Abstract
Studies on the freshwater crab Sinopotamon henanense have shown that acute and sub-chronic Cd2+ exposure induced differential alterations in the respiratory physiology and gill morphology. To elucidate Cd2+ toxicity under these two exposure conditions, crabs were acutely exposed to 7.14, 14.28, and 28.55 mg/L Cd2+ for 96 h and sub-chronically exposed to 0.71, 1.43, and 2.86 mg/L Cd2+ for 3 weeks. The Cd2+ accumulation, total metallothionein (MT), superoxide dismutase, and malondialdehyde (MDA) contents in the gill tissues were detected. Moreover, the glucose-6-phosphate dehydrogenase (G6PDH) activity, NADPH content, reduced glutathione (GSH), oxidized glutathione (GSSG), and GSH/GSSG ratio in the hepatopancreas were determined. The morphology of the X-organ–sinus gland complex was also observed. The results showed that sub-chronical Cd2+ exposure induced lower MT content and higher MDA level in the gills than in the acute exposure. In the hepatopancreas, acute Cd2+ exposure decreased the pentose phosphate pathway activity and NADPH content; however, an increased G6PDH activity and NADPH content were detected in sub-chronic Cd2+ exposure (2.86 mg/L). Morphological changes occurred in the sinus gland in crabs exposed to 2.86 mg/L Cd2+ for 3 weeks. The tightly packed structure composed by the axons, enlarged terminals, and glial cells, became loose and porous. Ultra-structurally, a large number of vacuoles and few neurosecretory granules were observed in the axon terminal. These effects added to our understanding of the toxic effects of Cd2+ and provide biochemical and histopathological evidence for S. henanense as a biomarker of acute or long-term waterborne Cd2+ pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd2+) is a heavy metal pollutant widely present in aquatic environments, entering organisms through breathing and feeding. Aquatic animals exposed to Cd2+ exhibit various stress responses: slowing the breathing or metabolic rate to reduce Cd2+ absorption (Vijayavel and Balasubramanian 2006); accelerating the synthesis of metallothionein (MT), a Cd2+ chelating protein, to reduce intracellular free Cd2+ (Li et al. 2015b; He et al. 2019; Matić et al. 2020; Yang et al. 2019, 2020); and activating the antioxidant defense system (Atli and Canli 2010; Zhou et al. 2016, 2017). The freshwater crab Sinopotamon henanense, an important representative of decapod crustaceans, is a highly relevant species in the context of metal toxicology (Xu et al. 2019a). Our previous study on the effects of acute and sub-chronic Cd2+ on the oxygen consumption and respiratory metabolism of S. henanense found that acute treatment increased oxygen consumption and promoted aerobic respiration, whereas sub-chronic treatment reduced the aerobic respiration rate (Xuan et al. 2013). The observation of acute and sub-chronic Cd2+-induced cellular damage in S. henanense also indicated that sub-chronic Cd2+ exposure caused more profound morphological damages in the gills than acute exposure did (Xuan et al. 2014). These results suggested that the crabs’ response strategies to the two exposure conditions were different. To supplement our knowledge of Cd2+ toxicity under the two exposure conditons, we selected three typical organs: the gills, hepatopancreas, and X-organ–sinus gland complex, representing the most susceptible organ, a detoxification organ, and a less susceptible organ, respectively, and evaluated and compared their responses to acute and sub-chronic Cd2+ exposure.
Several studies have shown that the histological and ultrastructural changes induced by Cd2+ were closely related to oxidative damage (Li et al. 2015a; Zhou et al. 2017; Das et al. 2019). In these cases, whether the evident morphological changes induced by sub-chronic Cd2+ exposure (2.86 mg/L Cd2+ for 3 weeks) were related to Cd2+-induced severe oxidative damage in the gills is unknown. To test this hypothesis, in this study, we detected and compared the Cd2+ accumulation, MT content, total superoxide dismutase (SOD) activity, and malondialdehyde (MDA) content in the gills of crabs exposed to acute and sub-chronic waterborne Cd2+. As the main detoxification organ for crabs, Cd2+ accumulation and MT levels in the hepatopancreas during acute and chronic Cd2+ exposure have been extensively reported (Silvestre et al. 2005; Ma et al. 2008), as well as the SOD activity and MDA content (Li et al. 2008). Nevertheless, the hepatopancreas is also the main site for another main antioxidant defense system, the glutathione system, especially for the synthesis of reduced glutathione (GSH), an important antioxidant. The ratio of GSH/GSSG (oxidized glutathione) could sensitively reflect the cellular redox status (Wang et al. 2008). NADPH was crucial for maintaining the restored state of GSH, and glucose-6-phosphate dehydrogenase (G6PDH) in the pentose phosphate pathway (PPP) is the main provider of NADPH in various detoxification pathways (Tian et al. 1999; Winzer et al. 2002; Pierron et al. 2007). These results showed that G6PDH and NADPH play crucial roles during the detoxification process in the hepatopancreas (Winzer et al. 2002). However, reports on their responses to Cd2+ are limited. This study analyzed and compared the G6PDH activity, NADPH content, and GSH/GSSG ratio between acute and sub-chronic Cd2+ exposure to clarify the response of these important biological indicators to Cd2+ and increase the understanding of Cd2+ toxicity.
The X-organ–sinus gland complex is one of the most important endocrine organs in crustaceans; it plays regulatory roles in energy metabolism, molting, growth, ion regulation, and reproduction (Böcking et al. 2002; Webster et al. 2012; Duangprom et al. 2017; Liu et al. 2019; Xu et al. 2019b; Sook Chung et al. 2020). This complex is located in the optic ganglia, surrounded by pigments and muscle and protected by the exoskeleton of the eyestalk, suggesting that it is less susceptible to Cd2+ toxicity. Although studies have shown that Cd2+ has a potential interference or inhibitory effect on the endocrine activity of crabs (Medesani et al. 2001, 2004), whether Cd2+ toxicity involves the histopathological alteration of this organ in crustaceans is unknown. In this study, we observed the morphology of this organ under two different Cd2+ exposure conditions.
Biochemical indicator detection of the gills and hepatopancreas and morphological observation of the X-organ–sinus gland complex could elucidate the toxic effects of Cd2+ and provide biochemical and histopathological evidence for S. henanense as a biomarker of acute or long-term waterborne Cd2+ pollution.
Materials and methods
Experimental animals and design
Crabs were purchased from the Dongan Aquatic Market in Taiyuan, China. Prior to experiments, crabs were acclimated for 2 weeks in glass aquarium filled with dechlorinated, carbon-filtered city tap water (pH 7.5, dissolved oxygen 8.0–8.3 mg/L). Ten crabs with 15.5 ± 1.5 g in one aquarium (50 cm × 30 cm × 25 cm) was as a reasonable stocking density. Temperature was kept at 20 ± 2 °C. Aquaria were shielded by a black plastic to reduce disturbance. Crabs were fed commercial feed three times a week.
Stock solution of the metal was prepared by dissolving CdCl2˙21/2H2O (analytical grade) in distilled water. Appropriate volumes of stock solution were added to 4 L city water to get a series of expected Cd2+ solutions. For acute exposure, crabs were allocated to control, 7.14, 14.28, and 28.55 mg/L Cd2+ solutions for 96 h, corresponding to 0, 1/16, 1/8, 1/4 of the Cd’s 96 h LC50 for S. henanense (Wang et al. 2008). For sub-chronic exposure, crabs were treated with controls, 0.71, 1.43, and 2.86 mg/L Cd2+ solutions for 21 days, corresponding to 0, 1/160, 1/80, and 1/40 of the Cd’s 96 h LC50 for S. henanense (Xuan et al. 2013). For each group, 10 males with similar weight of 15.3 ± 0.6 g were randomly divided into two aquariums.
During the experimental period, all groups were fed daily (1% of body weight) feedstuff containing 35% protein. The exposure medium was renewed every 48 h. All other conditions were kept the same as those used for acclimation.
Histology and ultra-structural sample preparation
At the end of the exposure, the crabs were anesthetized in ice bath. The eye stalk was cut off and the optic ganglia was separated from the exoskeleton in saline, then the pigments and muscles surrounding the ganglia were peeled off. The right optic ganglion of 5 crabs in the control or the treatment groups were used as the transmission electron microscope (TEM) sample, and the left optic ganglion were used as histology sample.
For histology sample, the ganglion was wrapped with gauze cloth, labeled, sealed, then fixed in Berne's fixative solution for 24 h. Samples were dehydrated with ethanol and a toluene series and embedded in paraffin. Serial sections (4 μm) were mounted on gelatin-coated glass slides and stained with hematoxylin and eosin. Ten to 20 slides were examined with a light microscope (Olympus BX51, Japan).
For TEM sample, immediately after being excised from the ganglion, the sinus gland was immersion-fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.4), postfixed in 1% osmium tetroxide and embedded in thin viscosity resin. Ultrathin sections were cut with an ultramicrotome (Leica UC-6, Germany), stained with uranyl acetate and lead citrate, and examined by TEM (JEM-1400, Japan).
Determination of Cd2+ accumulation in gills
At the end of the exposure, Cd2+ accumulation in gills was determined according to the method described by Jing et al. (2019). The experimental crabs were cryoanesthesized by putting them on ice for about 15 min, then, their gills (≥ 0.1 g) were immediately excised, weighed, and digested at room temperature overnight in the digestion bottles containing nitric acid and perchloric acid (4:1) (20 mL acid solution/0.2 g tissue), then the bottles were placed on the hot plate till the tissues became transparent. Finally, the tissue digestion fluid was diluted with distilled water, and Cd2+ concentration (μg/g) was determined by flame atomic absorption spectrometer (Varian AA240, USA).
Determination of MT content and calculation of Cd2+-binding potentials (CBP)
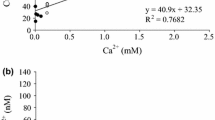
For the determination of MT content, we referred to the method of Ma et al. (2008): 400 μL Tris-HCl buffer (pH 8.6, 0.01 M) was added to 0.1 g wet tissue to prepare the homogenate, which was centrifuged at 12000g, 4 °C for 15 min, then 500 μL supernatant was added into equal volume of CdCl2 (20 μg/mL) solution. The mixture was placed at room temperature for 5 min, and added into 200 μL of freshly prepared 2% bovine hemoglobin (prepared with 0.2 M pH 3.7 acetic acid-sodium acetate), mixed well and ice bathed for 5 min, then the mixture was transferred to boiling water bath for 2 min and centrifuged at 10000g, 4 °C for 10 min. One milliliter of supernatant was added into 2 mL of Tris-HCl, mixed well, and Cd2+ concentration (C) was measured with flame atomic absorption spectrometer. MT content (μg/g) = C/112.4/6 × 6000.
Cd2+-binding potentials of MT (CBP) = Cd2+ accumulation in tissue/Cd2+ content (C) determined by cadmium-hemoglobin saturation method. If CBP > 1, Cd2+ in the tissue will not be completely bound by MT.
Determination of total SOD activity and MDA content
Commercial kits from Nanjing Jiancheng Bioengineering Institute were used to determine SOD activity (A001-1-2), MDA content (A003-1-2), and total tissue protein content (A045-2-2).
Determination of glucose-6-phosphate dehydrogenase (G6PDH) activity in hepatopancreas
For the enzyme assay, commercial kit (GENMED, Shanghai, China) was used. About 0.1 g of tissue was added to the homogenization buffer at a mass to volume ratio of 1:5, and homogenized on ice bath, then the homogenate was centrifuged at 12000 rpm, 4 °C for 12 min. Protein content of the supernatant was measured by Coomassie brilliant blue method (Jiancheng Nanjing Institute of Bio-Engineering, China); For G6PDH activity determination, the enzyme reaction solution in the kit was added to the supernatant, and the change rate of its absorbance at 340 nm was measured by micro-plate reader.
G6PDH activity (nmol NADPH/min/mg protein) = [△A340nm × reaction volume (ml)] ÷ [sample load (ml) × 6.22 (millimolar absorption coefficient) × light path (cm) × reaction time (min)] ÷ sample protein content (mg) × 103
Determination of reduced coenzyme II (NADPH) in hepatopancreas
NADPH concentration was detected according to the kit instruction (GENMED, Shanghai, China). The powdered NADPH-Na4 was prepared into a 20 mM stock solution with dilution buffer (same as the homogenization buffer in NADPH content measurement). The stock solution was further diluted to a series of concentrations: 1 mM, 0.5 mM, 0.25 mM, 0.125 mM, 0.0625 mM, 0.03125 mM, 0.015625 mM, and 0 mM, then the absorbance at 340 nm was measured. Five independent experiments were carried out and the average value was used to get a standard curve.
Method for determining the content of tissue NADPH: About 0.1 g of tissue was added to the homogenization buffer at a mass to volume ratio of 1:5. The homogenate was centrifuged at 9900 rpm, 4 °C for 10 min, then the absorbance of the supernatant at 340 nm (A1) was measured. Another 20 μL supernatant and 180 μL of reaction solution (without glutathione reductase (GR)) were added to the plate, and incubated at 25 °C for 5 min, then, 1 μL GR was added and incubated at 25 °C for 5 min, finally, the absorbance value (A2) was measured at 340 nm. The absorbance value of NADPH was A1-A2, and its content was calculated according to the NADPH standard curve above.
Determination of reduced glutathione (GSH) and oxidized glutathione (GSSG) in hepatopancreas
Commercial kits from Nanjing Jiancheng Bioengineering Institute were used to determine GSH (A006-2-1) and GSSG (A061-1-2) content.
Statistical analysis of the results
Statistical analyses were performed with SPSS 15.0 software. Data distributions and the homogeneity of variance were tested using the Kolmogorov-Smirnov and Levene tests, respectively. When the data satisfied the prerequisites for parametric tests (ANOVA), one-way ANOVA and Dunnet’s test were used to evaluate the significance of differences between the treatment and control groups. Otherwise, the Kruskal-Wallis H test was used. Numerical results are given as means ± SE. A probability value of less than 0.05 was regarded as significant. The figures were generated by Origin 8.0 software.
Results
Effect of acute and sub-chronic Cd exposure on Cd2 + accumulation in the gills of S. henanense and its stress responses
Compared to the control, both acute (28.55 mg/L for 96 h) and sub-chronic (2.86 mg/L for 21 days) Cd2+ exposures caused evident Cd2+ accumulation in the gills (Fig. 1a), and Cd2+ was not completely bound by MT (Fig. 1c). SOD activity was activated and the lipid peroxidation product MDA was significantly increased (Fig. 1d–e). There were no significant differences in Cd2+ accumulation, Cd2+-binding potential (CBP), and total SOD activity between acute and sub-chronic Cd2+ exposures. However, the MT level during acute exposure was significantly increased compared to that during sub-chronic exposure (Fig. 1b), and the MDA concentration showed the opposite results (Fig. 1e).
Effect of acute and sub-chronic Cd exposures on Cd2+ accumulation in the gills of S. henanense and its stress responses. a Cd2+ accumulation, b Metallothionein (MT) content, c Average Cd2+-binding potential (CBP) of MT. d Total superoxide dismutase (SOD) activity. e Malondialdehyde (MDA) content. *Significant differences between Cd2+-treated groups and the control (p < 0.05); #Significant differences between the acute and sub-chronic Cd2+-treated groups (p < 0.05) (mean ± SE, N = 5). Acute = 28.55 mg/L Cd2+ for 96 h, sub-chronic = 2.86 mg/L Cd2+ for 21 days
Effect of acute and sub-chronic Cd exposures on PPP activity and redox state in the hepatopancreas of S. henanense
The G6PDH activity was decreased significantly during acute Cd2+ exposure, and there was no conspicuous concentration–effect relationship (Fig. 2a). During sub-chronic Cd2+ exposures, the activity was enhanced with increased Cd2+ concentration, and a significant increase was detected in the group treated with 2.86 mg/L Cd2+ (Fig. 2b, p < 0.01). The NADPH contents in the acute groups were decreased with increased Cd2+ concentration, and significantly decreased under 14.28 and 28.55 mg/L Cd2+ exposure (Fig. 2c, p < 0.01). For sub-chronic Cd2+ exposure, there were no evident changes in NADPH content in the groups treated with 0.71 and 1.43 mg/L Cd2+; however, 2.86 mg/L of Cd2+ exposure induced a significant increase in NADPH content (Fig. 2d, p < 0.01).
After acute Cd2+ treatment, the GSH content and GSH/GSSG ratio were evidently decreased with increased Cd2+ concentration (Fig. 3a, e, p < 0.05), and there was no significant change in GSSG content (Fig. 3c, p > 0.05); For sub-chronic Cd2+ exposure, the GSH content was increased at the Cd2+ concentration of 0.71 mg/L and decreased at 2.86 mg/L (Fig. 3b). The GSSG content was increased in the groups with higher concentrations of Cd2+ (1.43 and 2.86 mg/L), accompanied by a decrease in the GSH/GSSG ratio (Fig. 3d, f).
Effect of acute and sub-chronic Cd exposure on the reduced glutathione (GSH) and oxidized glutathione (GSSG) contents and GSH/GSSG ratio in the hepatopancreas of S. henanense. a, c, e Acute group. b, d, f Sub-chronic group. a, b GSH content. c, d GSSG content. , f GSH/GSSG ratio; *p < 0.05; **p < 0.01 (mean ± SE, n = 5)
Effect of sub-chronic Cd exposure on the histology and ultrastructure of the sinus gland in S. henanense
Under the anatomical microscope, the sinus glands of the crab were milky white, flat, and spherical (Fig. 4a), located at the junction of the internal and terminal medullae of the optic ganglion (Fig. 4b). From the histological observation of the X-organ–sinus gland complex, the sinus gland was cystic, with the blood sinus cavity in the center, surrounded by a wall built up with axons, enlarged terminals, and glial cells. The axons, axon terminals, glial cells, and their protrusions were tightly wound inside the gland to form a solid structure, which fixes the shape of the gland. The axon nerve terminals were arranged radially along the central blood cavity (Fig. 4c). Many small blood sinuses could be observed in the gland wall, extending from the central blood cavity to the outer wall, so that the entire gland was bathed in the blood cavity (Fig. 4c). After the crabs were exposed to 2.86 mg/L waterborne Cd2+ for 21 days, the structure of the sinus glands was significantly looser than that in the control, and the fence structure that formed the wall of the sinus gland had disappeared. The tightly packed structure composed by the axons, enlarged terminals, and glial cells became loose and porous, and the sinus gland structure was near collapse (Fig. 4d–f).
Effect of sub-chronic Cd on the histology of the sinus gland in freshwater crab S. henanense. a Solid anatomy of the optic ganglion (× 50). The white flat spherical structure indicated by the arrow is the sinus gland (SG). b Histology of the optic ganglion of the normal group (× 100). The encircled structure is the SG, located at the junction of the internal (MI, medulla interna) and terminal medulla (MT, medulla terminalis) of the optic ganglion. c Enlarged structure of SG in the normal group (× 600) with a solid fence structure formed by tightly wound axons of neurosecretory cells, enlarged terminals, and glial cells. d–f Sub-chronically exposed group (2.86 mg/L Cd2+ for 21 days) at × 100 (d), × 200 (e), and × 600 (f) magnification. The fence structure forming SG wall disappeared, the tightly packed structure composed by the axons, enlarged terminals, and glial cells became loose and porous, and the SG structure was near collapse.
Under the electron microscope, different types of dense neuroendocrine granules around the blood sinus (Fig. 5a–c) wrapped in the enlarged axon tip of different neuroendocrine cells (Fig. 5b) were observed. Numerous microtubule structures and neurosecretory particles can be observed in the axons of neuroendocrine cells (Fig. 5d). A few mitochondria were also found in the axon tips (Fig. 5e–f).). Ultrastructure alterations in the X-organ–sinus gland complex were observed in the group treated with 2.86 mg/L Cd2+ for 21 days (Fig. 6a–d). Compared to the dense neuroendocrine granule distribution in the control group (Fig. 5a–c), Cd2+ exposure caused an evident reduction in neurosecretory particles around the blood sinus (Fig. 6a). Numerous vacuoles and partially damaged mitochondria were observed in the axon terminal (At) (Fig. 6b, c), and very few secreted particles were observed (Fig. 6d).
Ultrastructure of sinus gland in freshwater crab S. henanense. a × 5000, the small blood sinus (S) and the blood cells (hemocyte [Hc]) are shown. b × 10,000, many different types of neurosecretory granules (Ng) are distributed around the blood sinus (S). c × 6000, different types of Ng filled in the gap between two small sinuses (S). d × 10,000, nerve cell axons (A), containing microtubules (Mt), mitochondria (M), and Ng. e × 15,000, the enlarged axon terminal (At), containing a numerous Ng and a few mitochondria (M). f × 15,000, different types of axon terminals (At), containing a large number of different types of Ng
Effect of sub-chronic Cd on the ultrastructure of the sinus gland in freshwater crab S. henanense. a Sub-chronically exposed group (2.86 mg/L Cd2+ for 21 days) (× 8000), indicating that the neurosecretory granules distributed around the blood sinus are reduced; b sub-chronically exposed group (× 12,000) showing numerous vacuoles and partially damaged mitochondria (M) in the axon tip (At). c Sub-chronically exposed group (× 15,000) showing vacuolated axon tip and damaged mitochondria. d Sub-chronically exposed group (× 40,000) showing numerous vacuolated structures and few neurosecretory granules (Ng)
Discussion
Low MT content and high lipid peroxidation level in the sub-chronically exposed group may be responsible for the more profound gill damage
The Cd2+ accumulation in organisms is potentially correlated with the biological effects (Vijayavel and Balasubramanian 2006; Cheng et al. 2018; Yu et al. 2020). Our results showed that both the acute (28.55 mg/L Cd2+ for 96 h) and sub-chronic (2.86 mg/L Cd2+ for 21 days) Cd2+ treatments led to significant Cd2+ accumulation in the gills of S. henanense, and there was no significant difference between the two treatments. Nonetheless, we observed more profound tissue damage induced by sub-chronic Cd2+ exposure (2.86 mg/L Cd2+ for 21 days) (Xuan et al. 2014). It can be speculated that the differential damage between the two treatments may be related to the mechanism of Cd2+ clearance and stress resistance in this organ.
MT, as a type of cysteine-rich metal-binding protein, can be induced by multiple metals. It binds to heavy metals through thiol groups on cysteine residues, reducing or avoiding the binding of free heavy metal ions to biological macromolecules (Klaassen et al. 2009; Martinez-Finley and Aschner 2011; Pedersen et al. 2014). When the concentration and time of Cd2+ exposure continued to increase, the synthesis of MT reaches saturation or even declined, Cd2+ recruitment exceeds the binding capacity of MT, and free Cd2+ causes damage to the organisms (Li et al. 2015b). This study showed that MT synthesis in the acute group was significantly higher than that in the sub-chronic group. A similar result was observed by Silvestre et al. (2005), who reported that the MT-like proteins (MTLPs) seemed to be induced mainly in response to direct acute exposure in the anterior gill of the Chinese crab Eriocheir sinensis, and other sequestration and/or detoxification mechanisms might occur during long-term exposure. It was speculated that the acute Cd2+-induced increase in MT content was a result of the crab’s quick response. Nevertheless, the CBP showed that the Cd2+ recruitment exceeded the chelating capacity of MT during both exposures, suggesting that the decreased chelating capacity of MT was not the direct cause of gill tissue damage.
Numerous studies have shown that Cd could induce the generation of reactive oxygen species (ROS), which cause oxidative stress to the organisms and activates the antioxidant system (Wang et al. 2008; Zhou et al. 2016). When the generation rate of ROS exceeds the defense capacity of the antioxidant system, the ROS attack the polyunsaturated fatty acids in the biomembrane system, leading to lipid peroxidation and a series of oxidative damages (Zhou et al. 2017). Studies have shown that oxidative damage is a basic event in the process of tissue and cell damage. ROS can cause damage to major cell components, such as lipids, proteins, and nucleic acids, as well as induce apoptosis or necrosis (Rhee et al. 2013). Lipid peroxidation is the main mechanism of cell damage (Schuwerack and Lewis 2003). MDA is the final decomposition product of lipid peroxidation, and its content determines the degree of lipid peroxidation (Lei et al. 2011; Wang et al. 2011). In the present results, the significantly high MDA content in the sub-chronic group indicated that 2.86 mg/L Cd2+ exposure for 21 days induced more profound lipid peroxidation in the gills than acute Cd2+ exposure did. ROS mainly include superoxide anion radicals (O2–) and hydroxyl radicals (·OH). The enzyme that scavenges O2– in the organism is SOD, and its function is to disproportionate O2– to H2O2 and prevent O2– from invading the organism. In this study, the increased SOD activity did not show significant difference between the two exposure conditions, suggesting that the ability to remove O2– made no difference, further indicating that O2– was not the direct cause of differential damage to the gill structure. Increasing evidence has shown that MT has the ability to scavenge free radicals, and it scavenges ·OH better than O2– (Thornalley and Vâsàk 1985; Irato et al. 2001); thus, it is hypothesized that ·OH might be the main factor leading to lipid peroxidation of the gill tissue, and the higher MT content induced by the acute Cd2+ treatment (28.55 mg/L Cd2+ for 96 h) may be responsible for removing ·OH and reducing the gill damage in this group (data not shown). This inference requires further verification.
Differential PPP activity in the hepatopancreas of S. henanense may be an indicator of acute or long-term waterborne Cd2+ pollution
This study revealed a differential response of PPP activity between the acute and sub-chronic Cd2+ treatments. In the acute Cd2+ treatment, the G6PDH activity was significantly decreased, and there was no evident concentration–effect relationship. The reason for this may be that Cd2+ competitively inhibited Mg2+ bonding in the active center of G6PDH, resulting in the decreased activity of this enzyme. Correspondingly, the NADPH content also showed a downward trend. This is consistent with the results of Diaz-Flores et al. (2006) and Wang et al. (2008), indicating that the PPP is the main source of NADPH. Regarding the sub-chronic Cd2+ treatment, 2.86 mg/L Cd2+ exposure induced significant increases in both G6PDH activity and NADPH content, probably reflecting the higher demand for NADPH under such conditions. When Cd2+ enters the organism, GSH will combine with Cd2+ to form a GSH–Cd complex under the action of glutathione sulfur transferase to reduce the free Cd2+ concentration; GSH also converts hydrogen peroxide to water while it is oxidized to GSSG (Liu et al. 2008). To compensate for the large consumption of GSH during detoxification, GSH is further generated from GSSG under the action of enzymes and NADPH. In contrast, NADPH, as an important reducing agent necessary for energy synthesis, is required for a series of stress responses, such as metal chelation, antioxidation, immunity, and damage repair (Wang et al. 2008).
In this study, the GSH content during the sub-chronic Cd2+ treatments showed a difference, increasing first and then decreasing, suggesting that the low concentration (0.71 mg/L) of Cd2+ may activate another pathway for GSH synthesis. The over-expression of γ-cystine synthetase (γ-CST) would lead to increased GSH synthesis. Wang et al. (2008) found that the difference between GSH content and γ-CST activity was consistent in terms of time. Although the NADPH content was increased in the 2.86 mg/L Cd2+ treatment, the GSH content was decreased. This result was similar to that observed during acute Cd2+ exposures. The significantly increased GSSG content indicated that sub-chronic Cd2+ exposure induced the rapid consumption of GSH and an accelerated conversion of GSH to GSSG. The decreased NADPH content during acute Cd2+ exposure probably reflected the inhibition of GSH synthesis, while there was no significant change in GSSG content, suggesting that GSSG was preferentially discharged from cells to reduce oxidative stress (Schafer and Buettner 2001). Nevertheless, the decreased GSH/GSSG indicated that both acute and sub-chronic Cd2+ exposure caused oxidative stress in the hepatopancreas. GSH/GSSG is generally considered a potential indicator of oxidative stress (Lange et al. 2002). Overall, acute and sub-chronic Cd2+ exposure induced differential responses in PPP activity and NADPH content, which may be used as biological indicators for acute or long-term waterborne Cd2+ pollution.
Sub-chronic Cd exposure caused histological and ultrastructural alterations in the X-organ-sinus gland complex in S. henanense
The X-organ-sinus gland complex, located in the optic ganglion of the eyestalk, is an important endocrine organ of crustaceans that is responsible for the synthesis and secretion of crustacean hyperglycemic hormone (CHH), molt-inhibiting hormone (MIH), gonadotropin-inhibitory hormone (GnIH), and mandibular organ-inhibiting hormone (MOIH). Medesani et al. (2001, 2004) found that the Cd-induced hypoglycemic response of the South American river crab Chasmagnathus granulate could be restored to normal levels by injecting CHH. This indicated that Cd potentially interferes with or inhibits the synthesis or secretion of CHH. Although researchers at home and abroad have revealed the histology and ultrastructure of the crustacean X-organ–sinus gland complex (May and Golding 1983; Sun et al. 2001), there have been no reports on Cd-induced morphological changes in the complex. In this study, the complex from both acute (28.55 mg/L Cd2+ for 96 h) and sub-chronic (2.86 mg/L Cd2+ for 21 days) groups were subjected to histological observation and electron microscopy. However, we did not find significant differences between the acute group and the control. This could be because this complex is located in the optic ganglia, surrounded by pigments and muscle and protected by the exoskeleton of the eyestalk, suggesting that it is less susceptible to Cd2+ toxicity; in contrast, during acute Cd2+ exposure, oxygen consumption and aerobic respiration in S. henanense are enhanced to generate sufficient energy for detoxification (Xuan et al. 2013), and the metabolic activity of the organs responsible for detoxification and energy supply, such as the hepatopancreas, are increased. Accordingly, the activity of the sinus gland is reduced, as well as the absorption and accumulation of Cd; thus, tissue damage is not apparent. However, damage to the morphology of the sinus gland and decreased neurosecretory granule distribution in the axon terminal were observed during 2.86 mg/L Cd2+ exposure for 21 days. The main function of the X-organ is to produce and transport neuropeptide hormones to the sinus gland through the axon; the sinus gland is then responsible for storing and releasing these hormones and regulating the metabolic activity of crustaceans. CHH is a multifunctional hormone. Studies have shown that when the external environment (temperature, oxygen, ions) is changed, many crustaceans can adjust the concentration of CHH in the hemolymph to adapt (Lorenzon et al. 2000; Chung and Webster 2005; Chung and Zmora 2008; Kim et al. 2013; Zhang et al. 2020). Regardless of the conditions (aerobic or anaerobic), short-term stress could be resolved by the regulation of CHH, which is responsible for providing a continuous energy metabolism substrate for organisms (Chung et al. 2009). When the storage and release function of the sinus gland is damaged, the physiological function of the peptide hormones in the CHH family would be impaired, even leading to death.
In summary, our results provide a potential reason for the sub-chronic Cd2+ exposure-induced gill morphological damage and reduced respiratory metabolism reported in previous studies. In addition to the differences in respiratory metabolism, the PPP activity and NADPH content in the hepatopancreas, histological and ultrastructural alterations in the X-organ–sinus gland complex in S. henanense also showed differential responses to acute and sub-chronic Cd2+ exposure. These results elucidate the toxic effects of Cd2+ and provide biochemical and histopathological evidence for S. henanense as a bioindicator of acute or long-term waterborne Cd2+ pollution.
Data availability
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Atli G, Canli M (2010) Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol Environ Saf 73:1884–1889
Böcking D, Dircksen H, Keller R (2002) The crustacean neuropeptides of the CHH/MIH/GIH family: structures and biological activities. In: Wiese K (ed) The Crustacean Nervous System. Springer, Berlin, pp 84–97
Cheng L, Zhou JL, Cheng J (2018) Bioaccumulation, tissue distribution and joint toxicity of erythromycin and cadmium in Chinese mitten crab (Eriocheir sinensis). Chemosphere 210:267–278
Chung JS, Webster SG (2005) Dynamics of in vivo release of molt-inhibiting hormone and crustacean hyperglycemic hormone in the shore crab, Carcinus maenas. Endocrinology 146:5545–5551
Chung JS, Zmora N (2008) Functional studies of crustacean hyperglycemic hormone (CHHs) of the blue crab, Callinectes sapidus-the expression and release of CHH in eyestalk and pericardial organ in response to environmental stress. FEBS J 275:693–704
Chung JS, Bembe S, Tamone S (2009) Molecular cloning of the crustacean hyperglycemic hormone (CHH) precursor from the X-organ and the identification of the neuropeptide from sinus gland of the Alaskan Tanner crab, Chionoecetes bairdi. Gen Comp Endocrinol 162:129–133
Das S, Tseng LC, Chou C, Wang L, Souissi S, Hwang JS (2019) Effects of cadmium exposure on antioxidant enzymes and histological changes in the mud shrimp Austinogebia edulis (Crustacea: Decapoda). Environ Sci Pollut Res Int 26(8):7752–7762
Diaz-Flores M, Ibanez-Hernandez MA, Galvan RE (2006) Glucose-6-phosphate dehydrogenase activity and NADPH/NADP+ ratio in liver and pancreas are dependent on the severity of hyperglycemia in rat. Life Sci 78:2601–2607
Duangprom S, Kornthong N, Suwansaard S, Srikawnawan W, Chotwiwatthanakun C, Sobhon P (2017) Distribution of crustacean hyperglycemic hormones (CHH) in the mud crab (Scylla olivacea) and their differential expression following serotonin stimulation. Aquacultrue 468:481–488
He Y, Wang L, Ma W, Lu X, Li Y, Liu J (2019) Secretory expression, immunoaffinity purification and metal-binding ability of recombinant metallothionein (ShMT) from freshwater crab Sinopotamon henanense. Ecotoxicol Environ Saf 169:457–463
Irato P, Santovito G, Piccinni E (2001) Oxidative burst and metallothionein as a scavenger in macrophages. Immunol Cell Biol 79:251–254
Jing W, Lang L, Lin Z, Liu N, Wang L (2019) Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere 219:321–327
Kim BM, Jeong CB, Han J, Kim IC, Rhee JS, Lee JS (2013) Role of crustacean hyperglycemic hormone (CHH) in the environmental stressor-exposed intertidal copepod Tigriopus japonicus. Comp Biochem Physiol 158C:131–141
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238(3):215–220
Lange A, Ausseil O, Segener H (2002) Alterations of tissue glutathione levels and metallothionein mRNA rainbow in trout during single and combined exposure to cadmium and zinc. Comp Biochem Physiol 131C:231–243
Lei W, Wang L, Liu D (2011) Histopathological and biochemical alterations of the heart induced by acute cadmium exposure in the freshwater crab Sinopotamon yangtsekiense. Chemosphere 84:689–694
Li Y, Wang L, Liu N, Wang Q, He Y, Meng F (2008) Effects of cadmium on enzyme activity and lipid peroxidation of Sinopotamon yangtsekiense. Acta Hydrobiologica Sinica 3:373–379
Li N, Hou YH, Ma DD, Jing WX, Dahms HU, Wang L (2015a) Lead accumulation, oxidative damage and histopathological alteration in testes and accessory glands of freshwater crab, Sinopotamon henanense, induced by acute lead exposure. Ecotoxicol Environ Saf 117:20–27
Li Y, Wu H, Wei X, He Y, Li B, Li Y, Jing W, Wang L (2015b) Subcellular distribution of Cd and Zn and MT mRNA expression in the hepatopancreas of Sinopotamon henanense after single and co-exposure to Cd and Zn. Comp Biochem Physiol 167C:117–130
Liu N, Yan B, Li Y, Wang L (2008) The effect of Cd2+ on the glutathione system of the freshwater crab Sinopotamon yangtsekiense. Environ Sci 29(8):2302–2307
Liu A, Liu J, Chen X, Lu B, Zeng C, Ye H (2019) A novel crustacean hyperglycemic hormone (CHH) from the mud crab Scylla paramamosa in regulating carbohydrate metabolism. Comp Biochem Physiol 231A:49–55
Lorenzon S, Francese M, Ferrero EA (2000) Heavy metal toxicity and differential effects on the hyperglycemic stress response in the shrimp Palaemon elegans. Arch Environ Contam Toxicol 39:167–176
Ma W, Wang L, He Y (2008) Tissue-specific cadmium and metallothionein levels in freshwater crab Sinopotamon henanense during acute exposure to waterborne cadmium. Environ Toxicol 23:393–400
Martinez-Finley EJ, Aschner M (2011) Revelations from the nematode Caenorhabditis elegans on the complex interplay of metal toxicological mechanisms. J Toxicol. https://doi.org/10.1155/2011/895236
Matić D, Vlahović M, Ilijin L, Mrdaković M, Grčić A, Filipović A, Perićeri oviić V (2020) Metallothionein level, non-specific esterases, fitness-related traits and integrated biomarker response (IBR) in larvae of Lymantria dispar (Lepidoptera) originating from unpolluted and polluted locations after chronic cadmium treatment. Ecol Indic 112:106136
May BA, Golding DW (1983) Aspects of secretory phenomena within the sinus gland of Carcinus maenas (L.). Cell Tissue Res 228:245–254
Medesani DA, López Greco LS, Rodríguez EM (2001) Effects of cadmium and copper on hormonal regulation of glycemia by the eyestalks, in the crab Chasmagnathus granulata. Bull Environ Contam Toxicol 66:71–76
Medesani DA, López Greco LS, Rodríguez EM (2004) Disruption of endocrine regulation of glycemia levels by cadmium and copper in the estuarine crab Chasmagnathus granulata. Bull Environ Contam Toxicol 73:942–946
Pedersen KL, Bach LT, Bjerregaard P (2014) Amount and metal composition of midgut gland metallothionein in shore crabs (Carcinus maenas) after exposure to cadmium in the food. Aquat Toxicol 150:182–188
Pierron F, Baudrimont M, Bossy A (2007) Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquat Toxicol 81:304–311
Rhee JS, Yu IT, Kim BM, Jeong CB, Lee KW, Kim MJ, Lee SJ, Park GS, Lee JS (2013) Copper induces apoptotic cell death through reactive oxygen species-triggered oxidative stress in the intertidal copepod Tigriopus japonicus. Aquat Toxicol 132-133:182–189
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212
Schuwerack PMM, Lewis JW (2003) Cellular responses to increasing Cd concentrations in the freshwater crab, Potamonautes warreni, harbouring microbial gill infestations. Cell Tissue Res 313:335–346
Silvestre F, Duchêne C, Trausch G (2005) Tissue-specific cadmium accumulation and metallothionein-like protein levels during acclimation process in the Chinese crab Eriocheir sinensis. Comp Biochem Physiol 140 C:39–45
Sook Chung J, Christie A, Flynn E (2020) Molecular cloning of crustacean hyperglycemic hormone (CHH) family members (CHH, molt-inhibiting hormone and mandibular organ-inhibiting hormone) and their expression levels in the Jonah crab, Cancer borealis. Gen Comp Endocrinol 295:113522. https://doi.org/10.1016/j.ygcen.2020.113522.
Sun J, Liu A, Du Y (2001) Microscopic and ultrastructure of sinus glands of Eriocheir sinensis. Acta Zool Sin 47:27–31
Thornalley P, Vâsàk M (1985) Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta 827:36–44
Tian WN, Braunstein LD, Apse K (1999) Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Phys Cell Phys 276:C1121–C1131
Vijayavel K, Balasubramanian MP (2006) Changes in oxygen consumption and respiratory enzymes as stress indicators in an estuarine edible crab Scylla serrata exposed to naphthalene. Chemosphere 63:1523–1531
Wang L, Yan B, Liu N (2008) Effects of cadmium on glutathione synthesis in hepatopancreas of freshwater crab, Sinopotamon yangtsekiense. Chemosphere 74:51–56
Wang L, Xu T, Lei W (2011) Cadmium-induced oxidative stress and apoptotic changes in the testis of freshwater crab, Sinopotamon henanense. PLoS One 6:1–8
Webster SG, Keller R, Dircksen H (2012) The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. Gen Comp Endocrinol 175:217–233
Winzer K, VanNoorden CJF, Kohler A (2002) Glucose-6-phosphate dehydrogenase: the key to sex-related xenobiotic toxicity in hepatocytes of European flounder (Platichthys flesus L.)? Aquat Toxicol 56:275–288
Xu P, Guo H, Wang H (2019a) Identification and profiling of microRNAs responsive to cadmium toxicity in hepatopancreas of the freshwater crab Sinopotamon henanense. Hereditas 156:34
Xu L, Pan L, Zhang X, Wei C (2019b) Effects of crustacean hyperglycemic hormone (CHH) on regulation of hemocyte intracellular signaling pathways and phagocytosis in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 93:559–566
Xuan R, Wu H, Lin C, Ma D, Li Y, Xu T, Wang L (2013) Oxygen consumption and metabolic responses of freshwater crab Sinopotamon henanense to acute and sub-chronic cadmium exposure. Ecotoxicol Environ Saf 89:29–35
Xuan R, Wu H, Li Y, Wang J, Wang L (2014) Sublethal Cd-induced cellular damage and metabolic changes in the freshwater crab Sinopotamon henanense. Environ Sci Pollut Res 21(3):1738–1745
Yang HZ, Gu WJ, Chen W, Hwang JS, Wang L (2019) Metal binding characterization of heterologously expressed metallothionein of the freshwater crab Sinopotamon henanense. Chemosphere 235:926–934
Yang HZ, Wang L, He YJ, Jing WX, Ma WL, Chen CM, Wang L (2020) Analysis of spectrometry and thermodynamics of the metallothionein in freshwater crab Sinopotamon henanense for its binding ability with different metals. Chemosphere 246:125670. https://doi.org/10.1016/j.chemosphere.2019.125670
Yu D, Peng X, Ji C, Li F, Wu H (2020) Metal pollution and its biological effects in swimming crab Portunus trituberculatus by NMR-based metabolomics. Mar Pollut Bull 157:111307
Zhang X, Pan L, Wei C, Tong R, Li Y, Ding M, Wang H (2020) Crustacean hyperglycemic hormone (CHH) regulates the ammonia excretion and metabolism in white shrimp, Litopenaeus vannamei under ammonia-N stress. Sci Total Environ 723:138128
Zhou Y, Dahms HU, Dong F, Jing W, Wang L (2016) Immune-associated parameters and antioxidative responses to cadmium in the freshwater crab Sinopotamon henanense. Ecotoxicol Environ Saf 129:235–241
Zhou Y, Jing W, Dahms HU, Hwang JS, Wang L (2017) Oxidative damage, ultrastructural alterations and gene expressions of hemocytes in the freshwater crab Sinopotamon henanense exposed to cadmium. Ecotoxicol Environ Saf 138 L:130–138 Declarations
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
The work was supported by the Natural Science Foundation of China (NO. 31272319), PhD Start-up Fund of Shanxi Medical University (NO. 03201552), and Youth Fund of Shanxi Medical University (NO.02201626).
Author information
Authors and Affiliations
Contributions
R.X participated in each experiment in the present study and completed the writing of the manuscript; H.W participated in the histological and ultra-structural examination of the X organ-sinus gland complex; Y. L participated in the determination of cadmium accumulation and MT content in the gill; B.W participated in the analysis of the data. The design, execution, data analysis of all experiments in this study, and manuscript writing and revision were completed under the guidance of L.W. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xuan, R., Wu, H., Li, Y. et al. Comparative responses of Sinopotamon henanense to acute and sub-chronic Cd exposure. Environ Sci Pollut Res 28, 35038–35050 (2021). https://doi.org/10.1007/s11356-021-13230-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13230-z