Abstract
Introduction
The influence of sintering temperature on the physico-mechanical characteristics (such as water absorption, apparent porosity, bulk density, weight loss on ignition, firing shrinkage, and compressive strength), leachability, and microstructure of shale brick containing oil well-derived drilling waste (DW) was investigated.
Methods
The experiments were conducted at a temperature ranging from 950°C to 1,050°C with 30% DW addition.
Results
The results indicate that increasing the sintering temperature decreases the water absorption and apparent porosity and increases the shrinkage, density, and compressive strength of sintered specimens. Moreover, the physico-mechanical properties of samples sintered at 1,050°C meet the requirements of the MU20 according to GB/T 5101-2003 (in China). The heavy metal concentrations of the leachate are much lower than the current regulatory limits according to GB16889-2008.
Conclusion
The results from XRD and SEM show that increasing sintering temperature results in an increase of the high temperature liquid phase, which may have a significant effect on the densification process of the samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the petroleum industry, various types of waste materials are generated including oil well-derived drilling waste (DW), which contains significant concentrations of heavy metals and organic pollutants as well as others, making necessary treatment and confined disposal to manage them properly. Therefore, the petroleum industry is confronted with the disposal problem of the huge amount of accumulated waste materials produced over the years. At present, the main predominant waste disposal methods include landfilling, sealing in the pit, incinerating, microbiological processing, mud to cement, and stabilization/solidification (S/S) (Peng et al. 2007; Yang et al. 2003; Charles et al. 2006; Xu et al. 2006; Tuncan et al. 1997). But in the long run, harmful substances will leak to the outside world, which still will bring pollution to the environment.
Sintering treatment technology is becoming more attractive as an alternative method for the recovery and recycling of inorganic wastes and residues. The prospective benefits of using wastes and residues in the production of sintered brick include immobilizing heavy metals in the fired matrix, oxidizing organic matter, and destroying any pathogens during the firing process. So many researchers have successfully developed suitable products (such as lightweight aggregate, bricks, tiles, and other construction products) made from various wastes or residues by sintering such as reservoir sediment, municipal sludge, bottom ash, fly ash, red mud, reservoir sediment, etc. (Lin et al. 2005, 2006; Mangialardi 2001; Lin 2006; Zhang et al. 2007; Chiang et al. 2008; Weng et al. 2003; Taner 2006). At present, most domestic and overseas researches on oil well-derived drilling waste stress on detoxification treatment, but its resource is not utilized. Although, the recycling of oil well muds for the production of bricks has been recently reported (El-Mahllawy and Osman 2010), they used clay as the main material, which is one kind of key and decreasing resources. Actually, the Chinese government has already enacted a law to prohibit using clay to fabricate sintered solid clay brick. So, in this paper, shale was used as the main material instead of clay, and there is rarely research on the utilization of oil well-derived waste in the production of shale brick. This study will potentially provide further beneficial uses of oil well-derived waste.
Therefore, in the present paper, the feasibility of oil well-derived drilling waste used as a replacement material for producing shale brick was investigated. In addition, relationships among sintering temperature, microstructure, and characteristics of sintered shale brick production containing oil well-derived drilling waste were further discussed. For this purpose, experiments were conducted on physico-mechanical properties (water absorption, apparent porosity, bulk density, weight loss on ignition, firing shrinkage) and mechanical property (compressive strength) of sintered specimens. Mineralogy and microstructure of sintered specimens were also discussed by X-ray diffraction (XRD) and scanning electron microscopy (SEM) analysis, respectively.
2 Experiments
2.1 Raw materials
The oil well-derived drilling waste and shale used in this study were collected from Sichuan and Qinhuangdao in China, respectively. Prior to further applications, both the raw materials were dried and ground into fine powder until the particles could pass through a 75-μm mesh sieve. The specific surface area of DW and shale are 486 and 618 m2/kg, respectively; their particle size distributions determined by Mastersizer 2000E are shown in Fig. 1. The physical properties and Atterberg limits of raw materials are summarized in Tables 1 and 2, respectively. The chemical composition of the raw materials, as analyzed by XRF, is shown in Table 3.
As shown in Table 3, the major components in the shale are SiO2 (58.89%), Al2O3 (18.75%), and Fe2O3 (8.95%). DW has a high amount of BaO (30.04%) and SiO2 (58.89%) as well as CaO (18.24%), which will cause lime burst in the sintered bricks. Besides, a higher loss on ignition of DW is also not favored for the production of sintered bricks; this is related to the development of the porosity and the densification in the process of sintering and eventually has an effect on the compressive strength of the sintered products (Lin et al. 2005; Weng et al. 2003). Consequently, DW was mixed in the raw material at a relatively low proportion (30%) in this study.
2.2 Sample preparation
DW and shale were homogenized in a blender by the proportion of 30:70 (by weight), and the mixtures were cured with the addition of 15% water (by weight) in a humidity chamber with a temperature of 20 ± 1°C and a relative humidity of 98 ± 2% for 72 h. Cylindrical green samples were prepared by semi-dry pressing method at 20 MPa for 30 s with the size of φ50 × 40 mm. Green samples were dried at room conditions (25°C, 40% relative humidity) for 48 h and then oven dried at 105°C for 24 h to drive off the moisture prior to the sintering process.
The dried samples were sintered in an air-ventilated electrical furnace according to the following designed heating program. Heating rate was 2°C/min below 400°C, then 3°C/min from 400°C to the sintering temperature, and kept at sintering temperature for 1 h. Sintered specimens were cooled down to room temperature in the furnace. For each species, six samples were prepared for the following tests, and averages and the coefficient of variation were computed to provide statistics and error estimates.
2.3 Testing methods
Chemical and thermal analyses of raw materials were performed by XRF (Axios-Advanced) and TG/DSC (NETZSCH SAT 449C) techniques, respectively. Mineralogical analyses of raw materials and sintered specimens were investigated by X-ray diffraction (XRD-Rigaku D/Max–RB) using Cu-Kα radiation. Microstructure of sintered specimens was investigated by SEM. The particle size distribution of raw materials was determined by Mastersizer 2000E.
Water absorption, apparent porosity, and bulk density of samples soaked in distilled water for 24 h were tested using Shimadzu Balances (AUW220H) basing on the Archimedes principle (Wu 2002). Firing shrinkage was measured by a micrometer. Weight loss on ignition was measured by an electronic analytical balance (TIANYI FA2004). Compressive strength tests were performed using a compression testing machine (TYE-300) at a loading rate of 0.6 kN/s.
Heavy metal leachability experiment was carried out according to the solid waste-extraction procedure for leaching toxicity-acetic acid buffer solution method (HJ/T 300–2007) with samples damaged less than 5 mm, and the leaching solution was analyzed by inductively coupled plasma atomic-emission spectroscopy.
3 Results and discussion
3.1 Thermal analysis
In order to decide the appropriate sintering temperature of shale brick containing DW, the thermal analyses of DW and shale were investigated by TG/DSC. The results of TG/DSC are shown in Fig. 2.
The TG/DSC curves of DW (Fig. 2a) can be explained as follows: (1) An endothermic peak occurs at 82°C due to dehydration (loss of free moisture); (2) Two exothermic peaks occur at 335°C and 454°C with a mass loss due to the combustion of oil and other organic compounds; (3) A second endothermic peak, due to the decomposition of calcite occurs at 710°C; (4) Two endothermic peaks occur at about 1,170°C and 1,210°C with a mass loss of 9.5% due to the decomposition of barite (Ren and Feng 1986).
As shown in Fig. 2b, no obvious endothermic or exothermic peak occurs during the heating process, and the total mass loss was only 3.6%; the DSC curve becomes steep after 1,000°C, and the TG curve shows that there is no quality change; it means the emergence of a large amount of high temperature liquid phases, which can enhance the connection between particles of raw materials and are favorable for the melting of phases and reacting of components. According to the above analysis, three sintering temperatures (950°C, 1,000°C, and 1,050°C) were selected.
3.2 Physico-mechanical properties
The effects of sintering temperature on physical properties (such as water absorption, apparent porosity, bulk density, firing shrinkage, and weight loss on ignition) and compressive strength are shown in Table 4.
Water absorption is an effective index in evaluating the quality and densification of building brick. The less water infiltrates into the brick, the more the durability of the brick and resistance to the natural environment are expected. As shown in Table 4, the water absorption of sintered specimens decreases from 21.07% to 18.27% with the increasing of the sintering temperature from 950°C to 1,050°C, which is due to the apparent porosity of sintered specimens which decreases significantly with increasing sintering temperature similar to the results accepted by Lin Kae Long (Lin 2006). The smaller water absorption that occurred after sintering at the higher temperature (1,050°C) suggests that high temperature liquid phases occurred, which contributed to a decrease in the pore volume and thus the water absorption.
According to the results of bulk density, the bulk density increases from 1.67 to 2.00 g cm−3 with increasing temperature, which is closely related to the results of water absorption and apparent porosity. When the sample absorbs more water, the sample exhibits a higher apparent porosity, resulting in a light density.
The dimensional change in sintered specimens is a prominent indicator for the building brick quality. Table 4 reveals that the shrinkage rate increases significantly with the increasing of sintering temperature. The sintered specimens produce a significant densification, resulting in total volume shrinkage. Accordingly, a contrary trend occurs between the dimensional change and water absorption of sintered specimens (as shown in Table 4). It can be concluded that the better conditions for sintered specimens are higher shrinkage rate and lower water absorption.
The compressive strength is the most important engineering quality index for building materials, and the results indicate that the compressive strength is mainly affected by the sintering temperature. The sintering temperature increases the compressive strength through densification. With the increase of sintering temperature, the compressive strength of samples increases from 13.79 to 24.65 MPa. And sintered at 1,050°C, the strength measured of the samples meets the requirements of the MU20 according to the Chinese National Standard (GB/T 5101–2003 2003). The results indicate that the optimum sintering temperature for maximum compressive strength was 1,050°C, and oil well-derived drilling waste can be used as a replacement material for the production of shale brick.
All the above results indicate that sintering temperature is a key factor determining the brick quality, and compressive strength is closely related to the physical properties.
3.3 Heavy metal leachability
In the process of oil and gas drilling, some additives contain a lot of heavy metals such as chrome and zinc, bringing a large amount of Cr and Zn in the drilling mud. So, in this paper, a test of heavy metal leachability was carried out only on the heavy metals Cr and Zn, and the results of the specimens sintered at different temperatures are summarized in Table 5.
As shown in Table 5, the total leachate concentrations for all tested metals (Cr and Zn) in the sintered specimens reveal a decreasing tendency with the increase of the sintering temperatures, and the leachate concentrations for the tested metals in all sintered specimens are much lower than the permission range of standard value according to the Chinese National Standard (GB16889-2008). In the process of high temperature sintering, nonvolatile species become chemically bonded into the resultant matrix, rendering them nonleachable (Cortez et al. 1996). So, heat treatment has been identified as a potentially effective tool for immobilizing heavy metals into a nonleachable slag.
Actually, compared with the experimental condition, sintered specimens leaching in situ is a slow and gradually diluting process, and only few grains are below 5 mm, so the heavy metal immobilizing of sintered specimens could be more reliable in the practical environment, and a minimum of harmful materials can be contained and released in the environment.
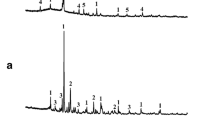
3.4 XRD analysis
Figure 3 shows the X-ray diffraction pattern of raw materials and the samples sintered at different temperatures (950°C, 1,000°C, 1,050°C). The major mineral phases are quartz (SiO2) and dolomite (CaMg(CO3)2) in shale. The DW is mainly composed of quartz (SiO2) and barite (BaSO4). XRD result comparisons for the as-received sediment samples and sintered specimens at different sintering temperatures indicate that the mineral species peaks of dolomite and barite disappeared due to their decomposition in the sintering process, which is in agreement with the result of weight loss on ignition(as shown in Table 2); the major mineral phases of the sintered specimens are quartz, and the intensities of quartz peak decreased with sintering temperature increased from 950°C to 1,050°C, which indicated that emergence of increasing amount of high temperature liquid phases, which are proved in the results of physico-chemical and mechanical properties.
3.5 SEM investigation
SEM investigations were conducted in order to get a better understanding of the morphology of the microstructure and the relationships between physico-mechanical properties and microstructure of the sintered specimens. The SEM results of the sintered specimens at different sintering temperatures are illustrated in Fig. 4.
It is clear that the high temperature liquid phases in sintered specimens, which are favorable for the melting and reacting of raw material components, increased in accordance with increasing sintering temperatures. Furthermore, the particles of sintered specimens sintered at 1,050°C are very closely connected together and have a relatively densified structure. That is, the densified matrix material sintered at 1,050°C shows better surface characteristics than that sintered at 1,000°C (as shown in Fig. 4). As previous results indicate, after being sintered at 1,050°C, the sintered specimens have a good quality, higher compressive strength, and lower leaching toxicity of the building brick than the specimens sintered at 950°C and 1,000°C.
3.6 Properties of standard fired common bricks
Standard fired common bricks were prepared with a size of 240 × 115 × 53 mm according to the Chinese National Standard (GB5101-2003) with the proportion of DW/shale = 30:70 by weight, and fired at 1,000°C. The properties of fired common bricks were listed in Table 6.
4 Conclusion
This study has demonstrated a feasible way of using oil well-derived drilling waste as a partial substitution for shale to produce good quality bricks. Sintered samples were heated to sintering temperatures varying from 950 to 1,050°C. Based on the experiment reported in this paper, the following conclusions and recommendations are drawn:
-
1.
The sintered temperature is a key factor determining the brick quality and compressive strength. Water absorption and apparent porosity decrease with the increase of the sintering temperature, while bulk density and firing shrinkage increase. Compressive strength of the samples increases with the increase of sintering temperature. The physico-mechanical properties of samples sintered at 1,050°C meet the requirements of the MU20 according to the Chinese National Standard (GB/T 5101–2003 2003).
-
2.
Increasing sintering temperature results in an increase of the high temperature liquid phase, which may be have a significant effect on the densification process of the samples.
-
3.
The leachate concentrations of Cr and Zn in all sintered specimens are lower and also in compliance with the permission range of standard value according to the Chinese National Standard (GB16889-2008 2008).
References
Charles GH, Adam D, John L, Kee M, Tim M (2006) An assessment model for the fate and environmental effects of offshore drilling mud discharges. Estuar Coast Shelf Sci 70:577–588
Chiang KY, Chien KL, Hwang SJ (2008) Study on the characteristics of building bricks produced from reservoir sediment. J Hazard Mater 159:499–504
Cortez R, Zaghloul HH, Stephenson LD, Smith ED (1996) Laboratory scale thermal plasma arc vitrification studies of heavy metal-laden waste. J Air Waste Manage 46:1075–1080
El-Mahllawy MS, Osman TA (2010) Influence of oil well drilling waste on the engineering characteristics of clay bricks. J Am Sci 6:48–54
GB/T 5101–2003 (2003) Chinese state standards for sintered common bricks. Chinese Standards Institute (in Chinese)
GB16889-2008 (2008) Standard for pollution control on the landfill site of municipal solid waste. Chinese Standards Institute (in Chinese)
HJ/T 300–2007 (2007) Solid waste-extraction procedure for leaching toxicity-acetic acid buffer solution method. Ministry of Environmental Protection (in Chinese)
Lin KL (2006) Feasibility study of using brick made from municipal solid waste incinerator fly ash slag. J Hazard Mater 137:1810–1816
Lin DF, Luo HL, Sheen YN (2005) Glazed tiles manufactured from incinerated sewage sludge ash and clay. J Air Waste Manage 55:163–172
Lin CF, Wu CH, Ho HM (2006) Recovery of municipal waste incineration bottom ash and water treatment sludge to water permeable pavement materials. Waste Manage 26:970–978
Mangialardi T (2001) Sintering of MSWI fly ash for reuse as a concrete aggregate. J Hazard Mater 87:225–239
Peng Y, Yang X, Sun CJ (2007) The overview of the way to recycle municipal domestic refuse. Environ Sci Manage 32:102–104
Ren YF, Feng L (1986) Mechanism of decomposition of barite in process of sintering Jiuquan barite-containing iron ore concentrate. Acta Metall Sinica 8:142–148
Taner K (2006) Use of boron waste as a fluxing agent in production of red mud brick. Build Environ 41:1779–1783
Tuncan M, Tuncan A, Koyuncu H (1997) Stabilization of petroleum contaminated drilling wastes by additives. Proceedings of the International Offshore and Polar Engineering Conference, Honolulu, Hawaii, 25–30 May, 950–953
Weng CH, Lin DF, Chiang PC (2003) Utilization of sludge as brick materials. Adv Environ Res 7:679–685
Wu HB (2002) Experiments of inorganic non-metallic materials, first edn. China, Beijing
Xu H, Wang B, Qian SS (2006) The solidification treatment experimental research on waste drilling fluid. Environ Sci Manage 7:139–141
Yang MJ, Liang WL, Jin QR (2003) Study on comprehensive treatment technique about waste drilling mud. J Mineralog Petrol 23:109–112 (in Chinese)
Zhang H, Zhao Y, Qi J (2007) Study on use of MSWI fly ash in ceramic tile. J Hazard Mater 141:106–114
Acknowledgments
The work described in the paper is funded by the National Natural Science Foundation of China (51002110) and National Key Technology R&D Program from the Ministry of Science and Technology of China (2011BAJ04B01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Li, XG., Lv, Y., Ma, BG. et al. Influence of sintering temperature on the characteristics of shale brick containing oil well-derived drilling waste. Environ Sci Pollut Res 18, 1617–1622 (2011). https://doi.org/10.1007/s11356-011-0526-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0526-0