Abstract
To explore whether sublethal cadmium (Cd) exposure causes branchial cellular damages and affects the metabolic activity in brachyuran crustaceans, the freshwater crab Sinopotamon henanense was exposed to 0.71, 1.43, and 2.86 mg/L Cd2+ for 3 weeks. Gill morphology, metabolic activity (activities of isocitrate dehydrogenase (IDH), cytochrome c oxidase (CCO) and lactate dehydrogenase (LDH), mRNA expression of CCO active subunit 1 (cco-1) and ldh, as well as ATP levels) in crab muscle were investigated. The results showed that sublethal Cd exposure caused profound morphological damages in the gills. The branchial epithelial cells were disorganized and vacuolized. Ultrastructurally, a decrease in number of apical microvilli, vacuolized mitochondria, and condensed chromatin were observed in gill epithelial cells. Correspondingly, the Cd exposure also induced downregulations of cco-1 and ldh mRNA expression and reduced activities of IDH, CCO, and LDH, in accordance with the lower ATP level in crab muscle. These results led to the conclusion that gill damage caused by sublethal Cd exposure could lead to an impairment of oxygen uptake of S. henanense, and the inhibition of metabolic activity decreases the oxygen demand of the crab and assists them to survive under the condition of lower oxygen availability. These effects add to our understanding on toxic effects of Cd and survival management of S. henanense subchronically exposed to sublethal Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a widespread pollutant in aquatic systems, largely increasing as a result of mining, smelting, and burning activities. Unlike organic compounds, Cd is not biodegradable and has a very long biological half-life. This metal has been found to cause a wide range of biochemical and physiological dysfunctions, as well as morphological effects in aquatic organisms (Sherwood et al. 2000; Rajotte and Couture 2002; Liu et al. 2011; Xuan et al. 2011; Wang et al. 2011).
In the aquatic environment, organisms usually face relatively low pollutant concentrations but over a rather long duration. Silvestre et al. (2006) indicated that the crab Eriocheir sinensis would physiologically acclimate under chronic Cd exposure conditions resulting in the upregulation of several antioxidant enzymes and a switchover of metabolic profiles. These acclimation mechanisms are helpful for crabs as a defense against Cd2+ exposure. The freshwater crab Sinopotamon henanense is one of the important representatives of decapod crustaceans and a species commonly found in the freshwater of China. They are metal-tolerant, and the median lethal concentration (LC50) value is relatively higher than that of other crustacean species (Wang et al. 2008; Lorenzon et al. 2000). This may be related to the acclimation mechanism evolved over long periods of time. Earlier results by our group showed that subchronically exposing the freshwater crab S. henanense to 2.86 mg/L Cd2+ for 21 days led to an obvious decrease of oxygen consumption (Xuan et al. 2013), which is determined by the tissue metabolic activity and oxygen uptake ventilation of the gills (Sokolova and Lannig 2008). Thus, metabolic depression, monitored as a drastically reduced oxygen consumption rate (Gorr et al. 2006), and potential damage in oxygen uptake ventilation of the gill were taken into account to investigate the decreased oxygen consumption.
For S. henanense, the cheliped muscle is the third main target for Cd accumulation and metallothionein induction following the gill and hepatopancreas (Ma et al. 2008), suggesting that the muscle is one of the tissues with high metabolic activity due to its functions in context with detoxification, defense against other organisms, escaping from adverse factors, feeding, etc. In order to provide energy to support these activities, a source of fuel must be continually supplied to the muscle via the circulatory system. Consequently, a source of oxygen is always available, and therefore, the muscle can make the most efficient use of the substrate by complete oxidation via citric acid (TCA) cycle. High numbers of mitochondria in muscle cells are the major characteristic for aerobic metabolism and ATP generation. The activity and gene expression of mitochondrial enzymes, such as the activity of NAD-isocitrate dehydrogenase (IDH) and cytochrome c oxidase (CCO), mRNA expression of genes encoding the CCO subunit 1 (cco-1), have been used in several studies to assess mitochondrial function and respiratory responses of aquatic organisms to Cd (Matozzo et al. 2001; Pierron et al. 2009; Dorts et al. 2011; Garceau et al. 2010; Ivanina et al. 2011; Navarro et al. 2011). There is an inverse relationship between glycolytic flux and oxygen availability during O2 deprivation, which marks the switch from aerobic (mitochondrial) to anaerobic (fermentative) metabolism for energy production (Storey 1985; Schmidt and Kamp 1996). To evaluate the metabolic activities of S. henanense under subchronic Cd exposure, the above-described mitochondrial enzymes, lactate dehydrogenase (LDH) activity, an indicator of anaerobic metabolism, and ldh mRNA expression regulated by hypoxia (Li and Brouwer 2007), as well as ATP level in crab muscle were also assayed.
The aims of the present study were to (1) explore whether sublethal Cd exposure causes cellular damages in S. henanense gills and is responsible for reduced oxygen uptake through observations of gill histological and cytological changes and (2) investigate changes in metabolic activity due to sublethal Cd exposure by analyzing parameters involved in aerobic and anaerobic metabolism in S. henanense muscle.
Material and methods
Experimental animals and design
Crabs were purchased from the Dongan Aquatic Market in Taiyuan, China. Prior to experimentation, crabs were acclimated for 2 weeks in glass aquaria (50 cm × 30 cm × 25 cm) filled with dechlorinated, carbon-filtered tap water (pH 7.5, dissolved oxygen 8.0–8.3 mg L−1). Temperature was kept at 20 ± 2 °C. Aquaria were shielded by black plastic sheets to reduce disturbance. During the acclimation period, crabs were fed three times a week.
Stock solution of the metal was prepared by dissolving 1.63 g CdCl2·2½H2O (analytical grade) in 50 mL of distilled water (20 g/L Cd2+). Appropriate volumes of stock solution were added to 4 L of tap water to obtain a series of sublethal concentrations of Cd2+ solutions (0.71, 1.43, and 2.86 mg/L). After acclimation, healthy intermolt individuals with a similar weight of 15.8 ± 0.8 g were exposed to the above Cd2+ solutions for 3 weeks. Two replicates were prepared for each concentration with five crabs in each replicate. Parallel controls were maintained along with the experiment. During the experimental period, all groups were fed daily (1 % of body weight). The exposure medium was changed every 48 h to maintain nominal Cd2+ concentration. All other conditions were kept the same as those during acclimation.
Sample preparation
At the end of exposure, the crabs were cryoanesthesized by placing them on ice for about 15 min. The gills from ten crabs in the control and 2.86 mg/L Cd2+ exposure groups (five crabs for each group) were immediately excised and fixed for histological and cytological examinations. Cheliped muscle from 20 crabs (five crabs used per concentration) was shock-frozen and stored in liquid nitrogen for biochemical parameter assays and mRNA expression analysis.
Histological examination with light microscopy
The gills were fixed for 24 h at room temperature by direct immersion in a 0.1-M phosphate buffer at pH 7.4 with 4 % paraformaldehyde. Samples were dehydrated with ethanol and a toluene series and embedded in paraffin. Serial sections (4 μm) were mounted on gelatin-coated glass slides and stained with hematoxylin and eosin. Ten to 20 slides were examined with a light microscope (Olympus BX51, Olympus, Tokyo, Japan).
Ultrastructure observation with transmission electron microscopy
Small pieces of 1 mm−3 in size taken from the gills were immersion-fixed in 2.5 % glutaraldehyde in phosphate buffer (pH 7.4) immediately after excision. Tissues were postfixed in 1 % osmium tetroxide and embedded in thin viscosity resin. Ultrathin sections were cut with an ultramicrotome (Leica UC-6, Leica Camera AG, Solms, Germany), stained with uranyl acetate and lead citrate, and examined by transmission electron microscope (JEM-1400, JEOL, Tokyo, Japan).
Measurements of biochemical parameters
Commercial kits were used to measure the activities of NAD-IDH, CCO, and LDH (GENMED, Shanghai, China). The experimental crabs were cryoanesthesized, and their cheliped muscle was immediately excised, weighted, and stored in liquid nitrogen overnight. Then, muscle samples were ground into a powder in liquid nitrogen. GENMED lysate was added to the tissue powder, vortexed for 30 s, and incubated on ice for 30 min with 30 s of vortexed turbulence per 10 min. Supernatant for the enzyme assays was obtained after centrifugation at 10,000×g for 10 min. Isocitrate and pyruvate were used as substrate for determining NAD-IDH and LDH activity, respectively, and activities of both enzymes were estimated based on absorbance changes at 340 nm. CCO activity was measured based on the oxidation of ferrocytochrome c to ferricytochrome c by CCO, resulting in a decrease in absorbance at 550 nm. ATP levels were determined using a commercial kit (Jiancheng Nanjing Institute of Bioengineering, China), based on the detection of creatine phosphate generated by creatine kinase catalyzing ATP and creatine in samples through phosphomolybdic acid colorimetry at 636 nm. Protein concentrations of the samples were measured according to Bradford (1976), using bovine albumin as a standard.
Quantitative real-time PCR
Total RNA was extracted from 50 to 100 mg of muscles using TRIzol Reagent (Transgene, Beijing, China), according to the manufacturer’s instructions. RNA quality was evaluated by electrophoresis on a 1 % agarose gel, and RNA concentrations as well as purity were determined by spectrophotometry (RNA/DNA Calculator, Eppendorf). First-strand cDNA was then synthesized from 5 μg of RNA, and partial coding of sequences for cco-1 and ldh was obtained using the TransScriptTM Two-Step RT-PCR SuperMix (Transgene, Beijing, China), according to the manufacturer’s instructions. Specific primers were deduced from alignment of cco-1 and ldh sequences available from NCBI for different crab species using DNAMAN software (Lynnon BioSoft, Vaudreuil, Quebec, Canada).
The mRNA expression for genes of cco-1 and ldh was determined using quantitative real-time PCR (qRT-PCR) using an ABI 7500 Real-Time PCR System (Applied Biosystem, Foster, CA, USA) and SYBR® Premix Ex TaqTM (TaKaRa, Shiga, Japan) according to the manufacturers’ instructions. The qRT-PCR reaction mixture consisted of 10 μl of 2× SYBR Green Master Mix, 0.4 μM of each forward and reverse primers, 2 μl of 50× diluted cDNA template, and water to adjust to 20 μl. Twenty microliters of reaction mixture was loaded into an ABI 7500 Real-Time PCR System and subjected to the following cycling: 30 s at 95 °C to denature DNA and activate Taq polymerase; 40 cycles of 5 s at 95 °C, 30 s at 55 °C, and 31 s at 72 °C. For each primer, serial dilutions of a cDNA standard were amplified in each run to determine amplification efficiency, and two negative controls were also amplified: non-reverse-transcribed total RNA as a control for contamination by genomic DNA and a template-negative sample, to control for any contamination of the reagent. Relative quantification of gene expression was achieved by concurrent amplification of the β-actin endogenous control. Specific primers used for qRT-PCR were cco-1F, 5′-CTGGTAGGCTTATTGG-3′, cco-1R, 5′-GAGTTAAAGAAGGAGGTA-3′, ldhF, 5′-GGAATGTG AAGATTGAGGC-3′, ldhR, 5′-AATGATGCCCTTGAAGATA-3′, β-actinF, 5′-ATGAAGGTTAT GACTGAACGAGG-3′, and β-actinR, 5′-GCAGCAGCTTGAGCCATTT-3′. The expected length of amplified product was 201 bp for cco-1, 150 bp for ldh, and 176 bp for β-actin.
Statistical analysis of the results
Statistical analyses were performed with SPSS 15.0 software. Data distributions and the homogeneity of variance were tested using the Kolmogorov–Smirnov and Levene tests, respectively. When the data satisfied the prerequisites for parametric tests (ANOVA), one-way ANOVA and Dunnet’s test were used to evaluate the significance of differences between the treatment and control groups. Otherwise, the Kruskal–Wallis H test was used. Numerical results are given as means ± SE. A probability value of less than 0.05 was regarded as significant. The figures were generated by Origin 8.0 software.
Results
Effect of sublethal Cd2+ exposure on S. henanense gill morphology
Light microscopic observations on the gills
The gills of S. henanense are dendrobranchiate, with an axis that supports numerous laminae at two angles (Fig. 1(A01)). The laminae consisted of cuticle, epithelial cells, and blood vessels (Fig. 1(A02, A03)). After 21 days of exposure to 2.86 mg/L Cd2+, the laminae became disorganized and irregular (Fig. 1(A11, B11)). In some laminae, the epithelial cells were swollen, resulting in narrowed hemolymph vessels and, in some cases, even the disappearance of the laminae sinus (Fig. 1(A12, A13)). In some other laminae, large spaces lacking any cytoplasm were observed beneath the cuticle, which separated the epithelial cells from the cuticle (Fig. 1(B12, A13)). Numerous vacuoles were noted in their epithelial cells (Fig. 1(A13, B13)). An increase in hemocytes was observed in the hemolymph sinus (Fig. 1(B13)).
Light micrographs of S. henanense gills. A 01 –A 03 : Control. (A 01 ) ×100, an axis supports numerous laminae at two angles. (A 02 ) ×200, the gill lamellae were composed of two single-layered epithelial sheets lined outside by the cuticle. (A 03 ) ×400. Note the thin epithelium (E) tightly connected with the cuticle (C), nucleus (N) of epithelial cells, and lamella sinus (S). A 11 –A 13 and B 11 –B 13 : 2.86 mg/L Cd treated for 21 days. A 11 (×100), A 12 (×200), and A 13 (×400): hemolymph sinus disappeared at some sites of the laminae due to the extrusion of swollen epithelial cells. B 11 (×100), B 12 (×200), and B 13 (×600): large spaces (sp) separated the epithelial cells from the cuticle, numerous vacuoles (v) were in epithelial cells as seen in A 13 , and the number of hemocytes (H) in the sinus increased
Transmission electron microscopy observation of gill epithelial cells
In control crabs, the apical microvilli of epithelial cells were in a dense arrangement (Fig. 2(A0)). The mitochondria were generally numerous with well-developed cristae (Fig. 2(B0)). In the nuclei, electron-dense heterochromatin was uniformly distributed and adherent to the continuous and intact nuclear envelope (Fig. 2(C0)). Exposure to 2.86 mg/L Cd2+ for 21 days resulted in important alterations of the epithelium cells, where the apical microvilli became partially dissolved (Fig. 2(B1), black arrow), ruptured (Fig. 2(C2), black arrow), and even lost (Fig. 2(A1)), where large spaces with no cytoplasm were observed beneath the cuticle, separating the epithelial cells from the cuticle (Fig. 2(A1, sp)). Partial dissolution of mitochondrial cristae and their vacuolation were observed. The nucleus chromatin of some epithelial cells was heavily marginated, and the nuclear envelope was basically disrupted. Most nuclear matrices were condensed with vacuoles around the nucleus.
Transmission electron micrographs of gill epithelial cells of S. henanense. A 0 –A 2 : Apical microvilli (MV) and cytoplasm. (A 0 ) Control, ×20,000. (A 1 ) Exposure to 2.86 mg/L Cd for 21 days. A large space separates the cuticle from the epithelium, ×25,000. (A 2 ) Exposure to 2.86 mg/L Cd for 21 days. Numerous vacuoles in epithelial cytoplasm, ×15,000. B 0 –B 2 : Mitochondria. (B 0 ) Control, ×6,000. (B 1 ) Exposure to 2.86 mg/L Cd for 21 days. Partial dissolution of cristae and vacuolation of mitochondria. Lysis of apical microvilli (arrow), ×30,000. (B 2 ) Exposure to 2.86 mg/L Cd for 21 days. Ruptured and dissolved mitochondria cristae, and the mitochondria were heavily vacuolated, ×40,000. C 0 –C 2 : Nuclei. (C 0 ) Control, ×10,000. (C 1 ) Exposure to 2.86 mg/L Cd for 21 days. Chromatin margination and nuclear envelope disruption, ×12,000. (C 2 ) Exposure to 2.86 mg/L Cd for 21 days. Chromatin condensation and nuclear membrane deformation. The microvilli expanded into the lacunae (arrow), and the mitochondria were heavily vacuolated, ×15,000. MV, microvilli; sp, space; M, mitochondria; V, vacuole
Effect of sublethal Cd2+ exposure on metabolic activities of S. henanense muscle
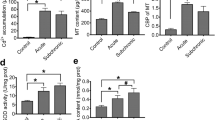
IDH activity increased in the 0.71-mg/L Cd2+ treatment (P < 0.05, Fig. 3a). With increase of Cd2+ concentrations, IDH activities decreased gradually, and a significant decrease in IDH activity appeared in the 2.86-mg/L Cd2+-treated group (P < 0.01, Fig. 3a). CCO and LDH activities also significantly decreased in the 2.86-mg/L group (P < 0.01 for CCO, Fig. 3b; P < 0.05 for LDH, Fig. 3d). The cco-1 and ldh gene expressions were changed in a similar manner, and significant downregulation of both genes was observed in the group exposed to 2.86 mg/L (0.16-fold of control for cco-1 gene expression level, P < 0.05, Fig. 3c; 0.26-fold of control for ldh gene expression level, P < 0.05, Fig. 3e). An obvious decrease in ATP content was found in the 2.86-mg/L Cd2+ treatment (P < 0.01, Fig. 3f).
Discussion
Gill damage could cause lower oxygen uptake
Unlike some other crabs (Johnson 1980; Silvestre et al. 2005), there were no morphologically distinct osmoregulatory and respiratory sites in the gills of S. henanense, which is supporting similar findings in the African freshwater crab Potamon niloticus (Maina 1990). For S. henanense gills, the epithelial cells over the vascular channels were very thin, and the thickest parts of these cells contain the nucleus, forming the translamellar extensions of the cells. This histological characteristic indicates that S. henanense gill structure compromises between the functions of osmoregulation and gas exchange (Maina 1990). The very thin parts of the epithelial cells represent the gas exchange barrier in crustaceans. This epithelium provides a simple diffusion of oxygen from the external medium across the epithelium to the continuously flowing hemolymph of the internal body. Thus, the thin epithelial cells would improve the gas exchange efficiency of the gills. After sublethal Cd2+ exposure, the gill lamellae of the experimental crabs became disorganized. A number of hemocytes appeared in the hemolymph vessel after Cd exposure. Hemocytes may play a defensive role under the condition of Cd exposure. Abundant large vacuoles in epithelial cells made the lamellae wider. The swollen epithelial cells and an increasing number of hemocytes decrease the space for the circulating hemolymph. The non-tissue spaces between cuticle and epithelial cells, and vacuoles in the epithelial cells could result in an increase in the water–hemolymph diffusion distance and a subsequent reduction of gill gas diffusion capacity. The apical microvilli-like structure in branchial epithelial cells may increase the functional lamellar area and produce microturbulances, enhancing the effectiveness of the exchange processes over the surface epithelia. The decrease of microvilli-like structure and changes of the epithelial cell surface may diminish the capacity for gas exchange. The lower number of these structures in Cd-exposed crabs suggests that the respiratory function of these cells is disturbed. The most pronounced effects of epithelial cells after Cd treatment are shown in their degenerated mitochondria. The damage of the mitochondria can severely affect the energy production of the gills and further impact the ventilation of the gills. Changes in the nuclei, especially chromatin condensation and marginalization following Cd exposure may indicate the progressive inactivation of the nuclear component, probably resulting from the inhibition of DNA repair and DNA methylation (Waisberg et al. 2003). Such changes in the nucleus also go along with symptoms of necrosis and would lead to a dysfunction of respiratory epithelial cells. The alterations of gill morphology suggest that the function of this organ is impaired by sublethal Cd2+ exposure, and the gill morphology damage could be responsible for the decrease of oxygen consumption of S. henanense subchronically exposed to Cd2+, which was shown in a previous study (Xuan et al. 2013). It is most likely that the cellular oxygen supply status and aerobic metabolism would be affected.
Sublethal Cd2+ exposure impairs the metabolic activities in S. henanense muscle
Sublethal Cd2+ exposure impairs the mitochondrial aerobic metabolism in S. henanense muscle
One of the main characteristics of muscle tissue is to have abundant mitochondria. Aerobic metabolism activity in the muscle is well represented by the activities of mitochondrial metabolism enzymes. The TCA cycle of the mitochondria is the most important metabolic pathway for energy production under aerobic conditions, as well as a key pathway for the production of small molecule precursors in anabolism. The hydrogens removed during the cycle are transferred to the electron transport chain, and the energy released during electron transport is conserved by the formation of ATP. The energy that is formed during the oxidation of glucose or fatty acids in the cycle is important in the muscle to support the mechanical activity. It has been suggested that the dehydrogenation of isocitrate in the operation of the TCA cycle for energy formation is catalyzed by the NAD+-linked IDH (Alp et al. 1976). CCO is the terminal enzyme in the mitochondrial electron transport chain, involving in the aerobic ATP generation by means of oxidative phosphorylation. The decreased IDH activity at higher Cd2+ concentration (2.86 mg/L) indicates that Cd may make the TCA cycle as the candidate for Cd-induced impairment of mitochondria substrate oxidation and further impair the aerobic metabolism and energy generation, demonstrated by the decreased CCO activity and ATP level in muscle tissues. A previous study on the addition of Cd to resting mitochondria induced rapid oxidation of mitochondrial NAD(P)H pools and consequently resulted in the disturbance of the redox status indicating interference at the level of dehydrogenases (Belyaeva et al. 2004), and the inhibition of mitochondrial matrix dehydrogenases was then considered as one mechanism responsible for oxidative damage and elevated ROS levels that are the earliest hallmarks of Cd exposure in the mitochondria (Ivanina et al. 2008). Hence, the decreased IDH activity at higher Cd2+ concentrations (2.86 mg/L) is also indicating possible oxidative damage in S. henanense muscle.
Differential response was observed when Cd2+ concentration was lower (0.71 mg/L), where IDH activity was increased, indicating an increased activity of the TCA cycle. This enhanced aerobic metabolism activity is in favor of energy production, amino acid precursor supply for detoxicated protein synthesis, etc. Our previous studies investigating the effects of Cd exposure on S. henanense have found an increased mobilization of carbohydrate and protein in the crab muscle (Xuan et al. 2011) and, also, an increased synthesis of metallothionein, a detoxicated protein for binding and storage of metals (Wu and Hwang 2003) in crab tissues (Ma et al. 2008). Taking into account that the TCA cycle is the common pathway for carbohydrate and protein catabolism and plays important role in the supply of precursor for anabolism, we consider that the present increased TCA cycle could mean a response of S. henanense to cope with the stress induced by lower Cd2+ concentration, including the demand for energy and detoxicated protein synthesis. This is consistent with earlier standpoint that organisms need more energy to detoxify the toxicants to minimize the adverse effect (Ansaldo et al. 2006; De Smet and Blust 2001). The unchanged CCO activities and ATP levels at this Cd2+ concentration most likely indicate a successful response of S. henanense to lower Cd2+ concentration, which may be collapsed at the higher Cd2+ concentrations (2.86 mg/L).
The cco-1 gene expression was significantly downregulated at the greatest exposure concentration. The decrease in the expression level of this gene involved in the mitochondrial respiratory chain was considered as a proceeding adaptation of organisms in the case of low oxygen level by reducing the activity of the major cellular consumer of oxygen—the mitochondria (Pierron et al. 2007). The present result corroborates with a previous report on decreased cco-1 gene expression level in wild yellow perch exposed to elevated Cd concentrations in the environment (Pierron et al. 2009). It also demonstrated that gene expression studies provide a promising field of investigation in ecotoxicology.
Sublethal Cd2+ exposure impairs the anaerobic metabolism in S. henanense muscle
Hypoxia tolerance is widespread amongst invertebrates, including some crustaceans (Schmitz and Harrison 2004), and anaerobic glycolysis has been considered as an adaptive strategy during O2 deprivation (Ebbesen et al. 2004). LDH is an important glycolytic enzyme, involved in the production of energy, being particularly important when a considerable amount of additional energy is rapidly required. A negative correlation between LDH activity and ambient oxygen levels for some aquatic organisms suggest a possible biochemical adjustment in response to the lowered oxygen levels (Wu and Lam 1997; Diamantino et al. 2001). This probably occurs also in situations of chemical stress. Expression of genes involved in glycolysis was reported to be regulated by the hypoxia-inducible factor under hypoxic conditions in both vertebrates and invertebrates (Li and Brouwer 2007). The here-observed decrease in LDH activity and downregulation of ldh gene expression indicated an inhibition of anaerobic glycolysis during the Cd2+-induced low oxygen availability. Similar glycolytic rate inhibition also has been shown in bivalve muscles and insect brains during episodes of oxygen deprivation (Wegener 1993; Grieshaber et al. 1994). One possible reason for this is that anaerobic glycolysis represents a short-term attempt of oxyregulatory tissues to compensate for continuing ATP utilization demands under hypoxic conditions, which soon amass toxic levels of end products, e.g., lactate (Xuan et al. 2011). If it still fails to meet the ATP demands for ionic and osmotic equilibrium, anaerobic glycolysis will produce an ultimately fatal ATP imbalance in the cell (Boutilier and St-Pierre 2000). So it seems that the inhibition of anaerobic metabolism in S. henanense muscle to some extent maintains the survival of crabs under lower oxygen availability.
In summary, results of this study demonstrate that subchronic exposure to 2.86 mg/L Cd2+ could cause low oxygen uptake of S. henanense by reducing the gas diffusing capacity, decreasing the functional lamellar area, narrowing the space for the circulating hemolymph, impairing the energy production, and inducing necrosis in the gills. Exposure to the same Cd2+ concentration also impaired metabolic activities in S. henanense muscle, including both aerobic and anaerobic metabolisms, evidenced by the decreased activities of IDH, CCO, and LDH; downregulations of cco-1 and ldh; and the poor ATP generation in muscle tissues. On the other hand, the impairment of metabolic activities would yet reduce the demand for oxygen and also reduce Cd2+ uptake from the water. This could be considered as an adaptive mechanism that could help the organism to survive under the condition of low oxygen availability.
References
Alp PR, Newsholme EA, Zammit VA (1976) Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J 154:689–700
Ansaldo M, Nahabedian DE, Holmes-Brown E, Agote M, Ansay CV, Guerrero V, Wider EA (2006) Potential use of glycogen level as biomarker of chemical stress in Biomphalaria glabrata. Toxicology 224:119–127
Belyaeva EA, Glazunov VV, Korotkov SM (2004) Cd2+-promoted mitochondrial permeability transition: a comparison with other heavy metals. Acta Biochim Pol 51:545–551
Boutilier R, St-Pierre J (2000) Surviving hypoxia without really dying. Comp Biochem Physiol 126A:481–490
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analy Biochem 72:249–254
de Smet H, Blust R (2001) Stress responses and changes in protein metabolism in carp Cyprinus carpio during cadmium exposure. Ecotoxicol Environ Saf 48:255–262
Diamantino TC, Almeida E, Soares AMVM, Guilhermino L (2001) Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 45:553–560
Dorts J, Kestemont P, Dieu M, Raes M, Silvestre F (2011) Proteomic response to sublethal cadmium exposure in a sentinel fish species, Cottus gobio. J Proteome Res 10:470–478
Ebbesen P, Eckardt KU, Ciampor F, Pettersen EO (2004) Linking measured intercellular oxygen concentration to human cell functions. Acta Oncologica 43:598–600
Garceau N, Pichaud N, Couture P (2010) Inhibition of goldfish mitochondrial metabolism by in vitro exposure to Cd, Cu and Ni. Aquat Toxicol 98:107–112
Gorr TA, Gassmann M, Wappner P (2006) Sensing and responding to hypoxia via HIF in model invertebrates. J Insect Physiol 52:349–364
Grieshaber M, Hardewig I, Kreutzer U, Portner H-O (1994) Physiological and metabolic responses to hypoxia in invertebrates. Rev Physiol Biochem Pharmacol 125:43–147
Ivanina AV, Habinck E, Sokolova IM (2008) Differential sensitivity to cadmium of key mitochondrial enzymes in the eastern oyster, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Comp Biochem Physiol 148C:72–79
Ivanina AV, Froelich B, Williams T, Sokolov EP, Oliver JD, Sokolova IM (2011) Interactive effects of cadmium and hypoxia on metabolic responses and bacterial loads of eastern oysters Crassostrea virginica Gmelin. Chemosphere 82:377–389
Johnson PT (1980) Histology of the blue crab Callinectes sapidus. A model for the decapoda. Praeger, New York
Li T, Brouwer M (2007) Hypoxia-inducible factor, gsHIF, of the grass shrimp Palaemonetes pugio: molecular characterization and response to hypoxia. Comp Biochem Physiol 147B:11–19
Liu D, Yan B, Yang J, Lei W, Wang L (2011) Mitochondrial pathway of apoptosis in the hepatopancreas of the freshwater crab Sinopotamon yangtsekiense exposed to cadmium. Aquat Toxicol 105:394–402
Lorenzon S, Francese M, Ferrero EA (2000) Heavy metal toxicity and differential effects on the hyperglycemic stress response in the shrimp Palaemon elegans. Arch Environ Contam Toxicol 39:167–176
Maina JN (1990) The morphology of the gills of the freshwater African crab Potamon niloticus (Crustacea: Brachyura: Potamonidae): a scanning and transmission electron microscopic study. J Zool 221:499–515
Ma WL, Wang L, He YJ, Yan T (2008) Cadmium accumulation and metallothionein biosynthesis in the freshwater crab Sinopotamon henanense. Acta Scientiae Circumstantiae 28:1192–1197 (in Chinese)
Matozzo V, Ballarin L, Pampanin DM, Marin MG (2001) Effects of copper and cadmium exposure on functional responses of hemocytes in the clam, Tapes philippinarum. Arch Environ Contam Toxicol 41:163–170
Navarro A, Faria M, Barata C, Piňa (2011) Transcriptional response of stress genes to metal exposure in zebra mussel larvae and adults. Environ Pollut 159:100–107
Pierron F, Baudrimont M, Gonzalez P, Bourdineaud J-P, Elie P, Massabuau J-C (2007) Common pattern of gene expression in response to hypoxia or cadmium in the gills of the European glass eel (Anguilla anguilla). Environ Sci Technol 41:3005–3011
Pierron F, Bourret V, St-Cyr J, Campbell PGC, Bernatchez L, Couture P (2009) Transcriptional responses to environmental metal exposure in wild yellow perch (Perca flavescens) collected in lakes with differing environmental metal concentrations (Cd, Cu, Ni). Ecotoxicology 18:620–631
Rajotte JW, Couture P (2002) Effects of environmental metal contamination on the condition, swimming performance, and tissue metabolic capacities of wild yellow perch (Perca flavescens). Can J Fish Aquat Sci 59:1296–1304
Schmitz A, Harrison JF (2004) Hypoxic tolerance in air-breathing invertebrates. Respir Physiol Neurobiol 141:229–242
Schmidt H, Kamp G (1996) The Pasteur effect in facultative anaerobic metazoa. Experientia 52:440–448
Sherwood GD, Rasmussen JB, Rowan DJ, Brodeur J, Hontela A (2000) Bioenergetic costs of heavy metal exposure in yellow perch (Perca flavescens): in situ estimates with radiotracer (137Cs) technique. Can J Fish Aquat Sci 57:441–450
Silvestre F, Trausch G, Devos P (2005) Hyper-osmoregulatory capacity of the Chinese mitten crab (Eriocheir sinensis) exposed to cadmium; acclimation during chronic exposure. Comp Biochem Physiol 140C:29–37
Silvestre F, Jean-F D, Dumont V, Dieu M, Raes M, Devos P (2006) Differential protein expression profiles in anterior gills of Eriocheir sinensis during acclimation to cadmium. Aquat Toxicol 76:46–58
Sokolova IM, Lannig G (2008) Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Clim Res 37:181–201
Storey K (1985) A re-evaluation of the Pasteur effect: new mechanisms in anaerobic metabolism. Mole Physiol 8:439–461
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192:95–117
Wang L, Yan B, Liu N, Li Y, Wang Q (2008) Effects of cadmium on glutathione synthesis in hepatopancreas of freshwater crab, Sinopotamon yangtsekiense. Chemosphere 74:51–56
Wang L, Xu T, Lei W, Liu D, Li Y, Xuan R, Ma J (2011) Cadmium-induced oxidative stress and apoptotic changes in the testis of freshwater crab, Sinipotamon yangtsekiense. PLoS ONE 6:1–8
Wegener G (1993) Hypoxia and posthypoxic recovery in insects: physiological and metabolic aspects. In: Hochachka P, Lutz P, Sick T, Rosenthal M, Thillart GVD (eds) Surviving hypoxia: mechanisms of control and adaptation. CRC, Boca Raton, pp 417–434
Wu RSS, Lam PKS (1997) Glucose-6-phosphate dehydrogenase and lactate dehydrogenase in the green lipped mussel (Perna viridis): possible biomarker for hypoxia in the marine environment. Water Res 31:2797–2801
Wu SM, Hwang PP (2003) Copper or cadmium pretreatment increase the protection against cadmium toxicity in tilapia larvae (Oreochromis mossambicus). Zool Stud 42:179–185
Xuan R, Wang L, Sun M, Ren G, Jiang M (2011) Effects of cadmium on carbohydrate and protein metabolisms in the freshwater crab Sinopotamon yangtsekiense. Comp Biochem Physiol 154C:268–274
Xuan R, Wu H, Lin CD, Ma DD, Li YJ, Xu T, Wang L (2013) Oxygen consumption and metabolic responses of freshwater crab Sinopotamon henanense to acute and sub-chronic cadmium exposure. Ecotox Environ Saf 89:29–35
Acknowledgments
We are grateful to Drs. Enmin Zou and Hans-U. Dahms for their critical readings of the manuscript. The work was supported by the Natural Science Foundation of China (no. 31272319, to Lan Wang) and a project jointly funded by the Research Fund for the Doctoral Program of Higher Education (doctoral category).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Xuan, R., Wu, H., Li, Y. et al. Sublethal Cd-induced cellular damage and metabolic changes in the freshwater crab Sinopotamon henanense . Environ Sci Pollut Res 21, 1738–1745 (2014). https://doi.org/10.1007/s11356-013-2068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2068-0