continuous area irrigated with wastewater worldwide (Jiménez and Chávez 2004). As a consequence of the long-term irrigation, antibiotics have accumulated in soil (Dalkmann et al. 2012). In order to improve the quality of the water used for irrigation, the Atotonilco wastewater treatment plant is currently under construction. With an operational capacity of 35 m3 s−1, this plant will treat by 2016 approximately 65 % of the wastewater coming from Mexico City before it reaches the fields. This will expose soils and sediments to wastewater of different properties, but it is not known whether they will still accumulate or become a net source of the already sorbed pharmaceuticals due to their desorption. The different properties among the soils and sediment, as well as the quality of the treated water will determine how fast this potential release of pharmaceuticals can take place, so that we used a kinetic model to assess desorption rates from the tested soils.
To explore the consequences of the change in wastewater quality and particularly the influence of surfactants present in wastewater for desorption of pharmaceuticals from soils and sediments, we performed batch experiments with three soils and one sediment from the Mezquital Valley and different water qualities. We hypothesized that (i) the smaller concentrations of pharmaceuticals in treated wastewater compared to untreated wastewater promote the desorption of antibiotics, (ii) anionic surfactants enhance desorption of pharmaceuticals because of competition for sorption sites and their solubilizing effect, and (iii) the overall desorption process can be subdivided into an instantaneous and a kinetic rate-limited process.
2 Materials and methods
2.1 Soils
The Mezquital Valley is located approximately 80 km to the north of Mexico City. The climate is semi-arid, with a mean annual precipitation of 400 mm in the driest part increasing to 800 mm in the wettest part, with a yearly average temperature range between 16 and 18 °C (Siebe 1994). Irrigation with untreated wastewater from Mexico City started in the beginning of the twentieth century (Siebe 1994) and has continuously expanded since then. Nowadays, about 73,600 farmers irrigate 900 km2 of agricultural land with the wastewater of the Mexico City Metropolitan Area (MCMA) (Siemens et al. 2008; Raschid-Sally and Jayakody 2008). Part of the wastewater discharged from the MCMA is stored in the Endhó reservoir, which has a capacity of approximately 190,000 m3. This ensures a supply of water to the farmers during the dry season but also serves unintentionally as a primary treatment step, during which a sedimentation process takes place. The wastewater used for irrigation in the Mezquital Valley contains pharmaceuticals that accumulate in the irrigated soils (Dalkmann et al. 2012) and likely also in the sediment of the Endhó reservoir (Siemens et al. 2008). We selected one sediment sample from the Endhó reservoir and three soil types for the desorption experiments, namely a Vertisol, a Leptosol, and a Phaeozem, which characteristics are detailed in Table 1. Samples from the first 20 cm were collected and transported to the laboratory at approx. 4 °C in cooling bags. They were kept at −21 °C until used. Because of potential oxidation processes during pretreatment of the originally reduced sediment samples under aerobic conditions, the in situ pH of the sediment could well be higher than the value reported in Table 1. On the other hand, the sample pretreatment under aerobic conditions reduced the possibility of pH shifts during the extraction of pharmaceuticals in the batch experiment.
2.2 Chemicals
According to the consumption data of Mexico (IMS 2005) and their ecotoxicological relevance, we selected ciprofloxacin (CIP) and sulfamethoxazole (SMX) as our model antibiotics. They were obtained from Sigma-Aldrich (Schnelldorf, Germany). Table 2 shows some physicochemical properties of these pharmaceuticals. Isotope-labeled ciprofloxacin (carboxyl-13C3, quinoline-15N, ≥98 % pure) and sulfamethoxazole (ring-13C6, ≥98 % pure) were provided by LGC Standards (Wesel, Germany) as internal standards. Sodium dodecyl benzenesulfonate was selected as model surfactant because it is widely used in household cleaning products. It was purchased from TCI (Eschborn, Germany). The aqueous solutions were prepared with deionized water (Millipore) and the organic solvents were all of HPLC grade. The inorganic salts used for the artificial wastewater were of analytical grade.
2.3 Desorption experiments
Desorption experiments with soils and sediment were performed with three extractants: artificial wastewater, artificial wastewater spiked with linear alkylbenzene sulfonate (LAS) (100 μg L−1) and treated wastewater (effluent) from a treatment plant. The experiments were performed in triplicate. The batch desorption experiment was conducted according to the OECD Guideline 106 for the testing of chemicals’ adsorption/desorption (OECD 2000). We chose a soil/solution ratio of 1:5 (m/m) and performed a sequential extraction of five steps within a total period of 336 h. Soils and sediment were freeze-dried and sieved through a 2 mm mesh before the desorption tests. We weighted 5 g of the corresponding soil or sediment in borosilicate centrifuge tubes. The material was then sterilized with an autoclave (121 °C, 20 min) in the centrifugation tubes to minimize microbial activity before adding 25 mL of the corresponding extracting solution. Sterilization of the soils and sediments was necessary to prevent LAS biodegradation. Soil properties suffer some changes by autoclaving; nevertheless, previous studies have suggested that such changes are significant but not dramatic (Berns et al. 2008). The mixture was shaken with an overhead shaker for the defined time for the corresponding extraction step, namely 24, 48, 72, 144, and 336 h. Subsequently, we separated supernatant and solids by centrifugation (2500×g, 40 min). The liquid phase was decanted for further analysis and replaced with enough fresh extracting solution in order to have again 25 mL of liquid phase for the following extraction step. The tubes were weighted before and after each extraction step to quantify the amount of added or withdrawn liquid.
2.4 Desorption kinetics
To describe the kinetic rate-limited desorption of the tested compounds, we used the following Eq. (1):
with t as contact time between the solid and liquid phase (h); Y 0 as the point of intersection with the axis of ordinates, representing the amount of instantaneously desorbed antibiotic at infinitesimal time (μg kg−1); Y e as the total desorbable amount at an infinite time, and (Y e − Y 0) as the amount of pharmaceuticals desorbed via a rate-limited process with the rate constant k (h−1). Parameters were estimated by plotting the cumulative amounts of desorbed SMX in the subsequent extractions steps against time and fitting these data to Eq. (1) using SigmaPlot version 11.0 (Systat Inc.).
2.5 Wastewater
Wastewater for the desorption experiments was collected from the treatment plant Cerro de la Estrella in Mexico City, which uses a conventional activated sludge process. The treatment plant receives water from Mexico City’s sewer system. After a tertiary treatment, the water is used to recharge the Xochimilco lakes. The treated wastewater from this plant was selected as a good model to emulate the water that the soils in the Mezquital Valley will receive in the near future after the implementation of the Atotonilco wastewater treatment plant, which uses also mainly a conventional activated sludge process. Once sampled, the water was transported to the laboratory in a cooling box (approx. 4 °C) and then kept at −21 °C until used for analysis. Artificial wastewater was prepared with inorganic salts to achieve an ion composition mimicking the composition of the wastewater used for irrigation in the Mezquital Valley (see Electronic Supplementary Material for a detailed composition of the artificial wastewater). We used this solution for the experiments with and without surfactants. The spiking LAS concentration was 100 μg L−1.
2.6 Analysis of supernatant
Prior to the analysis of sulfamethoxazole and ciprofloxacin concentrations, the supernatant samples were preconcentrated and cleaned up using solid-phase extraction (SPE). Approximately 20 mL of supernatant were diluted with 300 mL of Millipore water, and the pH was adjusted to 2.4 with 1 M HCl. An internal standard solution containing isotope-labeled sulfamethoxazole and ciprofloxacin was added, and subsequently, this solution was passed over a combination of a Chromabond SB (Macherey and Nagel, Germany) and an OASIS HLB (Waters, UK) cartridge. Subsequently, the HLB cartridges were eluted with 5 mL methanol, 5 mL acetonitrile, and 5 mL acetonitrile that were acidified with 0.1 % 12 M HCl. The eluents were combined and concentrated with a rotary evaporator to a volume of approximately 0.5 mL. One milliliter of 50 mM phosphoric acid/acetonitrile solution (80:20 v/v) was added and sonicated for 2 min. Then, the liquid was transferred to 1.5-mL Eppendorf tubes, centrifuged at 15,000g for 20 min and transferred to 2-mL amber autosampler vials. SPE of the supernatants and subsequent elution with methanol and acetonitrile likely extracted also at least partly the extracted sulfamethoxazole and ciprofloxacin that was associated with DOM in the original solution.
Concentrations of pharmaceuticals and surfactant were measured using liquid chromatography tandem mass spectrometry (LC-MS/MS) with a TSQ Quantum Ultra spectrometer (Thermo Finnigan, Dreieich, Germany) equipped with a heated electrospray ionization ion source (HESI). For the chromatographic separation of pharmaceuticals, we used an XBridge C18 (3.5 μm, 2.1 × 150 mm) column (Waters, Milford, MA, USA) together with a guard column (Sentry 2.1 × 10 mm, Waters, Milford, MA, USA) and a gradient of methanol (A) and Millipore water (B), both with a 0.1 % content of formic acid, and a flow of 300 μL min−1. The gradient started with 5 % A, increasing linearly to 60 % in the first 5 min. The fraction of A was then increased linearly to 80 % in the next 10 min. For 1 min, it was increased up to 90 % and then held for 1 min more. From the minute 16 to 17, the % A was reduced linearly to 5 % and held for 5 min more to take the system to the initial condition. The total run time was 22 min. Pharmaceuticals were measured in positive ion mode with a discharge current of 4.0 kV, a vaporizer and capillary temperatures of 390 and 217 °C, respectively, nitrogen as sheath gas and argon as collision gas (0.2 Pa). Routine limits of quantification (RLOQ = lowest concentration of standard used) were 250 ng kg−1 for CIP and 25 ng kg−1 for SMX.
3 Results
3.1 SMX desorption as affected by water quality
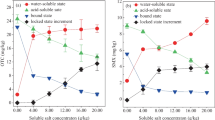
Artificial wastewater with and without the anionic surfactant extracted a total of 1115–2004 ng SMX kg−1 from the three soils after the five desorption steps (Fig. 1). The content of SMX extracted during each extraction step from soils with these extractants was always relatively low (<1000 ng kg−1), and there was no clear difference in the total amount of SMX extracted with or without surfactant, except for the Phaeozem (Fig. 1a). We observed no SMX desorption from the sediment with these extractants, because sediment did not contain significant amounts of SMX (Table 1).
With the treated wastewater as extractant, a net sorption, indicated as “negative desorption” in Fig. 1, was observed during some extraction steps, particularly for the Vertisol and the sediment sample. For all samples, we observed a net sorption of SMX during the second extraction step (Fig. 1a–c). From the third extraction onward, the behavior was different for the four substrates: we observed a net desorption from the three soils at least at one step, but not from the sediment. For the Phaeozem, we found a steady increase in the amount of desorbed SMX from the third step onward. For the Leptosol and Vertisol, we saw an increase at the third step followed by an irregular pattern by the last two steps. The sediment showed a different trend: we observed for every extraction step a SMX sorption from treated wastewater.
In the two cases for which we observed a net desorption of SMX from the soil with treated wastewater (Phaeozem and Leptosol), this liquid phase extracted around two times more SMX than artificial wastewater, regardless of the presence of the surfactant. Interestingly, the total amount of SMX desorbed from these two soils with treated wastewater after the five desorption steps corresponds to more than 90 % of the tightly bound fraction, determined previously (Dalkmann et al. 2012) by accelerated solvent extraction (Table 1).
3.2 CIP desorption as affected by water quality
Only small amounts of CIP were desorbed from soils or sediment with artificial wastewater, regardless of the presence or absence of the anionic surfactant, although previous studies demonstrated the presence of sorbed CIP on these soils (Table 1; Dalkmann et al. 2012). Therefore, the bars corresponding to these two extractants are virtually absent in Fig. 2. When using treated wastewater with a CIP concentration of 3.475 μg L−1 for the extractions, we found for all samples even an almost complete sorption of CIP. However, with each additional extraction step, the sorbed amount of CIP decreased slightly.
3.3 Desorption kinetics
We used Eq. (1) to describe the desorption kinetics of the pharmaceuticals from soil and sediments, but this was not feasible for some cases. As CIP was not significantly desorbed from the soils but quantitatively sorbed to the sediment, an analysis of its desorption kinetics was not possible. An analysis of the SMX desorption kinetics from the sediment sample was also not possible as no SMX was present in the sediment. The net sorption of SMX from treated wastewater hampered the interpretation of the desorption kinetics in contact with this extractant (Fig. 3). Nevertheless, Fig. 3 nicely illustrated the transient reduction of SMX desorption. Desorption of SMX into artificial wastewater with or without surfactant could be well described with the biphasic desorption model (Eq. (1), Fig. 3).
The instantaneous SMX desorption from the Phaeozem and Vertisol sample into artificial wastewater without surfactant was close to zero (Table 3). Regarding to the effect of the anionic surfactant on this first instantaneous desorption phase, we found a greater desorption of SMX from Phaeozem and Vertisol compared with the extraction without surfactant (Fig. 3). Rate constants for the kinetic desorption of SMX decreased in the order Phaeozem > Vertisol > Leptosol, regardless of the presence of surfactant. Remarkably, the desorption rate constant we estimated for the extraction with surfactant was always higher than the rate constant for the extraction without surfactant.
4 Discussion
4.1 SMX desorption as affected by water quality
Despite long-term exposure to wastewater, we did not detect any SMX in sediment even with an accelerated solvent extraction, which accesses the tightly bound fraction. This was possibly due to the biodegradation of SMX by facultative anaerobic bacteria in lake sediment, as reported by Zhang et al. (2013). Therefore, no SMX was desorbed from sediment with artificial wastewater, either with or without surfactant.
The treated wastewater used in our experiments contained 1.045 μg L−1 SMX and 3.475 μg L−1 CIP. During conventional wastewater treatment, many organic compounds are degraded but some emerging contaminants like pharmaceuticals can persist (Hirsch et al. 1999). Considerable concentrations of sulfamethoxazole (Carballa et al. 2004) and ciprofloxacin (Lindberg et al. 2006) in the effluent of wastewater treatment plants after activated sludge treatment have also been reported in other studies; hence, there is doubt that the wastewater treatment in the Mezquital Valley will succeed in eliminating pharmaceutical loads completely. This is the reason why we observed a “negative desorption” when we used this extractant (Fig. 1). Although pharmaceuticals have accumulated in soils in Mezquital Valley due to long-term wastewater irrigation (Dalkmann et al. 2012), they can still sorb SMX and CIP as Dalkmann et al. (2014b) observed in sorption batch experiments. The sorption degree of SMX was different in the soils and sediments, but at the second desorption step, we observed a net sorption to all the three soils (Fig. 1a–c). This might be explained by a breakdown of soil aggregates during the first and second extraction steps, exposing new surfaces and sorption sites that were previously unavailable to the SMX-containing extractant.
The sediment showed a very different behavior than the soils because (i) the sediment did not contain originally any SMX, as discussed above, and (ii) the sediment had a high OC content (Table 1), promoting SMX sorption by hydrophobic interactions as suggested for SMX and other sulfonamides, although the predominant SMX species at the pH of the experiments is the anionic form (Sukul et al. 2008; Zhang et al. 2010; Leal et al. 2013; Zheng et al. 2013). The neutral and cationic species of SMX did not play a significant role in our experiments as the pH of the soils and the sediment were always above the pKa2 (Table 2). Also, SMX sorption to the Vertisol was likely promoted by a larger clay content and—in tendency—also higher organic matter content compared with the Leptosol and Phaeozem (Table 1). Overall, the amount of desorbed or sorbed SMX was negatively related to the organic matter content of the soils and the sediment (Pearson r = −0.96, p < 0.05), highlighting the importance of maintaining or even raising organic matter contents of irrigated soils in the Mezquital Valley after the implementation of wastewater treatment.
A main difference between treated and artificial wastewater was the dissolved organic carbon concentration. While artificial wastewater was prepared only with inorganic salts and, in the case of the LAS-spiked solution, a low concentration of anionic surfactant (100 μg L−1 LAS; ~0.06 mg L−1 DOC), we measured a DOC concentration of 9.5 mg L−1 for the treated wastewater. Haham et al. (2012) reported a reduced sorption of the sulfonamide sulfapyridine to soils with low organic carbon contents when the liquid phase was enriched with dissolved organic carbon. They concluded that this effect might be caused by competition between sulfapyridine and dissolved organic carbon for binding sites or through direct interactions between the organic compound and the dissolved organic carbon. Additionally, Chefetz et al. (2008) suggested a competition of the anionic form of naproxen with dissolved organic matter for binding sites in the soil organic matter to explain the higher mobility of the pharmaceutical in topsoil from column experiments. A reduced sorption of sulfonamides was also observed when manure was applied to soil as it increased the DOM concentration (Thiele-Bruhn and Aust 2004). Based on these independent observations, we suggest that the combined effect of the competition between dissolved organic matter in treated wastewater and sulfamethoxazole for sorption sites, and the sorption of the sulfonamide by DOM reducing its activity and increasing its concentration in the liquid phase, promoted the desorption of sulfamethoxazole from the Phaeozem and Leptosol with lower clay and Fe oxide contents than the Vertisol and lower organic matter contents than the sediment in our experiments. This explains why treated wastewater extracted around two times more SMX than artificial wastewater, regardless of the presence of the surfactant.
4.2 CIP desorption as affected by water quality
Due to the strong sorption of CIP in soils (Dalkmann et al. 2014a), we observed only small amounts of desorbed CIP with artificial wastewater, with or without surfactant. As artificial wastewater did not contain any CIP, no sorption from these solutions was possible. This high affinity of CIP for soils also led to an almost complete sorption on the solid phases from treated wastewater. Given the CIP concentration in wastewater and the volume used in the experiments, a maximum of approximately 75,000 ng kg−1 of CIP could be sorbed after the five extraction steps (assuming a complete sorption). Figure 2 shows that all samples sorbed approximately 70,000 ng kg−1 of CIP, reflecting the strong binding of CIP to soils and sediments.
According to a kinetic study (Dalkmann et al. 2014b), CIP is sorbed mainly to negatively charged clay minerals via a cation exchange mechanism in soils with pH values in the range of 7.1–7.5. This process could be enhanced at smaller pH values, as CIP would be present mainly as cation, like Vasudevan et al. (2009) observed in Vertisols with a lower pH (3.0–5.5); nevertheless, the soils we tested have a pH between 6.76 and 7.48, so CIP was present mainly as zwitterion (Table 2). Anyhow, we observed a high affinity of CIP for all the soils and the sediment tested.
4.3 Desorption kinetics
Desorption of SMX from the three soils with artificial wastewater could be well described by Eq. (1) (Fig. 3). The model encompasses an instantaneous desorption of the SMX sorbed to easily accessible sorption sites on the surface of soil and sediment particles and a slower rate-limited desorption process, e.g., from the micropores of the solid, that is characterized by a rate constant k (h−1). According to this theory, most of the desorbed SMX extracted from these soils was located in poorly accessible sites, because of the very small values of the instantaneous SMX desorption from the Phaeozem and Vertisol sample into artificial wastewater without surfactant (Table 3). This is probably due to the long-term accumulation of this compound in the crop fields in the Mezquital Valley, allowing diffusion to poorly accessible pores and binding sites upon aging (e.g., Rosendahl et al. 2011). Instantaneous desorption from the Leptosol into artificial wastewater was likely facilitated by its small contents of soil organic matter and clay (Table 1) as well as iron oxides (0.68 mg g−1 oxalate-extractable iron; Siebe 1994). Therefore, the soil with the lowest OC, Fe oxide, and clay contents released SMX at the highest rate, regardless of the presence of surfactant (Table 3).
The greater desorption of SMX from Phaeozem and Vertisol with artificial wastewater with surfactant compared with the extraction without surfactant (Fig. 3) suggests a promoting effect of even a concentration of 100 μg L−1 of anionic LAS on the rapid desorption of anionic SMX that can be explained by (an)ion exchange reactions at soil organic matter or iron oxide surfaces. Thiele-Bruhn et al. (2004) suggested that beside the soil organic matter, pedogenic oxides are relevant for sulfonamide sorption. In addition, Förster (2011) described the contribution of iron oxides to the process of intraparticle diffusion of sulfadiazine. Thus, weak sorption of SMX in the Leptosol poorest in iron oxide, clay, and organic matter probably prevented a promoting effect of LAS on the rapid desorption of SMX in this soil.
This and the larger desorption rate constants for the extractions with surfactant compared to those without surfactant for the three soils, reflect again the competitive sorption of both compounds and perhaps also an effect of the surfactant on soil organic matter conformation (and “rigidity”) or its dispersing effect on aggregates (Schaumann and Thiele-Bruhn 2011), which could both accelerate the release of SMX from poorly accessible binding sites.
5 Conclusions
Strong sorption likely prevents desorption and release of CIP from the Mezquital Valley soils and sediments after the implementation of wastewater treatment. However, Leptosols and Phaeozems with particularly lower contents in clay and organic matter can turn into net sources of SMX when they are irrigated with treated wastewater in the future. SMX release from sediments is limited by their small SMX contents.
The desorption of SMX from soils in contact with artificial wastewater can be subdivided into an instantaneous desorption and a slower rate-limited desorption, and it has been the latter step that controlled the ongoing release of the sulfonamide by surfactants and treated wastewater from the soils.
References
Asano T, Levine AD (1996) Wastewater reclamation, recycle and reuse: past, present and future. Water Sci Technol 33:1–14
Barron L, Havel J, Purcell M, Szpak M, Kelleher B, Paull B (2009) Predicting sorption of pharmaceuticals and personal care products onto soil and digested sludge using artificial neural networks. Analyst 134:663–670
Berns AE, Philipp H, Narres HD, Burauel P, Vereecken H, Tappe W (2008) Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur J Soil Sci 59:540–550
Carballa M, Omil F, Lema JM, Llompart M, García-Jares C, Rodríguez I, Gómez M, Ternes T (2004) Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res 38:2918–2926
Chefetz B, Mualem T, Ben-Ari J (2008) Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 73:1335–1343
Chen H, Gao B, Li H, Ma LQ (2011) Effects of pH and ionic strength on sulfamethoxazole and ciprofloxacin transport in saturated porous media. J Contam Hydrol 126:29–36
Chen Z, Ngo HH, Guo W (2013) A critical review on the end uses of recycled water. Crit Rev Environ Sci Technol 43:1446–1516
Dalkmann P, Broszat M, Siebe C, Willaschek E, Sakinc T, Huebner J, Amelung W, Grohmann E, Siemens J, Liles MR (2012) Accumulation of pharmaceuticals, Enterococcus, and resistance genes in soils irrigated with wastewater for zero to 100 years in central Mexico. PLoS ONE 7(9):e45397. doi:10.1371/journal.pone.0045397
Dalkmann P, Siebe C, Amelung W, Schloter M, Siemens J (2014a) Does long-term irrigation with untreated wastewater accelerate the dissipation of pharmaceuticals in soil? Environ Sci Technol 48:4963–4970
Dalkmann P, Willaschek E, Schiedung H, Bornemann L, Siebe C, Siemens J (2014b) Long-term wastewater irrigation reduces sulfamethoxazole sorption, but not ciprofloxacin binding, in Mexican soils. J Environ Qual 43:964–970
Deblonde T, Cossu-Leguille C, Hartemann P (2011) Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health 214:442–448
Edser C (2006) Latest market analysis. Focus Surfactants 5:1–2
FAO (1992) Wastewater treatment and use in agriculture. Pescod MB. FAO irrigation and drainage paper 47. Food and Agricultural Organization, Rome, 125 pp
FAO (2007) Coping with water scarcity. Challenge of the twenty-first century. Food and Agricultural Organization, Rome, 29 pp
FAO (2011) The state of the world’s land and water resources for food and agriculture. Food and Agricultural Organization, Rome, 47 pp
FAO (2012) Coping with water scarcity. An action framework for agriculture and food security. Food and Agricultural Organization, Rome, 78 pp
Fatta-Kassinos D, Kalavrouziotis LK, Koukoulakis PH, Vasquez MJ (2011) The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci Total Environ 409:3555–3563
Figueroa-Diva RA, Vasudevan D, MacKay AA (2010) Trends in soil sorption coefficients within common antimicrobial families. Chemosphere 79:786–793
Förster M (2011) Sequestration of sulfadiazine in soils: sorption and sequestration of sulfadiazine in soils and soil fractions. Dissertation, University of Bonn, 97 pp
Gomez V, Ferreres L, Pocurull E, Borrull F (2011) Determination of non-ionic and anionic surfactants in environmental water matrices. Talanta 84:859–866
González S, Petrovic M, Barceló D (2007) Removal of a broad range of surfactants from municipal wastewater – comparison between membrane bioreactor and conventional activated sludge treatment. Chemosphere 67:335–343
Haham H, Oren A, Chefetz B (2012) Insight into the role of dissolved organic matter in sorption of sulfapyridine by semiarid soils. Environ Sci Technol 46:11870–11877
Hanjra MA, Blackwell J, Carr G, Zhang F, Jackson TM (2012) Wastewater irrigation and environmental health: implications for water governance and public policy. Int J Hyg Environ Health 215:255–269
Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118
IMS (2005) IMS chemical country profile Mexico. Database. IMS Health Incorporated, London
Jiménez B, Chávez A (2004) Quality assessment of an aquifer recharged with wastewater for its potential use as drinking source: “El Mezquital Valley” case. Water Sci Technol 50:269–276
Jiménez B, Mara D, Carr R, Brissaud F (2010) Wastewater treatment for pathogen removal and nutrient conservation: suitable systems for use in developing countries. In: Drechsel P, Scott CA, Raschid-Sally L, Redwood M, Bahri A (eds) Wastewater irrigation and health: assessing and mitigating risk in low-income countries. Earthscan, London, pp 149–169
Katsoyiannis A, Samara C (2007) The fate of dissolved organic carbon (DOC) in the wastewater treatment process and its importance in the removal of wastewater contaminants. Environ Sci Pollut Res Int 14:284–292
Leal RMP, Alleoni LRF, Tornisielo VL, Regitano JB (2013) Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 92:979–985
Lindberg RH, Olofsson U, Rendahl P, Johansson M, Andersson BAV (2006) Behavior of fluoroquinolones and trimethoprim during mechanical, chemical, and active sludge treatment of sewage water and digestion of sludge. Environ Sci Technol 40:1042–1048
OECD (2000) Test No. 106: adorption - desorption using a batch equilibrium method, OECD guidelines for the testing of chemicals, section 1. OECD Publishing, Paris
Pedrero F, Kalavrouziotis I, Alarcón JJ, Koukoulakis P, Asano T (2010) Use of treated municipal wastewater in irrigated agriculture—review of some practices in Spain and Greece. Agric Water Manag 97:1233–1241
Qadir M, Wichelns D, Raschid-Sally L (2010) The challenges of wastewater irrigation in developing countries. Agric Water Manag 97:561–568
Raschid-Sally L, Jayakody P (2008) Drivers and characteristics of wastewater agriculture in developing countries-results from a global assessment. International Water Management Institute, Colombo
Rosendahl I, Siemens J, Groeneweg J, Linzbach E, Laabs V, Herrmann C, Vereecken H, Amelung W (2011) Dissipation and sequestration of the veterinary antibiotic sulfadiazine and its metabolites under field conditions. Environ Sci Technol 45:5216–5222
Schaumann GE, Thiele-Bruhn S (2011) Molecular modeling of soil organic matter: squaring the circle? Geoderma 166:1–14
Siebe C (1994) Akkumulation, Mobilität und Verfügbarkeit von Schwermetallen in langjährig mit städtischen Abwässern bewässerten Böden in Zentralmexiko. Hohenheimer Bodenkundliche Hefte Nr. 17, 213 pp
Siemens J, Huschek G, Siebe C, Kaupenjohann M (2008) Concentrations and mobility of human pharmaceuticals in the world’s largest wastewater irrigation system, Mexico City-Mezquital Valley. Water Res 42:2124–2134
Sukul P, Lamshöft M, Zühlke S, Spiteller M (2008) Sorption and desorption of sulfadiazine in soil and soil-manure systems. Chemosphere 73:1344–1350
Thiele-Bruhn S, Aust MO (2004) Effect of pig slurry on the sorption of sulfonamide antibiotics in soil. Arch Environ Contam Toxicol 47:31–39
Thiele-Bruhn S, Seibicke T, Schulten HR, Leinweber P (2004) Sorption of sulfonamide pharmaceutical antibiotics on whole soils and particle size fractions. J Environ Qual 33:1331–1342
UNDP (2006) Human development report 2006. Beyond scarcity: power, poverty and the global water crisis. Palgrave Macmillan, New York
UNESCO (2009) Water in a changing world. The united nations world water development report 3. United Nations Educational, Scientific and Cultural Organization, Paris
Vasudevan D, Bruland GL, Torrance BS, Upchurch VG, MacKay AA (2009) pH-dependent ciprofloxacin sorption to soils: interaction mechanisms and soil factors influencing sorption. Geoderma 151:68–76
Vo PT, Ngo HH, Guo W, Zhou JL, Nguyen PD, Listowoski A, Wang XC (2014) A mini-review on the impacts of climate change on wastewater reclamation and reuse. Sci Total Environ 494–495:9–17
WHO (2006) Guidelines for the safe use of wastewater, excreta and graywater. Volume 1: Policy and regulatory aspects. 100 pp
Yu Z, Yediler A, Yang M, Schulte-Hostede S (2012) Leaching behavior of enrofloxacin in three different soils and the influence of a surfactant on its mobility. J Environ Sci 24:435–439
Zhang D, Pan B, Zhang H, Ning P, Xing B (2010) Contribution of different sulfamethoxazole species to their overall adsorption on functionalized carbon nanotubes. Environ Sci Technol 44:3806–3811
Zhang Y, Xu J, Zhong Z, Guo C, Li L, He Y, Fan W, Chen Y (2013) Degradation of sulfonamides antibiotics in lake water and sediment. Environ Sci Pollut Res 20:2372–2380
Zheng H, Wang Z, Zhao J, Herbert S, Xing B (2013) Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ Pollut 181:60–67
Acknowledgments
We acknowledge funding for a scholarship for M.C. by the German Academic Exchange Service (DAAD, Ref. Nr. A/11/79684). Furthermore, this work was jointly co-funded by the German Research Foundation (DFG, grant no. SI 1106/5-1,2) and the Mexican Consejo Nacional de Ciencia y Tecnología (CONACyT, grant no. I 0110-193-10). We thank to Dr. Alexander Correa-Metrio and his team for supporting the sediment sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Manuel Carrillo and Gianna Carina Braun contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Carrillo, M., Braun, G.C., Siebe, C. et al. Desorption of sulfamethoxazole and ciprofloxacin from long-term wastewater-irrigated soils of the Mezquital Valley as affected by water quality. J Soils Sediments 16, 966–975 (2016). https://doi.org/10.1007/s11368-015-1292-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1292-2